Providencia rettgeri septicaemia in farmed crocodiles
Suresh Benedict A and Catherine M Shilton BA Berrimah Veterinary Laboratories
Department of Primary Industries and Fisheries, Northern Territory
Makagon Road, Berrimah
NT 0828, Australia
Tel: +61 9 8999 2249
Email: suresh.benedict@nt.gov.au
B Berrimah Veterinary Laboratories
Department of Primary Industries and Fisheries, Northern Territory
Makagon Road, Berrimah
NT 0828, Australia
Tel: +61 9 8999 2249
Email: cathy.shilton@nt.gov.au
Microbiology Australia 37(3) 114-117 https://doi.org/10.1071/MA16039
Published: 10 August 2016
Bacterial septicaemia is a major cause of morbidity and mortality in farmed saltwater crocodiles (Crocodylus porosus) in the Northern Territory. Providencia rettgeri is the most common aetiological agent. Efficacy of antibiotic treatment is dubious and there are high levels of resistance to antibiotics commonly used by farms, underlining the need for exploration of new approaches to managing the disease.
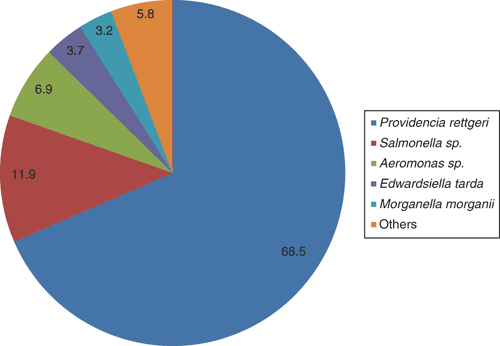
Saltwater crocodile farming is a growing industry in Australia, with an annual gross value of over $50 million, the main product being high quality skins for the luxury leather market. In the Northern Territory, there are several farms, the largest having approximately 40 000 crocodiles. Berrimah Veterinary Laboratories (BVL) is situated within 30 km of the four largest farms, facilitating a close collaborative relationship. Each year, BVL receives from 50–100 farmed crocodile diagnostic submissions, the vast majority concerning hatchling to juvenile crocodiles (2–8 months of age). In this age group, a very common cause of death is bacterial septicaemia, with Providencia rettgeri predominating (Figure 1).
|
Grossly, affected crocodiles may exhibit regional subcutaneous and serosal vascular congestion and oedema (Figure 2a, b). Despite young crocodiles being offered food every 2–3 days, septicaemic crocodiles invariably have no stomach content at necropsy, indicating that affected crocodiles are inappetent. Histological findings include fibrinous pyogranulomatous cellulitis and polyserositis, intravascular coagula of fibrin, macrophages and degranulating heterophils, and acute multifocal splenic necrosis and heterophil infiltration (Figure 2c).
Definitive diagnosis in cases of suspected septicaemia is achieved by bacterial culture of at least two, aseptically sampled, blood filtering organs (lung, liver, spleen or lung). Tissues are homogenised aseptically in physiological saline and a swab soaked in homogenised samples is inoculated on tryptic soy agar with sheep’s blood and MacConkey agar for aerobic culture. The plates are incubated at 35°C and are examined for bacterial growth after overnight and 48 hours incubation. The predominant colony type is then selected for biochemical testing. In cases of P. rettgeri septicaemia, the bacterium is typically present as a moderate to heavy growth in pure culture.
Providencia rettgeri appears non-haemolytic on sheep’s blood agar and is an aerobic, Gram-negative bacillus, oxidase negative and catalase positive (Figure 3). A commercial kit, Microbact 24E (Oxoid Ltd), is routinely used for biochemical identification and the test results after overnight incubation at 35°C are as follows: negative reactions for lysine decarboxylase, ornithine decarboxylase, H2S production, ONPG, acetoin production, gelatin liquefaction, malonate inhibition, arginine dihydrolase and fermentation of xylose, sorbitol, sucrose, lactose, arabinose and raffinose; positive reactions for indole, urease, citrate utilisation, TDA and fermentation of glucose, mannitol, adonitol and inositol; mostly positive but occasional negative reactions for fermentation of rhamnose and salicin (octal codes: 06331212, 06331012, 06331210 or 06331010).
