Nanoparticle sample preparation and mass spectrometry for rapid diagnosis of microbial infections
Andrea Ranzoni A , Hanna Sidjabat B and Matthew A Cooper A CA Institute for Molecular Bioscience, The University of Queensland, Brisbane, Australia
B University of Queensland Centre for Clinical Research, The University of Queensland, Brisbane, Australia
C Email: m.cooper@uq.edu.au
Microbiology Australia 34(4) 170-174 https://doi.org/10.1071/MA13057
Published: 10 October 2013
In vitro diagnostics encompasses a wide range of medical devices and assays, which aim to provide reliable and accurate diagnosis of disease. This can be achieved by detecting a target, for example, a protein biomarker or a pathogen bacterium, and/or host factors such as cytokines induced in an inflammatory response. Detection involves an assay to capture target molecules and distinguish them from other substances in an ex vivo sample matrix. Selective capturing can be achieved using affinity probes, such as antibodies or small molecules, often coupled to a label, for example, an enzyme or a particle, to facilitate detection in complex matrixes (Figure 1). Today, the combination of nanoparticle approaches for sample preparation/concentration, with high information content, rapid analysis by mass spectrometry, is changing the way we detect and identify pathogenic bacteria in the diagnosis of microbial infection.
Most diagnostic assays are still carried out in centralised laboratories with equipment operated by dedicated laboratory personnel. Decentralised and rapid testing is important for time-critical diagnoses, where time affects morbidity and mortality outcomes for the patient, particularly in the case of bacteria sepsis. The trend towards decentralisation poses demanding constraints on assay technologies. Target molecules are present in body fluids in minute concentrations. To achieve high sensitivity detection, the physics, biology and chemistry of the sensing device, or biosensor, needs to be well integrated and controlled to transduce the presence of specific analytes into a measurable signal. This challenge is even harder when trying to detect bacteria, due to the incredible diversity of the genotype and phenotypes between species and within species. Detection of most pathogens is still carried out by culturing a clinical sample, a process susceptible to contamination, with a long lead time to diagnosis.
Matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS) has been adopted as a fast and reliable method for bacterial detection and identification, based on the characteristic mass spectrometry profiles for each bacterial species1. Databases have been developed covering commonly encountered pathogenic microorganisms for use in routine bacterial identification with results in <2 h. MALDI-TOF MS can also be used to identify microorganisms directly from clinical specimens, especially urine and blood2,3. Recently, a combination of a nanoparticle immunoassay with MALDI-TOF has been used to identify dengue and influenza viruses4,5. Agrawal recently reported on a wide range of particle-based nanotechnologies for rapid detection of protein biomarkers in food and beverages6. This review provides an overview on recent developments in nanotechnology that address key challenges in modern microbiology, with an emphasis on nanoparticle-based diagnostics and mass-spectrometry profiling of bacteria.
