Bairnsdale ulcer in humans and animals
Janet Fyfe A B and Carolyn O’Brien CA Victorian Infectious Diseases Reference Laboratory
North Melbourne, Vic., Australia
B WHO Collaborating Centre for Mycobacterium ulcerans
Victorian Infectious Diseases Reference Laboratory
North Melbourne, Vic., Australia
Tel: +61 3 9342 2617
Fax: +61 3 9342 2666
Email: Janet.Fyfe@mh.org.au
C Faculty of Veterinary Science
The University of Melbourne
Parkville, Vic., Australia
Tel: +61 3 9533 8955
Email: cob@catvet.net.au
Microbiology Australia 34(4) 189-191 https://doi.org/10.1071/MA13066
Published: 2 October 2013
Buruli/Bairnsdale ulcer (BU) is a destructive skin disease caused by Mycobacterium ulcerans. Although BU in humans has been reported in over 30 countries (especially in west and sub-Saharan Africa), in Australia sporadic cases and several focal outbreaks have been reported since the 1940s. Victoria is the only region in the world to report BU in animals. The majority of animal disease occurs in arboreal marsupials, particularly koalas and common ringtail possums. Severely affected animals may develop systemic disease, with granulomatous lesions in the liver and lungs. Significant levels of M. ulcerans DNA can be detected in the gut contents and faeces of clinically affected and non-affected individuals. Investigations into the potential role of these animals in the ecology and transmission of the infection are ongoing.
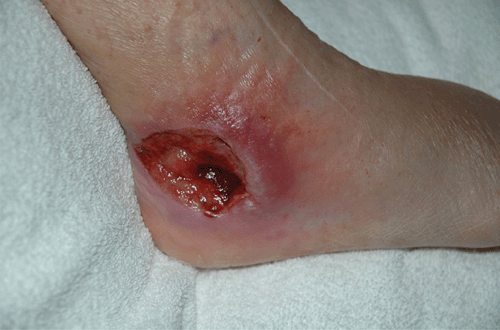
The term ‘Bairnsdale ulcer’ was first used to describe a cluster of unusual skin ulcers in residents of the Bairnsdale district in East Gippsland, Victoria, in the 1940s1. The cause of this infection, now known internationally as Buruli ulcer (BU), is the environmental bacterium, Mycobacterium ulcerans. Although the ecology and mode of transmission has not been determined, outbreaks are highly focal, with endemic and non-endemic communities often in close proximity2. Lesions include large skin ulcers (Figure 1), plaques, sub-cutaneous nodules and oedema due to the production of the destructive polyketide toxin, mycolactone. Buruli ulcer, a WHO-designated ‘neglected tropical disease’, is the third most important mycobacterial disease of humans after tuberculosis and leprosy. It has been reported in over 30 countries, mainly in tropical and subtropical areas of west and sub-Saharan Africa.
|
Laboratory confirmation of BU is generally performed directly from swab or tissue specimens via molecular methods (gel-based or real-time PCR targeting the insertion sequence IS2404)3. Culture of M. ulcerans may take up to 12 weeks.
Australia is the only developed country reporting significant local transmission of M. ulcerans. Since the initial cases in Bairnsdale, foci of infection have been reported in tropical far north Queensland, with over 60 cases in 2011. In temperate coastal Victoria, several outbreaks have been documented in the past two decades: Phillip Island (1992–1995), the Frankston/Langwarrin region (1990–1997 with ongoing sporadic cases) and several towns on the Bellarine Peninsula (1998–present). The current outbreak in Point Lonsdale, on the Bellarine Peninsula, is the largest on record in Australia, with over 150 cases diagnosed since 2002. Recently, increasing numbers of cases have been linked to the nearby towns of Barwon Heads (62 cases since 2006), Ocean Grove (30 cases since 2005) and Queenscliff (15 cases since 2009).
