Microbiology of chloroethene degradation in groundwater
Sayali S Patil A , Eric M Adetutu A and Andrew S Ball A BA School of Applied Sciences
RMIT University
PO Box 71
Vic. 3083, Australia.
B Corresponding author. Tel: +61 3 9925 7122
Fax: +61 3 9925 7110
Email: andy.ball@rmit.edu.au
Microbiology Australia 35(4) 211-214 https://doi.org/10.1071/MA14067
Published: 3 November 2014
Industrial development, population growth and urbanisation have all contributed to an increase in the release of chemical pollutants into the environment. Consequently, many natural resources show some degree of anthropogenic impact, including the widespread contamination of groundwater aquifers by hazardous wastes1. This is particularly significant because groundwater represents about 98% of the available freshwater on the planet. The fact that we are already using approximately 50% of readily available freshwater makes groundwater protection and clean-up of paramount importance. Increasing incidences of aquifer contamination by chloroethene solvents is of current concern throughout Australia. Further, due to the adverse effects of chloroethene contaminants to environmental and human well-being, it is of upmost importance to understand the potential for the natural microbial population within the groundwater to degrade the chloroethene to innocuous byproducts.
Introduction to chlorinated hydrocarbons
Chlorinated ethenes or chloroethene are common groundwater pollutants; in 1995 trichloroethene (TCE) was found to be the second most common hazardous waste in the US2. TCE does not occur in the environment naturally, it is an anthropogenic chemical3. It was widely used as a metal degreaser, in paints, dry cleaning, paint strippers and carpet shampoos. Improper handling and disposal of these compounds has led to severe soil and groundwater contamination4. TCE is a clear liquid, denser than water, which allows it to sink forming a dense-non-aqueous-phase-liquid (DNAPL). Since the partition coefficient (Log Kow) is 2.61, TCE is hydrophobic and so is only slightly soluble in water, aiding the formation of the DNAPL.
TCE has been found to be a carcinogen in model animals such as rats and mice, causing kidney, lung and liver cancers5. It has been found to strongly correlate with a higher incidence of renal cancer in humans when exposed to TCE as metal degreasers6. People exposed to TCE due to their occupation were also found to experience an increase in the prevalence of non-Hodgkin’s lymphoma6.
Environmental fate and prevalence of chlorinated solvents
TCEs degrade slowly with half-lives in the natural environment of 80–800 days7, and due to the formation of DNAPLs at the bottom of aquifers, TCE exhibits sustained release over time into groundwater8. TCE was found in breast milk in Arizona, USA at concentrations of 6 µg/L, which is above the EPA maximum contaminant level (MCL) of 5 µg/L. The MCL for cis-DCE and VC are 70 µg/L and 1.2 µg/L respectively. TCE had also been found in foods such as decaffeinated coffee and eggs, demonstrating the scope of products that can lead to TCE ingestion9.
Chloroethene contaminant detoxification: the microbiology
The discovery of microorganisms in the mid-1990s that gain energy from the process called reductive dechlorination of chloroethene led to a turning point from a predominantly co-metabolic view of chloroethene biodegradation to the concept of chloroethenes serving as primary substrates for microbial metabolism10. Most of the chlorinated compounds have a synthetic origin and have not been in contact with microorganisms through evolutionary periods of time11. As a result, chlorinated solvents are not frequently metabolised by indigenous organisms. Nevertheless, several biotransformation mechanisms have been identified that could be exploited for degradation.
The main biotransformation pathways for chlorinated ethenes are explained below12:
-
Aerobic oxidation: the pollutant serves as the primary substrate for growth. Oxygen serves as the electron acceptor. Aerobic metabolism is limited to the less chlorinated compounds such as chloromethane, dichloromethane, chloroethane, 1, 2-DCA and VC.
-
Aerobic co-metabolism: in addition to oxygen, an electron donor must also be present. In general, the fewer the Cl atoms, the better the co-metabolic process will work. Toluene, methane, propane, butane and phenol have all been used as primary substrates to support such co-metabolic transformation.
-
Anaerobic oxidation: in this mechanism, the chlorinated organic serves as an electron donor for growth. Only a few chlorinated aliphatics are amenable to this treatment (i.e. dichloromethane; 1, 2-dichloroethene; cis- and trans-DCE and VC). Nitrate and sulphate can serve as electron acceptors in such cases and dichloromethane can also be fermented. Nevertheless, degradation rates are relatively slow and this process has not yet been demonstrated or exploited for site remediation.