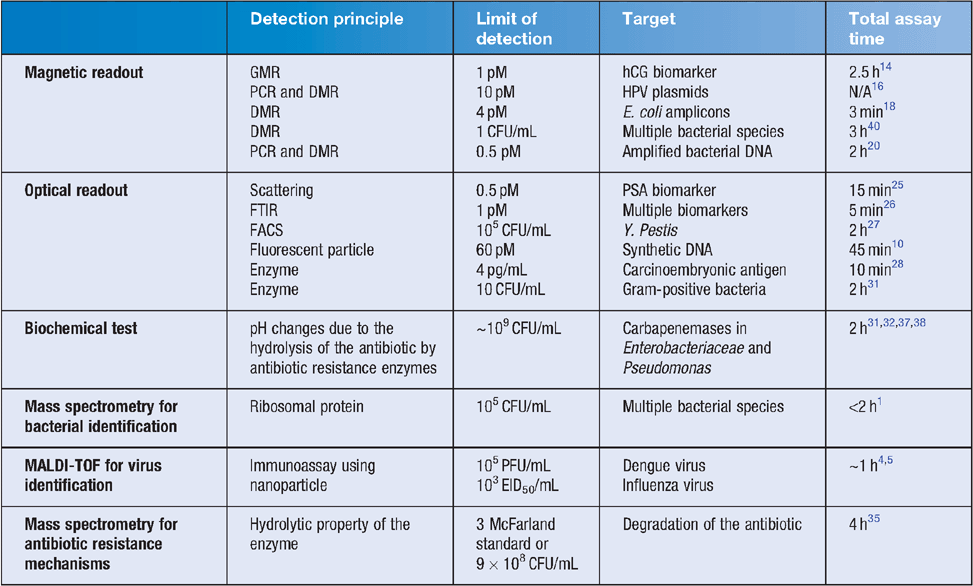
Magnetic nanoparticles can be decorated with a high density of capture probes due to their extremely high surface-to-volume ratio7. Additionally, they can be externally manipulated by controlled magnetic fields in a background of a complex biological matrix. The bioactive coating on the surface captures and magnetically ‘tags’ the target, enabling magnetic enrichment8, washing and re-suspension into a clean matrix or buffer to facilitate detection. Sensitive particle-based diagnostic nanotechnologies require efficient suppression of the background arising from non-specific interactions originating from the sample matrix. As a consequence, a great deal of research has focused on the development of robust surface architectures to extend assay applicability to complex matrixes. Hydrophilic polymer coatings (e.g. polyethylene glycol) are extensively exploited to reduce biofouling and to space the bioactive probes from the surface of the particle, which ameliorates risk of steric hindrance for analyte capture9–11. Once tagged, the target can be detected magnetically or optically (Table 1). Magnetic detection encompasses the measurement of stray magnetic fields induced into the particles by an external field, for example, by means of giant magneto-resistance (GMR) and diagnostic magnetic resonance (DMR)12,13. The assay architecture is similar in most of the recent literature and is based on several steps14–17. The capture probe is immobilised on the sensor surface and binds the target. Subsequently, a biotinylated reporter probe binds the sequestered targets. Finally, streptavidin-coated magnetic particles label the captured targets and enable detection. With this approach, Osterfeld et al. demonstrated multiplexed detection of bacterial protein biomarkers in buffer at a concentration of 1 pg/mL and Xu et al. reported detection of synthetic DNA at a concentration of 10 pM14,16. By combining magnetic tagging and detection with PCR amplification, Koets et al. demonstrated detection of 4–250 pM of genomic double-stranded bacterial DNA18. With a similar approach, Escherichia coli strains have been detected with a lower limit of detection (LoD) of 10–1000 CFU/mL17.
Diagnostic magnetic resonance takes advantage of the change in opto-magnetic properties of the particles upon target-induced aggregation. In particular, clusters of magnetic particles have increased efficiency to dephase the spins of surrounding water molecules, thus inducing a shift in the spin–spin relaxation time (T2), which is measurable using nuclear magnetic resonance (NMR). A major advantage of this technology is the ability to work with turbid or opaque matrixes such as whole blood. This approach has been widely exploited by T2 Biosystems to sensitively capture protein biomarkers, nucleic acids and bacteria19. By combining PCR amplification with detection using NMR, they recently demonstrated sensitive detection (0.5 pM of amplified DNA) and phenotyping of bacteria within 2 h20.