In Victoria, case presentation is seasonal, with most diagnosed in winter and spring4. Based on a recent study involving 23 patients with only brief exposures to an endemic area, the mean incubation period was estimated to be 4.5 months5, suggesting that transmission likely occurs in summer and autumn. Incidence appears to increase in years recording greater than average rainfall in the summer months. Studies implicate the involvement of insect vectors, including mosquitoes, although this remains to be proven6–9. There is no evidence implicating human-to-human transmission.
Although human BU occurs worldwide, naturally occurring M. ulcerans infections in animals have only been reported in Victoria. Cases have been described in domestic animals, including two horses10, a cat11, two alpacas12 and several dogs13. However, most disease occurs in free-living marsupials. In the 1980s, approximately 6% (11/200) of koalas (Phascolarctos cinereus) on Raymond Island, near Bairnsdale, were clinically affected14,15, and after 20 apparently disease-free years, another three cases were diagnosed in 2009–2010 from this location. The cutaneous lesions observed on the koalas occurred at sites typically associated with injuries caused by fighting and mating15. Between 1998 and 2003, at least 11 cases of BU were identified in a second species of arboreal mammal, the common ringtail possum (Pseudocheirus peregrinus). Like koalas, ringtail possums have strict nutritional requirements, are caecotrophic16 and rarely come to the ground. Infected possums were first identified in East Cowes on Phillip Island, following the human outbreak in the same location17. Possums with single or multiple cutaneous lesions, uncharacteristically sitting on the ground, were reported to wildlife officers and referred to local vets for diagnosis and euthanasia. In most instances, M. ulcerans was cultured from lesions. Occasional ringtail possum cases have been identified on Phillip Island until 2010, despite the lack of recent human cases.
Following the onset of the human BU outbreak, we began an intensive environmental survey to determine the ecology and mode of transmission of M. ulcerans at Point Lonsdale. Using optimised DNA extraction and real-time PCR to detect three independent M. ulcerans-specific DNA targets18, low levels of M. ulcerans DNA were detected in a range of samples, including mosquitoes, soil, dry vegetation and biofilm from a pond. However, the highest concentration of M. ulcerans DNA, confirmed by VNTR typing as indistinguishable from the strain(s) causing human infection, was detected in the faeces of common ringtail and common brushtail possums (Trichosurus vulpecula)19. Possum faeces (particularly from ringtails, which are present at a high population density20) are abundant under the trees where the animals live and feed and also on the roofs of houses. Surveys of Point Lonsdale indicated that M. ulcerans DNA was detectable in up to 50% of samples from ringtail possums, with estimated bacterial loads ranging from 10 to 107 M. ulcerans/gram of faecal material21. Results of similar surveys, in locations reporting only sporadic cases, demonstrate a much lower percentage of PCR-positive possum faeces and no M. ulcerans DNA has been detected in samples collected in non-endemic locations. During 2008–2010, examination of trapped and hand-caught possums at Point Lonsdale indicated that around 30% of the common ringtails had at least one ulcerative skin lesion (Figure 2), generally on the tail or a foot, with several animals having multiple lesions at these and additional sites. Oedematous lesions were also found on some animals. Significantly, all possums with active lesions also had detectable M. ulcerans DNA in their faeces, as did several healthy animals without lesions. In contrast, the percentage of common brushtail possums with M. ulcerans disease at this location was much lower, with only one of 21 animals caught having a lesion on one digit. However, five of these animals had detectable M. ulcerans DNA in their faeces on at least one occasion (several brushtail possums were captured multiple times)

|
Systemic M. ulcerans infection has been observed in several marsupial species, including ringtail possums, koalas and a long-footed potoroo (Potorous longipes). In all cases, M. ulcerans was detected using PCR in the liver and spleen at necropsy, and in the majority, was confirmed by culture. Six ringtail possums caught during the Point Lonsdale study underwent necropsy and we observed that only those with multiple skin lesions had systemic infection. Interestingly, primary lesions of the gastrointestinal tract (such as those seen in Johne’s disease in ruminants and tuberculosis in some mammals (including brushtail possums infected with Mycobacterium bovis in New Zealand) were absent (or transient and undetected). However, all had M. ulcerans DNA in their gastrointestinal tracts, with equivalent estimated bacterial loads in each gut compartment determined using real-time PCR (O’Brien et al., unpubl. data). Although the viability of M. ulcerans in the gut contents of these animals is yet to be confirmed using culture, it is intriguing to speculate that the organism might be able to colonise the gastrointestinal tracts of marsupials. We are yet to determine whether possums and other marsupial species are uniquely susceptible to infection/colonisation with M. ulcerans due to their immunobiology and/or ecology and the potential role of these animals as reservoirs of M. ulcerans is currently under investigation.