-
Anaerobic reductive dechlorination: in this process, the compound serves as an electron acceptor. All chlorinated aliphatics are susceptible to anaerobic, co-metabolic, reductive dechlorination. This requires a suitable electron donor and it works mainly under sulphate-reducing or methanogenic conditions. An exception is carbon tetrachloride, which can also be dechlorinated under denitrifying conditions.
Current research activity is focused on dehalorespiration, where PCE, TCE, DCE and VC serve as terminal electron acceptors in support of microorganism growth. There are two reductive dehalogenation mechanisms. The first is hydrogenolysis (hydrodehalogenation), which involves replacing halogen atoms such as Cl, Br and F by a hydrogen atom. This is illustrated in Figure 1 for the stepwise reduction of TCE via DCE to VC and ultimately to ethene. The other reductive dehalogenation mechanisms are dihaloelimination, which involves the simultaneous removal of two halogen atoms after two electrons are transferred. Reductive dechlorination generally decreases the toxicity and enhances the solubility (bioavailability) of the pollutant, but there are exceptions where the toxicity can be accentuated (e.g. TCE reduction to VC). Reductive dechlorination is often a co-metabolic reaction since the microorganisms that catalyse it cannot harvest the energy released by the redox process. Recently however, many bacterial strains have been found that can utilise PCE and TCE as a terminal electron acceptor during respiration using H2, formate, acetate and pyruvate as electron donor. This process is known as halorespiration and it can be mediated by species such as Desulfomonile tiedjei, Dehalobacter restrictus, Desulfitobacterium and Dehalococcoidese thenogenes11. PCE and TCE readily undergo reductive dechlorination but the efficiency of the reaction decreases with decreasing degree of chlorination. Some dechlorinators sequentially dechlorinate PCE to TCE, some preferentially to cis-DCE and some to VC. However, the conversion of DCE and VC as electron acceptor to non-toxic ethene is principally mediated by Dehalococcoides species-affiliated bacteria. Conversely, the tendency for aerobic oxidation of chlorinated ethenes increases with decreasing number of chlorine atoms of the molecule. Both metabolic and cometabolic oxidation of lower chlorinated ethenes have been reported. However, mineralisation of DCE and VC tends to increase with higher reduction potential.

|
Dehalorespiring bacteria
All of the known dehalorespiring microorganisms are bacteria and their dehalogenation capacities are highly strain dependent13. Anaerobic bacteria that grow with chloroethenes as final electron acceptors include Dehalobacter, Dehalococcoides, Desulfitobacterium, Desulfuromonas, Geaobacter and Sulfurospirillum. The well-studied organisms, Sulfurospirillum multivorans and Dehalobacter restrictus PER-K23 dechlorinate PCE to cis-DCE11. S. multivorans is a Gram-negative anaerobic spirilum, which belongs to the ε- subdivision of Proteobacteria. The Dehalobacter genus belongs to Firmicutes and is allied with the genus Desulfitobacterium. However, dehalorespiration is the sole system of energy production in the genus Dehalobacter. Although these strains can utilise PCE or TCE as electron acceptors, they cannot completely dechlorinated cis-DCE or VC to ethene. One genus of particular interest for such bioremediation is ‘Dehalococcoides’ (Dhc), obligate anaerobes that cannot use oxygen, nitrate or sulphate as electron acceptors. They are Gram-positive, coccoid cells closely related to a member of the Chloreflexi phylum (green non-sulphur bacteria), which possess diverse dehalogenation ability, grow robustly in mixed cultures and are present globally in microbial populations14. Dhc species are of particular interest as members of the genus are the only known bacteria capable of complete reduction of chlorinated ethenes (PCE and TCE) to ethene (Figure 1). Dehalococcoides ethenogenes 195 and Dhcsp.FL2 respectively dechlorinated PCE and TCE to ethene15. However, these two strains are unable to use VC as an electron acceptor. Thus, the slow dechlorination of VC to ethene is considered to proceed in a co-metabolic fashion uncoupled to energy production16. In contrast, four other Dhc strains, BAV1, VS, GT and KB1/VC can use VC as the electron acceptor in their dehalorespiration and can dechlorinated VC to ethene efficiently17. In the genus Dhc, dehalorespiration is solely an energy preservation system. These isolates exhibit a metabolic specialisation, using only H2 as an electron donor and chlorinated compounds as electron acceptors to support growth.