Magnetic particles can also be combined with fluorescent reporter probes (e.g. quantum dots, particles or enzymes) with measurement of scattering and fluorescence21. Scattering of light occurs when electromagnetic radiation encounters matter: the radiation couples to the electronic cloud in the particle, which results in an induced electric dipole. The oscillating induced dipole moment is manifested as a source of electromagnetic radiation, thereby resulting in scattered light. The phenomenon has been exploited to demonstrate solution-based detection of 1 pM of ovalbumin in buffer by turbidimetry in 3 minutes22: clusters of particles scatter more light than single particles, therefore target-induced aggregation results in a measurable decrease in transmitted light. The strong background arising from single particles limits the sensitivity of the approach23. Magnetically controlled rotation of linear clusters overcomes this limitation: rod-shaped clusters exhibit a time-dependent cross-section during one period of rotation, thus modulating the scattered light intensity24. Single spherical particles expose the same cross section to an incoming light beam, suppressing the amplitude of the modulation. With this approach, 0.5 pM of prostate specific antigen (PSA) in undiluted plasma could be detected in less than 15 minutes in one step25. Bruls et al. report detection of 1 pM of cardiac troponin and biomarkers of illicit drugs in less than 5 minutes26. The target is rapidly captured by magnetic nanoparticles and magnetically focussed on the sensor surface where secondary antibodies against multiple targets are immobilised. The unbound particles are magnetically removed and the light scattered by the bound magnetic particles is measured using frustrated total internal reflection. Optical scattering from magnetic particles was recently shown to improve the sensitivity in detecting and sorting bacteria. Sorting bacteria using flow cytometry enables sensitive and selective analysis and sorting of subpopulations. However, it has always been a difficult task due to the small size of bacteria, relative to mammalian cells. For such small objects, a light scatter signal is on the threshold detection level. The use of magnetic particles allows for enrichment of the bacteria from a large sample volume and consequent signal amplification27.
Several research groups have exploited magnetic labelling for enriching or extracting targets from a complex matrix followed by fluorescent detection aided by an enzyme or another fluorescent label (e.g. fluorescent nanoparticle or quantum dot). Amplification-free detection of 60 pM of nucleic acids has been demonstrated in a total assay time of 45 minutes in a three-step format10. Magnetic nanoparticles sequester the target sequence and, after a washing step, fluorescent nanoparticles decorated with the complementary sequence bind to the target. The final washing step removes unbound fluorescent labels and enables detection of the fluorescent intensity. With a similar approach, Wang et al. report magnetic tagging and chemiluminescent detection of a cancer biomarker in serum at a concentration as low as 4 pg/mL in less than 10 minutes28. Gu and co-workers developed vancomycin conjugated magnetic nanoparticles to capture several Gram-positive pathogens (e.g. vancomycin-resistant enterococci)29,30. In a further refinement, fluorescent vancomycin stained the enriched bacteria for the detection of 10 CFU/mL from blood samples within 2 hours using fluorescence microscopy31.
Mass spectrometry has been traditionally used for identification of bacteria and yeast1, and also recently with viruses. Notably, virus detection is usually used in combination with an immunocapture approach using nanoparticles such as those reviewed above5. For bacterial identification, MALDI-TOF can reduce the turnaround time to up to 1 day earlier to obtain accurate diagnostic results with cost savings of ~50% against culture-based and PCR-based protocols32. MALDI-TOF is capable of providing fragmentation data at a rate of less than 1 second per sample. Samples can also be processed in batch mode; for example, 192 samples in one batch for the Vitek MS system in 1 hour, with a further hour required for analysis and identification (Table 1).
The current approach to determine the bacterial susceptibility to antibiotics in a clinical diagnostic setting is by using the disk susceptibility test (DST) and minimal inhibition concentration using Vitek (Biomerieux). Recently, matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS) has been explored as a fast and reliable method for bacterial identification, based on the characteristic protein profiles for each bacterial species33,34. Databases have been developed that include commonly encountered pathogenic microorganisms, thus allowing the use of this method in routine bacterial identification in <2 hours. However, the antibiotic susceptibility patterns of the causative pathogens, which are essential to ensure appropriate and accurate antibiotic prescription, are usually only available 24–48 hours after collection of a specimen. Several recent studies explored the use of mass spectrometry in identifying carbapenemases and other β-lactamases by measuring the degradation of the antibiotic, with results produced in 4 hours35,36. However, this approach does not allow for identification of the specific carbapenemase involved, because it only measures the hydrolysis of carbapenems, which occurs with any carbapenemases37–39.