References
[1] MacCallum, P. et al. (1948) A new mycobacterial infection in man. J. Pathol. Bacteriol. 60, 93–122.| A new mycobacterial infection in man.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaH1c%2FitVarug%3D%3D&md5=ccf80f2d0f0e5fab3c3f48f7244e280cCAS | 18876541PubMed |
[2] Merritt, R.W. et al. (2010) Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS NTD 4, e911.
[3] Lavender, C.J. and Fyfe, J.A. (2013) Direct detection of Mycobacterium ulcerans in clinical specimens and environmental samples. Methods Mol. Biol. 943, 201–216.
| Direct detection of Mycobacterium ulcerans in clinical specimens and environmental samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXksFyntb0%3D&md5=277a447165cf9fab02e63f6112839c06CAS | 23104291PubMed |
[4] Boyd, S.C. et al. (2012) Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med. J. Aust. 196, 341–344.
| Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population.Crossref | GoogleScholarGoogle Scholar | 22432674PubMed |
[5] Trubiano, J.A. et al. (2013) The incubation period of Buruli ulcer (Mycobacterium ulcerans infection). PLoS NTD (in press).
[6] George, K.M. et al. (1999) Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283, 854–857.
| Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhtFSguro%3D&md5=67decad42e4389cc4d17cdbe8cd4c71eCAS | 9933171PubMed |
[7] Johnson, P.D. et al. (2007) Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg. Infect. Dis. 13, 1653–1660.
| Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia.Crossref | GoogleScholarGoogle Scholar | 18217547PubMed |
[8] Quek, T.Y. et al. (2007) Risk factors for Mycobacterium ulcerans infection, southeastern Australia. Emerg. Infect. Dis. 13, 1661–1666.
| Risk factors for Mycobacterium ulcerans infection, southeastern Australia.Crossref | GoogleScholarGoogle Scholar | 18217548PubMed |
[9] Lavender, C.J. et al. (2011) Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in southeastern Australia. PloS NTD 5, e1305.
[10] van Zyl, A. et al. (2010) Mycobacterium ulcerans infections in two horses in south-eastern Australia. Aust. Vet. J. 88, 101–106.
| Mycobacterium ulcerans infections in two horses in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3ntlOhtw%3D%3D&md5=143cf32213ce64954f3e6e400051366fCAS | 20402694PubMed |
[11] Elsner, L. et al. (2008) Localised Mycobacterium ulcerans infection in a cat in Australia. J. Feline Med. Surg. 287, 250–255.