The in vitro antimicrobial susceptibility testing on P. rettgeri is performed by disc diffusion method using the Clinical and Laboratory Standards Institute guidelines1,2. The three antibiotic treatments, namely sulphafurazole, tetracycline and sulphamethoxazole with trimethoprim, are routinely tested at BVL on crocodilian bacterial isolates as requested by the local crocodile farmers, since these are the antibiotics added to food for treatment. A total of 139 P. rettgeri isolates were tested between 2010 and 2015, of which 44% were sensitive to all three antibiotic treatments mentioned above, 21% were resistant to all three antibiotic treatments, 21% were resistant only to tetracycline and 14% were resistant only to sulphonamides. Antimicrobial resistance in crocodilian bacterial isolates may be due to development either of resistance in response to antibiotics used at the farm, or selective pressure for innately resistant bacteria in the environment. The high level of antibiotic resistance, and the fact that antibiotics are being used in food to treat septicaemic crocodiles that are likely not eating, are clear indications of the need for an alternative approach to the use of antibiotics to overcome this problem.
Bacteria belonging to the genus Providencia, family Enterobacteriaceae, are opportunistic pathogens that have been isolated from a range of environments and hosts including humans3. P. rettgeri has been associated with a variety of infections in humans including, travellers’ diarrhoea4, urinary tract infection, especially in certain types of immunocompromised patients5, hospital -acquired and community-acquired neuroinfection6, ocular infections7 and peritonitis8. Although P. rettgeri is a relatively uncommon isolate in septicaemic reptiles9, it has been found to be a frequent isolate from some reptilian environments, either as a part of their normal flora or in opportunistic infections10. The bacterium has also been noted to cause granulomatous pneumonia and hepatitis in a crocodile monitor lizard11, respiratory tract infection in a ball python12, septicaemia and meningitis in American alligators13, and meningitis in hatchling saltwater crocodiles14.
The occurrence of bacterial septicaemia in farmed crocodiles in the Northern Territory seems to be influenced by age and climate. The infection is rare in crocodiles older than one year of age, suggesting that adaptive immunity likely plays a role in resistance to infection. In young crocodiles, after hatching in the late wet season, the majority of infections do not occur until the onset of the dry season, in which there are relatively low atmospheric and/or water temperatures (average daily maximum temperature is 33°C during both seasons, but minimum temperature averages 18°C during the dry season compared to 24°C during the wet season) (Figure 4). The bactericidal function of crocodilian complement, an important component of the innate immune system, significantly decreases at temperatures below 15°C and above 30°C15,16. The experimental bacterial killing assay on crocodile plasma revealed that juvenile crocodiles have a more established innate ability to neutralise Escherichia coli compared with P. rettgeri17. Avenues for further investigation include characterisation of P. rettgeri virulence factors, determination of environmental factors on crocodile farms that may be promoting its presence and ability to cause infection, and efficacy of alternative methods to antibiotics to decrease the environmental and intestinal load and impact of P. rettgeri in crocodiles, such as alternative pen cleaning and water treatment methods and/or use of probiotics or vaccination.
Acknowledgements
The authors thank the major crocodile farms in the Darwin region for many interesting submissions and collaborations over the years. Numerous other staff at BVL were involved in the cases detailed in this report. Gregory P Brown, a post-doctoral fellow at The University of Sydney, performed the statistics.
References
[1] Wayne, P.A. (2013) CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria isolated From Animals: Approved Standard – Fourth Edition. CLSI document VET01-A4. Clinical and Laboratory Standards Institute 33, 1–71.[2] Wayne, P.A. (2013) CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; second informational supplement. CLSI document VET01-S2. Clinical and Laboratory Standards Institute 33, 15–29.
[3] Galac, M.R. and Lazzaro, B.P. (2011) Comparative pathology of bacteria in the genus Providencia to a natural host, Drosophila melanogaster. Microbes Infect. 13, 673–683.
| Comparative pathology of bacteria in the genus Providencia to a natural host, Drosophila melanogaster.Crossref | GoogleScholarGoogle Scholar | 21354324PubMed |
[4] Yoh, M. et al. (2005) Importance of Providencia species as a major cause of travellers’ diarrhoea. J. Med. Microbiol. 54, 1077–1082.
| Importance of Providencia species as a major cause of travellers’ diarrhoea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Cgu7nP&md5=0bf51251c0f758bc22b1bf6c35befb9dCAS | 16192440PubMed |
[5] Arroyo, J.C. et al. (1977) Proteus rettgeri infections: a review. J. Urol. 117, 115–117.
| 1:STN:280:DyaE2s%2FnsVGgtw%3D%3D&md5=4232746999dbc742431bc13823d252cdCAS | 583779PubMed |
[6] Maiti, T.K. et al. (2013) Providencia rettgeri: an unusual cause of central nervous system infections. Am. J. Med. Sci. 346, 158–159.
