Fatigue after infection: aetiology and pathophysiology
Andrew R LloydInflammation and Infection Research Centre
School of Medical Sciences
University of New South Wales
Sydney, NSW 2052, Australia
Tel: 02 9385 2534
Fax: +61 2 9385 1390
Email: a.lloyd@unsw.edu.au
Microbiology Australia 34(3) 142-144 https://doi.org/10.1071/MA13047
Published: 4 September 2013
Individuals suffering from acute infections typically experience systemic symptoms, including fever and musculoskeletal pain, as well as fatigue. Acute infections are also accompanied by increased slow wave sleep and stereotyped behavioural responses, including reduced motor activity, social withdrawal and anorexia. These manifestations are collectively termed the ‘acute sickness response’1. The action of pro-inflammatory cytokines, such as interleukin (IL)-1 and IL-6 on the central nervous system mediate the key features of the response2. In the great majority of cases, these constitutional symptoms, including fatigue, resolve in parallel with the fever. However, in a minority, a disabling fatigue state may persist for weeks, months or (rarely) years. Many of the features of the acute sickness response remain evident in the prolonged fatigue state.
Following acute Q fever, Epstein–Barr virus (EBV) infection or Ross River virus (RRV) infection approximately 30% of individuals experience severe post-infective fatigue and other symptoms lasting 3 months, and 10% of cases meet diagnostic criteria for chronic fatigue syndrome (CFS) when evaluated by a physician and psychiatrist at 6 months following the infection3. Infection with several other viral and non-viral pathogens have been linked to a subsequent prolonged fatigue state (i.e. lasting 1 month or more), including cytomegalovirus (CMV), influenza, toxoplasmosis, brucellosis and leptospirosis4–6. In contrast, non-specific viral infections (upper respiratory tract infections and gastroenteritis) have been specifically shown not to be associated with an increased incidence of prolonged fatigue7. In addition, inappropriate attribution of chronic fatigue to a coincidental preceding infection is common. For instance, reported prevalence rates of persistent fatigue obtained from surveys taken 12–24 months following RRV infection are considerably higher than those identified in prospective studies8.
Evaluation of a patient with post-infective fatigue relies on a thorough history, careful physical examination and judicious laboratory investigations. The assessment should include a review of the accuracy of the original infective diagnosis, both on clinical and epidemiological grounds, and the laboratory investigations conducted at the time. A characteristic feature of the fatigue state is a prolonged exacerbation triggered by relatively minor physical or even cognitive activities. This should be differentiated from muscle weakness (neuromuscular disease), dyspnoea (cardiac or respiratory disease), somnolence (primary sleep disorders) and loss of motivation and anhedonia (major depression). This assessment is important as a documented infection might have triggered the onset, but other factors may perpetuate the illness, such as depression or sleep–wake cycle disorder9.
The physical examination should include a careful assessment for signs of persisting infection with the triggering pathogen, as chronic infection might provide an alternative explanation for the fatigue state. The most important infections in this regard are the non-viral pathogens, such as Q fever, in which chronic, localised infection might occur in the form of endocarditis or hepatitis, which in turn might cause a prominent fatigue syndrome. The remainder of the physical examination is aimed at identifying signs indicative of an alternative medical diagnosis.
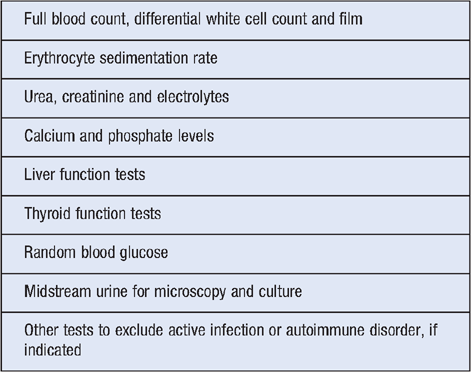
Laboratory investigations are primarily intended to exclude alternative medical diagnoses and rule out classical features of infection or inflammation (Table 1)9. Further specific investigations might be warranted, such as: phase I anti-Q fever antibody testing and PCR for suspected Q fever endocarditis; sexually transmitted infection (STI) screening in those with sexual exposure; or computed tomography (CT) scan of the sinuses if chronic sinusitis is suspected.
|
Given the association with infection at onset, many studies have sought evidence of persistence of the pathogen as a driver for ongoing symptoms in post-infective fatigue, and in chronic fatigue syndrome. These investigations have been both comprehensive and uniformly negative5. A notable recent example has been the flurry of interest in relation to the report in Science describing the detection of sequences of xenotropic murine leukemia virus-related virus (XMRV), as well as putative infectious virus in the blood of the majority of patients with chronic fatigue syndrome (67%) compared with a small proportion (3.7%) of healthy individuals10. Multiple subsequent studies failed to replicate the finding11–15, which was ultimately shown to be laboratory contamination with murine genomic DNA16.