Sample preparation is crucial in obtaining the best spectra of microorganisms using MALDI-TOF. The use of nanoparticle-based techniques could give the most favourable detection limit by extracting the microorganisms from complex materials, such as blood. We foresee integrated workflows with sample preparation using a combination of nanoparticle-based systems coupled to MALDI-TOF to allow simple, rapid and high-throughput application in IVD.
References
[1] Seng, P. et al. (2009) Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 49, 543–551.| Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVOmsrzM&md5=cde6b680b1495ae9ccaa5701a434ec92CAS | 19583519PubMed |
[2] Köhling, H.L. et al. (2012) Direct identification of bacteria in urine samples by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and relevance of defensins as interfering factors. J. Med. Microbiol. 61, 339–344.
| Direct identification of bacteria in urine samples by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and relevance of defensins as interfering factors.Crossref | GoogleScholarGoogle Scholar | 22275503PubMed |
[3] Ferreira, L. et al. (2011) Rapid method for direct identification of bacteria in urine and blood culture samples by matrix-assisted laser desorption ionization time-of-flight mass spectrometry: intact cell vs. extraction method. Clin. Microbiol. Infect. 17, 1007–1012.
| Rapid method for direct identification of bacteria in urine and blood culture samples by matrix-assisted laser desorption ionization time-of-flight mass spectrometry: intact cell vs. extraction method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtV2hsrjF&md5=a58b9bd1a25fff9f874069c7a97b2ad5CAS | 20718803PubMed |
[4] Chou, T.-C. et al. (2011) Rapid and specific influenza virus detection by functionalized magnetic nanoparticles and mass spectrometry. J. Nanobiotechnology 9, 52.
| Rapid and specific influenza virus detection by functionalized magnetic nanoparticles and mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvFyqsb0%3D&md5=79c83193f56c79221aea4d0bcf86dba1CAS | 22088100PubMed |
[5] Chen, S. et al. (2013) Impurities preparation of sodium tanshinone IIA sulfonate by high-speed counter-current chromatography and identification by liquid chromatography/multistage tandem mass spectrometry. J. Chromatogr. A 1288, 28–34.
| Impurities preparation of sodium tanshinone IIA sulfonate by high-speed counter-current chromatography and identification by liquid chromatography/multistage tandem mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXkt12qsbs%3D&md5=f12840ab207af7893c2ab31f0252a43aCAS | 23522260PubMed |
[6] Agrawal G.K. et al 2013 Biomarker discovery and applications for foods and beverages: proteomics to nanoproteomics. J. Proteomics
[7] Schrand, A.M. et al. (2010) Metal-based nanoparticles and their toxicity assessment. Wiley Interdisciplinary Reviews–Nanomedicine and Nanobiotechnology 2, 544–568.
| Metal-based nanoparticles and their toxicity assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFOnsrbJ&md5=48beb9ee7a2a6b932c45abef3f6fcde1CAS | 20681021PubMed |
[8] Pappert, G. et al. (2010) Immunomagnetic nanoparticle-based sandwich chemiluminescence-ELISA for the enrichment and quantification of E. coli. Microchimica Acta 168, 1–8.
| Immunomagnetic nanoparticle-based sandwich chemiluminescence-ELISA for the enrichment and quantification of E. coli.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1WksLg%3D&md5=c3e35dce5bb68bd40040e5300b2d2181CAS |
[9] Bagwe, R.P. et al. (2006) Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir 22, 4357–4362.
| Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XivF2qtbY%3D&md5=2fce6e86314ff42503f2b31f7b2f2029CAS | 16618187PubMed |
[10] Thomson, D.A.C. et al. (2012) Oligonucleotide and polymer functionalized nanoparticles for amplification-free detection of DNA. Biomacromolecules 13, 1981–1989.
| Oligonucleotide and polymer functionalized nanoparticles for amplification-free detection of DNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xnt1yiurg%3D&md5=afb71fa3b311b6dbdb77a02cc53c72e3CAS |
[11] Gu, H. et al. (2006) Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem. Commun. , 941–949.