Reductive dehalogenases (RDases)
Reductive dechlorination reactions are catalysed by the reductive dehalogenases (RDases). RDases are a class of enzymes found mostly in Dhc species and other dechlorinating organisms that catalyse the following reaction13:
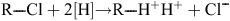
Hydrogenases are a crucial part of the reaction mechanism because they supply electrons to the reaction from H2. In anoxic environments, the above reaction is thermodynamically favourable and chlorinated compounds can act as electron acceptors. However, it has been observed previously that hydrogenases are oxygen sensitive, whereas RDases may retain some activity following exposure to oxygen18. ‘Dehalorespiration’ is defined as the process whereby energy from the above reaction is conserved and coupled to ATP synthesis in a chemo-osmotic mechanism11. Dechlorinating organisms obtain energy from the process and in many cases dechlorination activity can be linked to growth19. The hydrogenases spilt hydrogen into protons, driving the proton gradient that is utilised for ATP synthesis; and electrons (e–) are carried through the electron transport chain to the dechlorination reaction, where the chlorinated substrate acts as a terminal electron acceptor. Reactions are proposed to take place with a coronoid co-factor and 2 Fe-S clusters.
Although many putative dehalogenases exist, few have been purified and characterised. Those relevant to TCE dechlorination are listed in Table 1 along with their distribution among Dhc isolates. A TCE dehalogenase, encoded by the tceA gene was first discovered in Dehalococcoides ethenogenes strain 195 and is thought to be co-transcribed with the tceB gene encoding a small membrane anchor. This gene has a wide distribution among a range of environmental samples and those that contain tceA can degrade TCE, although not all TCE-degrading organisms contain tceA. Two VC-RDases have been discovered, originating from two different isolates –vcrA from strain VS and bvcA from strain BAV120. These are believed to be the distinguishing feature of Dhc from other dechlorinating organisms.

|
In conclusion, chloroethenes represent a serious threat to both human health and the environment. Microbial communities naturally present in contaminated groundwater have been found that are capable of the complete mineralisation of chloroethenes using a variety of mechanisms of which anaerobic reductive dechlorination represents the most ecologically significant process. Recent advances in molecular microbial ecology have led to greater understanding of the mechanisms underpinning the degradation process. This will lead to improvements in the management and remediation of contaminated groundwater.
References
[1] Lovley, D.R. (2001) Bioremediation: anaerobes to the rescue. Science 293, 1444–1446.| Bioremediation: anaerobes to the rescue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmsVektbo%3D&md5=1739d7fb41f10f896cf983a45e7e82dfCAS | 11520973PubMed |
[2] Department of Sustainability, Environment, Water, Population and Communities. (2011) Air toxics and indoor air quality in Australia. Australian Government, Canberra, ACT. http://www.environment.gov.au/archive/atmosphere/airquality/publications/sok/intro.html
[3] Department of Sustainability, Environment, Water, Population and Communities. (2011) ACT state of the environment report, vol. 2. Australian Government, Canberra, ACT.
[4] Bureau Veritas (2011) Groundwater installation and round 5 groundwater monitoring event. Site report.
[5] Dekant, W. (2001) Does exposure to trichloroethene in low doses constitute a cancer risk to humans? Hum. Ecol. Risk Assess. 7, 657–675.
| Does exposure to trichloroethene in low doses constitute a cancer risk to humans?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvF2rsLk%3D&md5=a397bf179f71a65502dda991c69ebf4bCAS |
[6] Brüning, T. et al. (2003) Renal cell cancer risk and occupational exposure to trichloroethylene: results of a consecutive case-control study in Arnsberg, Germany. Am. J. Ind. Med. 43, 274–285.
| Renal cell cancer risk and occupational exposure to trichloroethylene: results of a consecutive case-control study in Arnsberg, Germany.Crossref | GoogleScholarGoogle Scholar | 12594774PubMed |
[7] Druschel, S.J. and Kinner, N.E. (2013) Improvements to TCE microcosm protocols for biostimulation in bedrock aquifers. Remediation 23, 73–84.
| Improvements to TCE microcosm protocols for biostimulation in bedrock aquifers.Crossref | GoogleScholarGoogle Scholar |
[8] Dugat-Bony, E. et al. (1995) Bioremediation Engineering, Design and Application. USA, McGraw-Hill, Inc.
[9] Hirzel, S. (1994) Trichloroethene. Germany, German Chemical Society.