[12] O’Brien, C.R. et al. (2013) Mycobacterium ulcerans infection in two alpacas. Aust. Vet. J. 91, 296–300.
| Mycobacterium ulcerans infection in two alpacas.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sjktVOhtQ%3D%3D&md5=368fc0ef13d8b15504a1de91b6bb71aeCAS |
[13] O’Brien, C.R. et al. (2011) Localised Mycobacterium ulcerans infection in four dogs. Aust. Vet. J. 89, 506–510.
| Localised Mycobacterium ulcerans infection in four dogs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC387gs1Kiuw%3D%3D&md5=d83eada286c6b658c6dcbca40c1561fbCAS | 22103951PubMed |
[14] Mitchell, P.J. et al. (1984) Skin ulcers caused by Mycobacterium ulcerans in koalas near Bairnsdale, Australia. Pathology 16, 256–260.
| Skin ulcers caused by Mycobacterium ulcerans in koalas near Bairnsdale, Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M%2FoslCqtA%3D%3D&md5=0548afb096d74c7db93e0643f1150903CAS | 6514393PubMed |
[15] Mitchell, P.J. et al. (1987) Epidemiology of Mycobacterium ulcerans infection in koalas (Phascolarctos cinereus) on Raymond Island, southeastern Australia. J. Wildl. Dis. 23, 386–390.
| 1:STN:280:DyaL2szhtF2rug%3D%3D&md5=f4e68c63d5bc3d517ce9f4462274065fCAS | 3625894PubMed |
[16] Chilcott, M.J. and Hume, I.D. (1985) Coprophagy and selective retention of fluid digesta: their role in the nutrition of the common ringtail possum, Pseudocheirus peregrinus. Aust. J. Zool. 33, 1–15.
| Coprophagy and selective retention of fluid digesta: their role in the nutrition of the common ringtail possum, Pseudocheirus peregrinus.Crossref | GoogleScholarGoogle Scholar |
[17] Johnson, P.D. et al. (1995) Mycobacterium ulcerans infection on Phillip Island, Victoria. Med. J. Aust. 162, 221–222.
| 1:STN:280:DyaK2M7ot12nsg%3D%3D&md5=a3fc5efe527395a3eda82dcbbf092f24CAS | 7877550PubMed |
[18] Fyfe, J.A. et al. (2007) Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Appl. Environ. Microbiol. 73, 4733–4740.
| Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXptVyht7w%3D&md5=4679c64179ab670a675339564e7af768CAS | 17526786PubMed |
[19] Lavender, C.J. et al. (2008) Evaluation of VNTR typing for the identification of Mycobacterium ulcerans in environmental samples from Victoria, Australia. FEMS Microbiol. Lett. 287, 250–255.
| Evaluation of VNTR typing for the identification of Mycobacterium ulcerans in environmental samples from Victoria, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1ymtrjI&md5=1da17387139991b7a9f454a2e6ca2a25CAS | 18754785PubMed |
[20] Legione, A.R. (2010) The distribution and prevalence of Mycobacterium ulcerans in common ringtail possums (Pseudocheirus peregrinus) and common brushtail possums (Trichosurus vulpecula) in Victoria [Master of Science (Zoology)]. Melbourne: The University of Melbourne.
[21] Fyfe, J.A. et al. (2010) A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS NTD 4, e791.
Biographies
Dr Janet Fyfe gained her PhD from the University of Edinburgh in 1985. On returning to Australia, she studied gene regulation in Neisseria gonorrhoeae at Monash University with John Davies for 10 years. In 2001 Dr Fyfe took up the position as Senior Molecular Biologist in the Mycobacterium Reference Laboratory at the Victorian Infectious Diseases Reference Laboratory (VIDRL). Dr Fyfe has a particular interest in the diagnosis and epidemiology of mycobacterial diseases in humans and animals.
Carolyn O’Brien graduated from The University of Melbourne in 1994. She completed a residency program in Small Animal Medicine at the University of Sydney in 2003 and concurrently completed a Masters degree in Veterinary Clinical Studies in the epidemiology and treatment of fungal infections (specifically Cryptococcus) in cats and dogs. She successfully completed the Fellowship examinations of the Australian and New Zealand College of Veterinary Scientists in Feline Medicine in 2004. Carolyn is currently undertaking a PhD project investigating the ecology and epidemiology of environmental mycobacteria, specifically looking at Mycobacterium ulcerans infection in animal species, and the causative agents of feline leprosy.