Similarly, in Australia there has been ongoing interest in the possibility of locally acquired Lyme borreliosis as a cause for both acute manifestations and protracted fatigue17,18. However, these reports are largely based on serological testing, including western blot based on IgM positivity, which is recognised to largely reflect false positives when sampling is undertaken outside the acute phase, or IgG detection but with insufficient IgG bands to meet Centers for Disease Control criteria for a positive result19. Very few cases in non-travellers have been supported by PCR evidence of Borrelia sequences, and none have been independently validated (to exclude laboratory contamination). These data are juxtaposed against a thorough survey by microscopy, immunohistochemistry and PCR of approximately 12,000 ticks collected in coastal areas of New South Wales20. No evidence of Borrelia burgdorferi or any other spirochaete was detected in likely tick vectors. Furthermore, in patients with well-diagnosed acute Lyme disease followed by a post-infective fatigue syndrome there is no evidence of persistence of the pathogen21 or clear response to antimicrobial therapy22,23.
In addition to the data arguing against the possibility of a unique pathogen or abnormal persistence of a recognised microbe, several studies have examined the possibility of an exaggerated or protracted immune response as a driver of post-infective fatigue, including excessive pro-inflammatory cytokine production. Although cytokine levels correlate with fatigue and other symptoms during the acute, febrile phase2, case-control studies in the post-infective phase do not show a similar association with fatigue24,25. In combination, these findings are consistent with the most widely accepted hypothesis for the pathogenesis of post-infective fatigue, which is a central nervous system disorder triggered by acute infection associated with sensitisation to normal physiological signals from the body, including fatigue and pain5.
References
[1] Vollmer-Conna, U. (2001) Acute sickness behaviour: an immune system-to-brain communication? Psychol. Med. 31, 761–767.| Acute sickness behaviour: an immune system-to-brain communication?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2Fit1ymtg%3D%3D&md5=2c62d945926c982c13523e47131e9c8cCAS | 11459374PubMed |
[2] Vollmer-Conna, U. et al. (2004) Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol. Med. 34, 1289–1297.
| Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2M%2FmvVeluw%3D%3D&md5=c21818673c87f5341396a9314cb2bbf2CAS | 15697055PubMed |
[3] Hickie, I. et al. (2006) Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 333, 575–578.
| Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 16950834PubMed |
[4] Everts, R. et al. (2009) Chronic fatigue syndrome complicating leptospirosis. J. Occup. Health Safety – ANZ 25, 209–212.
[5] Lloyd, A.R. et al. (2009) Chronic fatigue and post-infective fatigue syndromes. In Clinical Virology (Third edn) (Richman, D., et al., eds). ASM Press.
[6] Prins, J.B. et al. (2006) Chronic fatigue syndrome. Lancet 367, 346–355.
| Chronic fatigue syndrome.Crossref | GoogleScholarGoogle Scholar | 16443043PubMed |
[7] Wessely, S. et al. (1995) Postinfectious fatigue: prospective cohort study in primary care. Lancet 345, 1333–1338.
| Postinfectious fatigue: prospective cohort study in primary care.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M3nt1ansQ%3D%3D&md5=c0829c2648f29cf071a5676e91f91f0eCAS | 7752755PubMed |
[8] Harley, D. et al. (2001) Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin. Microbiol. Rev. 14, 909–932.
| Ross River virus transmission, infection, and disease: a cross-disciplinary review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MrjvVymug%3D%3D&md5=2f006896052ae9f434932f768e33d99bCAS | 11585790PubMed |
[9] Fukuda, K. et al. (1994) The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 121, 953–959.
| The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M%2FmvFCiug%3D%3D&md5=a32155a9c4029a3546c0d7d5a85dc42eCAS | 7978722PubMed |
[10] Lombardi, V.C. et al. (2009) Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science 326, 585–589.
| Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ymsrbI&md5=40a6ced20649658ad811146c3dbb8f30CAS | 19815723PubMed |
[11] Erlwein, O. et al. (2010) Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome. PLoS ONE 5, e8519.
| Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome.Crossref | GoogleScholarGoogle Scholar | 20066031PubMed |
[12] Simmons, G. et al. (2011) Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: a multi-laboratory study. Science 334, 814–817.
| Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: a multi-laboratory study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVagtrnK&md5=d7196b59759855bd7b27f665653a45e0CAS |
[13] Tang, S. et al. (2011) Absence of detectable XMRV and other MLV-related viruses in healthy blood donors in the United States. PLoS ONE 6, e27391.