| Providencia rettgeri: an unusual cause of central nervous system infections.Crossref | GoogleScholarGoogle Scholar | 23811573PubMed |
[7] Koreishi, A.F. et al. (2006) Ocular infections caused by Providencia rettgeri. Ophthalmology 113, 1463–1466.
| Ocular infections caused by Providencia rettgeri.Crossref | GoogleScholarGoogle Scholar | 16797710PubMed |
[8] Wang, T.K.M. et al. (2014) Providencia rettgeri peritonitis in a patient on peritoneal dialysis with perforated appendicitis. Perit. Dial. Int. 34, 569–570.
| 1:CAS:528:DC%2BC2cXptlKgsbs%3D&md5=9e1f406f6fd8a338096c171826baaab2CAS |
[9] Jacobson, E.R. (2007) Bacterial diseases of reptiles. In Infectious Diseases and Pathology of Reptiles (Jacobson, E.R., ed.), pp. 461–526, CRC Press.
[10] Shotts, E.B. (1981) Bacterial diseases of alligators: an overview. In Proceedings of the First Annual Alligator Production Conference, University of Florida, Gainesville, pp. 36-41.
[11] Kycko, A. et al. (2013) Granulomatous pneumonia and hepatitis associated with Providencia rettgeri infection in a crocodile monitor lizard (Varanus salvadorii). Acta Vet. Hung. 61, 51–58.
| Granulomatous pneumonia and hepatitis associated with Providencia rettgeri infection in a crocodile monitor lizard (Varanus salvadorii).Crossref | GoogleScholarGoogle Scholar | 23439291PubMed |
[12] Myers, D.A. et al. (2009) Saccular lung cannulation in a ball python (Python regius) to treat a tracheal obstruction. J. Zoo Wildl. Med. 40, 214–216.
| Saccular lung cannulation in a ball python (Python regius) to treat a tracheal obstruction.Crossref | GoogleScholarGoogle Scholar | 19368267PubMed |
[13] Camus, A. et al. (2002) Providencia rettgeri-associated septicaemia and meningoencephalitis in juvenile farmed American alligators Alligator mississippiensis. J. Aquat. Anim. Health 14, 149–153.
| Providencia rettgeri-associated septicaemia and meningoencephalitis in juvenile farmed American alligators Alligator mississippiensis.Crossref | GoogleScholarGoogle Scholar |
[14] Ladds, P.W. et al. (1996) Providencia rettgeri meningitis in hatchling saltwater crocodiles (Crocodylus porosus). Aust. Vet. J. 74, 397–398.
| Providencia rettgeri meningitis in hatchling saltwater crocodiles (Crocodylus porosus).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2Fps1Cntg%3D%3D&md5=5960be9c7a7313d3aa62d2b3787437e8CAS | 8941426PubMed |
[15] Merchant, M. et al. (2003) Antibacterial properties of serum from the American alligator (Alligator mississippiensis). Comp. Biochem. Physiol. B 136, 505–513.
| Antibacterial properties of serum from the American alligator (Alligator mississippiensis).Crossref | GoogleScholarGoogle Scholar | 14602158PubMed |
[16] Merchant, M. and Britton, A. (2006) Characterization of serum complement activity of saltwater (Crocodylus porosus) and freshwater (Crocodylus johnstoni) crocodiles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 143, 488–493.
| Characterization of serum complement activity of saltwater (Crocodylus porosus) and freshwater (Crocodylus johnstoni) crocodiles.Crossref | GoogleScholarGoogle Scholar | 16487736PubMed |
[17] Finger, J.W. et al. (2015) Reference levels for corticosterone and immune function in farmed saltwater crocodile (Crocodylus porosus) hatchlings using current code of practice guidelines. Gen. Comp. Endocrinol. 212, 63–72.
| Reference levels for corticosterone and immune function in farmed saltwater crocodile (Crocodylus porosus) hatchlings using current code of practice guidelines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitVCksL4%3D&md5=c77a94015ae3bfc252b38f120baafa54CAS | 25644211PubMed |
Biographies
Suresh Benedict completed his doctoral degree on Leptospira and Leptospirosis from the University of Madras, India. He has worked as bacteriologist at BVL since 2001 and his interest comprises working on various tropical veterinary bacterial isolates including Burkholderia pseudomallei.
Catherine M Shilton received her veterinary training at the University of Guelph, in Canada, including a graduate degree in zoo and wildlife medicine and pathology. She has worked as a veterinary pathologist at BVL since 2002, enjoying the challenge of the variety of species submitted and pathologies present.