| Biofunctional magnetic nanoparticles for protein separation and pathogen detection.Crossref | GoogleScholarGoogle Scholar |
[12] Hall, D.A. et al. (2010) GMR biosensor arrays: a system perspective. Biosens. Bioelectron. 25, 2051–2057.
| GMR biosensor arrays: a system perspective.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXks1Kht7w%3D&md5=f043a1a435a76b38a5461e1a11bbc48aCAS | 20207130PubMed |
[13] Haun, J.B. et al. (2010) Magnetic nanoparticle biosensors. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology 2, 291–304.
| Magnetic nanoparticle biosensors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtlWgtLo%3D&md5=494740e249d66ac53ef59af4345dffdaCAS | 20336708PubMed |
[14] Osterfeld, S.J. et al. (2008) Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl. Acad. Sci. USA 105, 20 637–20 640.
| Multiplex protein assays based on real-time magnetic nanotag sensing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXks1GmsA%3D%3D&md5=567d6d25ee5b9fb6ac012d3b4c5f6e5bCAS |
[15] Maalouf, R. et al. (2008) Comparison of two innovative approaches for bacteria detection: paramagnetic nanoparticle and self assembles multilayer processes. Microchimica Acta 163, 157–161.
| Comparison of two innovative approaches for bacteria detection: paramagnetic nanoparticle and self assembles multilayer processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlWltb7L&md5=d9749867e6d171ca0b52d9bfb7f0ba87CAS |
[16] Xu, L. et al. (2008) Giant magnetoresistive biochip for DNA detection and HPV genotyping. Biosens. Bioelectron. 24, 99–103.
| Giant magnetoresistive biochip for DNA detection and HPV genotyping.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotlCrsrY%3D&md5=4048021c1b672e0ab5976add24e777b7CAS | 18457945PubMed |
[17] Mujika, M. et al. (2009) Magnetoresistive immunosensor for the detection of Escherichia coli O157:H7 including a microfluidic network. Biosens. Bioelectron. 24, 1253–1258.
| Magnetoresistive immunosensor for the detection of Escherichia coli O157:H7 including a microfluidic network.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVersQ%3D%3D&md5=ed7aced7e23f07bfafa212807deed4e5CAS | 18760584PubMed |
[18] Koets, M. et al. (2009) Rapid DNA multi-analyte immunoassay on a magneto-resistance biosensor. Biosens. Bioelectron. 24, 1893–1898.
| Rapid DNA multi-analyte immunoassay on a magneto-resistance biosensor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXit1Oltrw%3D&md5=a5c9f389edb91cd4fe495fbe9a04ed13CAS | 19028086PubMed |
[19] Skewis, L.R. et al. (2013) Nuclear magnetic resonance nanotechnology: applications in clinical diagnostics and monitoring. In Encyclopedia of Analytical Chemistry, pp. 1–24, John Wiley & Sons, Ltd.
[20] Chung, H.J. et al. (2013) A magneto-DNA nanoparticle system for rapid detection and phenotyping of bacteria. Nat. Nanotechnol. 8, 369–375.
| A magneto-DNA nanoparticle system for rapid detection and phenotyping of bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntVeitLc%3D&md5=17124284a43d963e19723d4a8e45d4bcCAS | 23644570PubMed |
[21] Chekina, N. et al. (2011) Fluorescent magnetic nanoparticles for biomedical applications. J. Mater. Chem. 21, 7630–7639.
| Fluorescent magnetic nanoparticles for biomedical applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtFent7c%3D&md5=848449f9f190be0d856294395d4ea310CAS |
[22] Baudry, J. et al. (2006) Acceleration of the recognition rate between grafted ligands and receptors with magnetic forces. Proc. Natl. Acad. Sci. USA 103, 16 076–16 078.