| Absence of detectable XMRV and other MLV-related viruses in healthy blood donors in the United States.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFyntbjK&md5=7e092331c9f65a270c02c07a691ee4beCAS | 22110639PubMed |
[14] Zhou, Y. et al. (2012) Development and application of a high-throughput microneutralization assay: lack of xenotropic murine leukemia virus-related virus and/or murine leukemia virus detection in blood donors. Transfusion 52, 332–342.
| Development and application of a high-throughput microneutralization assay: lack of xenotropic murine leukemia virus-related virus and/or murine leukemia virus detection in blood donors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XktFKhsrw%3D&md5=58e6f85b3b59eedd7af5a3b1b32513b1CAS | 22239212PubMed |
[15] Alter, H.J. et al. (2012) A multicenter blinded analysis indicates no association between chronic fatigue syndrome/myalgic encephalomyelitis and either xenotropic murine leukemia virus-related virus or polytropic murine leukemia virus. MBio 3, e00266-12.
| A multicenter blinded analysis indicates no association between chronic fatigue syndrome/myalgic encephalomyelitis and either xenotropic murine leukemia virus-related virus or polytropic murine leukemia virus.Crossref | GoogleScholarGoogle Scholar | 22991430PubMed |
[16] Kearney, M.F. et al. (2012) Multiple sources of contamination in samples from patients reported to have XMRV infection. PLoS ONE 7, e30889.
| Multiple sources of contamination in samples from patients reported to have XMRV infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjt1Krs70%3D&md5=4ccc29e0c621fe98e95c34f6e6e68e60CAS | 22363509PubMed |
[17] Mayne, P.J. (2011) Emerging incidence of Lyme borreliosis, babesiosis, bartonellosis, and granulocytic ehrlichiosis in Australia. Int. J. Gen. Med. 4, 845–852.
| Emerging incidence of Lyme borreliosis, babesiosis, bartonellosis, and granulocytic ehrlichiosis in Australia.Crossref | GoogleScholarGoogle Scholar | 22267937PubMed |
[18] Mayne, P.J. (2012) Investigation of Borrelia burgdorferi genotypes in Australia obtained from erythema migrans tissue. Clin. Cosmet. Investig. Dermatol. 5, 69–78.
| Investigation of Borrelia burgdorferi genotypes in Australia obtained from erythema migrans tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVKkurvI&md5=36e8f377254cdb591dedaece199bab43CAS | 22956879PubMed |
[19] Centers for Disease Control and Prevention 1995 Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR 44 590 591
[20] Russell, R.C. et al. (1994) Lyme disease: a search for a causative agent in ticks in south-eastern Australia. Epidemiol. Infect. 112, 375–384.
| Lyme disease: a search for a causative agent in ticks in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c3gtVejsw%3D%3D&md5=36567d6441f096e0110d14428b703eb8CAS | 8150011PubMed |
[21] Marques, A. (2008) Chronic Lyme disease: a review. Infect. Dis. Clin. North Am. 22, 341–360.
| Chronic Lyme disease: a review.Crossref | GoogleScholarGoogle Scholar | 18452806PubMed |
[22] Krupp, L.B. et al. (2003) Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology 60, 1923–1930.
| Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXksVGisLg%3D&md5=027f76fc74f104efc320bcc842deddb5CAS | 12821734PubMed |
[23] Fallon, B.A. et al. (2008) A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 70, 992–1003.
| A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjs1Kjtr0%3D&md5=5ab5b6b1ada21f5423e18f9e66742bf7CAS | 17928580PubMed |
[24] Vollmer-Conna, U. et al. (2007) Post-infective fatigue syndrome is not associated with altered cytokine production. Clin. Infect. Dis. 45, 732–735.
| Post-infective fatigue syndrome is not associated with altered cytokine production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFahtrrJ&md5=93e7c33b6727419386d13cc71d8f06e6CAS | 17712757PubMed |
[25] Cameron, B. et al. (2010) Serum cytokine levels in post-infective fatigue syndrome. Clin. Infect. Dis. 50, 278–279.
| Serum cytokine levels in post-infective fatigue syndrome.Crossref | GoogleScholarGoogle Scholar | 20034348PubMed |
Biography
Professor Andrew Lloyd AM, BBS, MD, FRACP is an infectious diseases physician and immunology researcher. He leads a multi-disciplinary team of clinicians and scientists in research studying the biological basis of inflammation in human infectious diseases, including studies of hepatitis C infection and post-infective fatigue states. He is a NHMRC Practitioner Fellow and his research is supported by a NHMRC Program, Partnership and Project Grants. He is the Director of Infection and Inflammation Research Centre (IIRC) in the School of Medical Sciences at the University of New South Wales. Professor Lloyd was awarded an Australia Medal (AM) for his research achievements in infectious diseases and for his work in establishing the hepatitis service in the NSW prisons.