[10] Sharma, P.K. and McCarty, P.L. (1996) Isolation and characterization of a facultatively aerobic bacterium that reductively dehalogenates tetrachloroethene to cis-1,2- dichloroethene. Appl. Environ. Microbiol. 62, 761–765.
| 1:CAS:528:DyaK28XhsVWru7Y%3D&md5=ad2343ce15a25d097acf5e065ecab9a5CAS | 16535267PubMed |
[11] Holliger, C. et al. (1998) Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol. Rev. 22, 383–398.
| Reductive dechlorination in the energy metabolism of anaerobic bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXksFCqtw%3D%3D&md5=74928e6e079d1998ba639a70d157541fCAS |
[12] Imfeld, G. (2009) PhD Thesis. Faculty of Science, University of Neuchatel, Switzerland.
[13] Futagami, T. et al. (2008) Biochemical and genetic bases of dehalorespiration. Chem. Rec. 8, 1–12.
| Biochemical and genetic bases of dehalorespiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjvVamsr0%3D&md5=0cff1987abb5eb101831df29acea4339CAS | 18302277PubMed |
[14] Ernst, T. (2009) Use of Dehalococcoides to bioremediate groundwater contaminated with chlorinated solvents. MMG 445 Basic Biotechnology 5, 72–77.
[15] Maymó-Gatell, X. et al. (1997) Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276, 1568–1571.
| Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene.Crossref | GoogleScholarGoogle Scholar | 9171062PubMed |
[16] Maymó-Gatell, X. et al. (1999) Reductive dechlorination of chlorinated ethenes and 1,2-dichloroethane by ‘Dehalococcoides ethenogenes’ 195. Appl. Environ. Microbiol. 65, 3108–3113.
| 10388710PubMed |
[17] Sung, Y. et al. (2006) Geobacter lovleyi sp. nov.strain SZ, a novel metal-reducing and tetrachloroethene-dechlorinating bacterium. Appl. Environ. Microbiol. 72, 2775–2782.
| Geobacter lovleyi sp. nov.strain SZ, a novel metal-reducing and tetrachloroethene-dechlorinating bacterium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XktFansrk%3D&md5=a6388b5bd6a15d983fb516605a1f2009CAS | 16597982PubMed |
[18] Jayachandran, G. et al. (2004) Studies on hydrogenase activity and chlorobenzene respiration in Dehalococcoides sp. strain CBDB1. Arch. Microbiol. 182, 498–504.
| 1:CAS:528:DC%2BD2cXhtVeju73O&md5=3c140c80812833b9a904b0793370805eCAS | 15490122PubMed |
[19] Adrian, L. et al. (2007) Growth of Dehalococcoides strains with chlorophenols as electron acceptors. Environ. Sci. Technol. 41, 2318–2323.
| Growth of Dehalococcoides strains with chlorophenols as electron acceptors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhvFKqt74%3D&md5=aa19a58b657267c56a55c48f36d428eaCAS | 17438781PubMed |
[20] Krajmalnik-Brown, R. et al. (2004) Genetic identification of a putative vinyl chloride reductase in Dehalococcoides sp. strain BAV1. Appl. Environ. Microbiol. 70, 6347–6351.
| Genetic identification of a putative vinyl chloride reductase in Dehalococcoides sp. strain BAV1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXosl2htrw%3D&md5=4df75d588f739ef14fc68efd09b7156fCAS | 15466590PubMed |
Biographies
Dr Sayali Patil received her PhD degree in Environmental Biotechnology from Flinders University in South Australia, Adelaide. Dr Patil has produced publications in the field of Environmental Microbiology and Biotechnology and given proffered presentations at several international conferences. Dr Patil has worked as a Project Manager at the South Australian Research and Development Institute (SARDI) for two years.
Dr Eric Adetutu is a Research Fellow at RMIT, University. He obtained his PhD from the University of Essex, UK. His research interests include microbiology of extreme environments (including caves), rumen microbiology, bioremediation and the application of next generation sequencing tools to the study of pristine and disturbed environments.
Professor Ball is a graduate of Liverpool University in the UK (BSc, 1983; PhD, 1986). He has been working in the field of environmental microbiology since 1983 with a focus on biogeochemical cycling and the degradation of pollutants in the environment. Professor Ball currently teaches in the fields of environmental microbiology and biotechnology in the School of Applied Science, RMIT University in Melbourne, Australia. He is also Director of the Centre for Environment, Sustainability and Remediation (EnSuRe) at RMIT University.