| Acceleration of the recognition rate between grafted ligands and receptors with magnetic forces.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Wht77P&md5=7f7cc4027d253a01e4887ce5099f6e22CAS |
[23] Park, S.Y. et al. (2010) Magneto-optical biosensing platform based on light scattering from self-assembled chains of functionalized rotating magnetic beads. Nano Lett. 10, 446–451.
| Magneto-optical biosensing platform based on light scattering from self-assembled chains of functionalized rotating magnetic beads.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1WjsLbI&md5=bf9a774cf7013886fe1e998d68a18e05CAS | 20038151PubMed |
[24] Ranzoni, A. et al. (2011) Frequency-selective rotation of two-particle nanoactuators for rapid and sensitive detection of biomolecules. Nano Lett. 11, 2017–2022.
| Frequency-selective rotation of two-particle nanoactuators for rapid and sensitive detection of biomolecules.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvFalurw%3D&md5=70bfb3112b4fc18594ef0ab42638842cCAS | 21449535PubMed |
[25] Ranzoni, A. et al. (2012) One-step homogeneous magnetic nanoparticle immunoassay for biomarker detection directly in blood plasma. ACS Nano 6, 3134–3141.
| One-step homogeneous magnetic nanoparticle immunoassay for biomarker detection directly in blood plasma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjs1Clt7g%3D&md5=eba25326d47507ab6467b20892c413f0CAS | 22414272PubMed |
[26] Bruls, D.M. et al. (2009) Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab Chip 9, 3504–3510.
| Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVOhtbvK&md5=ce6cfaf3c78c00672f9d53a0b383e88dCAS | 20024029PubMed |
[27] Zahavy, E. et al. (2012) Application of nanoparticles for the detection and sorting of pathogenic bacteria by flow-cytometry. Adv. Exp. Med. Biol. 733, 23–36.
| Application of nanoparticles for the detection and sorting of pathogenic bacteria by flow-cytometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVWhtLbO&md5=caf54627b3a12bb062cec41dcc4cb32bCAS | 22101709PubMed |
[28] Wang, C. et al. (2011) Highly sensitive rapid chemiluminescent immunoassay using the DNAzyme label for signal amplification. Analyst (Lond.) 136, 4295–4300.
| Highly sensitive rapid chemiluminescent immunoassay using the DNAzyme label for signal amplification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1ags7jI&md5=b46c18b80ff950d443ff86012c4d8d66CAS |
[29] Gu, H.W. et al. (2003) Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 3, 1261–1263.
| Presenting vancomycin on nanoparticles to enhance antimicrobial activities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmsFOitr0%3D&md5=f9ae5423367581859c435b9ba923aee2CAS |
[30] Gu, H.W. et al. (2003) Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentration. J. Am. Chem. Soc. 125, 15702–15703.
| Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptlOgtbk%3D&md5=8ac3a5f6f6f2fd02d882ba1f1ef78552CAS |
[31] Gao, J. et al. (2006) Combining fluorescent probes and biofunctional magnetic nanoparticles for rapid detection of bacteria in human blood. Adv. Mater. 18, 3145–3148.
| Combining fluorescent probes and biofunctional magnetic nanoparticles for rapid detection of bacteria in human blood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXit1ChsA%3D%3D&md5=ef1dbc31ad56f8369e1c3915f8b9f211CAS |
[32] Tan, K.E. et al. (2012) Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J. Clin. Microbiol. 50, 3301–3308.
| Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38fkvFCjtw%3D%3D&md5=723acefdcf83f6f3ef57ae75e9c96622CAS | 22855510PubMed |
[33] Bizzini, A. et al. (2010) Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J. Clin. Microbiol. 48, 1549–1554.
| Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnt1Cgtbg%3D&md5=0540db70567cd53bf1ff3c4f79da0693CAS | 20220166PubMed |
[34] Warscheid, B. and Fenselau, C. (2004) A targeted proteomics approach to the rapid identification of bacterial cell mixtures by matrix-assisted laser desorption/ionization mass spectrometry. Proteomics 4, 2877–2892.
| A targeted proteomics approach to the rapid identification of bacterial cell mixtures by matrix-assisted laser desorption/ionization mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovVynsbw%3D&md5=ba0e803e1675029a7c8ff9d5adb6fd1aCAS | 15378756PubMed |
[35] Hrabak, J. et al. (2012) Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 50, 2441–2443.
| Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionization-time of flight mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFCmsLvK&md5=a53c071ed2f70d1b4beb2ebab72dac57CAS | 22553235PubMed |
[36] Hrabak, J. et al. (2011) Carbapenemase activity detection by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 49, 3222–3227.
| Carbapenemase activity detection by matrix-assisted laser desorption ionization-time of flight mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1Gltb7N&md5=3cd29887bbfac52452da81ab4dd02db0CAS | 21775535PubMed |
[37] Dortet, L. et al. (2012) Rapid detection of carbapenemase-producing Pseudomonas spp. J. Clin. Microbiol. 50, 3773–3776.
| Rapid detection of carbapenemase-producing Pseudomonas spp.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslSnsbfP&md5=dc1cc48fe7494274bd98e2dcd037f3d9CAS | 22972829PubMed |
[38] Nordmann, P. et al. (2012) Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 18, 1503–1507.
| Rapid detection of carbapenemase-producing Enterobacteriaceae.Crossref | GoogleScholarGoogle Scholar | 22932472PubMed |
[39] Dortet, L. et al. (2012) Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob. Agents Chemother. 56, 6437–6440.
| Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslKls7vO&md5=c6ab649bd6cc5f5e58399ea825001ef5CAS | 23070158PubMed |
[40] Neely, L.A. et al. (2013) T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Science Translational Medicine 5, 182ra54.
| 1:CAS:528:DC%2BC3sXnsFWktbc%3D&md5=794ed0f47b74e5ffd28da6dd8bfa941bCAS | 23616121PubMed |
Biographies
Dr Andrea Ranzoni obtained his MSc in Physical Engineering from the Politecnico of Milan, Italy. He joined the laboratories of Philips Research in The Netherlands as a Marie Curie Research Fellow to perform experimental work on the development of opto-magnetic nanotechonolgies for biomarker detection in complex matrixes. He was awarded his PhD in Applied Physics from Eindhoven University of Technology and is currently Postdoctoral Fellow at the Institute for Molecular Bioscience at the University of Queensland. His current interests are the development of novel bionanotechnologies for magnetic nanoparticle based on in vitro diagnostics. He is author of five peer-reviewed publications and co-inventor in six international patents.
Dr Hanna Sidjabat obtained her PhD and Masters in molecular microbiology from the University of Queensland, Australia. She was a Postdoctoral Fellow at the Division of Infectious Diseases, University of Pittsburgh, Pennsylvania, USA. Dr Sidjabat is currently working as Research Officer at the University of Queensland Centre for Clinical Research, Australia. Dr Sidjabat’s research interests include: (1) the investigation of mechanisms of plasmid-mediated antimicrobial resistance in Gram-negative bacteria and molecular epidemiology of multidrug-resistant Gram-negative bacteria (MDR-GNB); (2) understanding the mechanisms of spread of antibiotic resistance through their genomes and proteomes; and (3) development of a proteomic-based approach for screening antibiotic-resistant enzymes.
Professor Matthew Cooper obtained his PhD in Organic Chemistry and BSc and Honours Degree (1st Class), from the University of Adelaide, Australia. He was a Postdoctoral Fellow with Professor Dudley Williams at the Cambridge Centre for Molecular Recognition, University of Cambridge, UK, and was a Senior Consultant at Biacore AB (Reporting to the CEO) in Sweden. Professor Cooper was also a Postdoctoral Fellow with Professor Chris Abell, Cambridge Centre for Molecular Recognition at the University of Cambridge, UK. He is a NHMRC Australia Fellow and Professor of Chemical Biology at the Institute of Molecular Bioscience, and is an Affiliate Professor in the School of Chemistry and Molecular Biology at The University of Queensland.