The Yersinia story: A proof of the Laurentia and Australia continents link
Jeanette PhamThe CDS Reference Laboratory
Department of Microbiology
SEALS Randwick Campus
The Prince of Wales Hospital
Randwick, NSW 2031, Australia
Tel: +61 2 9382 9046
Fax: +61 2 9382 9098
Email: jeanette.pham@sesiahs.health.nsw.gov.au
Microbiology Australia 34(1) 51-54 https://doi.org/10.1071/MA13016
Published: 20 March 2013
Predominant virulent European Yersinia enterocolitica 4/O:3 produce two types of β-lactamases, enzyme A and enzyme B and belong to phage type VIII (4/O:3/VIII). Y. enterocolitica 4/O:3 isolated in Canada and Australia are identical producing only enzyme A and belonging to the same phage type IXb (4/O:3/IXb/A). Their failure to express enzyme B is due to same defect in ampC gene. Rare European Y. enterocolitica 4/O:3 strains lacking enzyme B all have the same defect in ampC gene which is different from that observed in Canadian and Australian strains. The difference in defective ampC gene between European and Canadian/Australian 4/O:3 shows that Canadian/Australian 4/O:3 are not European 4/O:3 that are defective in the expression of enzyme B. The presence of the predominant and unique Y. enterocolitica subtype 4/O:3 at two areas far apart on the earth can only be explained by a common geographical origin. This provides a microbiological proof of linkages between Paleoproterozoic and Mesoproterozoic geological features in north western Canada and Australia presenting a new insight and perspective to both Geology and Microbiology.
The bug
Yersinia pestis, Yersinia pseudotuberculosis and Yersinia enterocolitica are the three most important species bacteria of the genus Yersinia named after the Swiss-born French physician and bacteriologist Alexander Yersin. Dr Alexander Yersin was sent to Hong Kong by Institut Pasteur, Paris and French government to investigate the bubonic plague that was sweeping through China in 1894. The bacteriologist isolated the bacillus, Y. pestis, from both rodents and affected patients linking the illness to the intermediate host. Y. pseudotuberculosis causes serious illness in young cattle while Y. enterocolitica mainly causes gastro-enteritis in human and more serious infections in susceptible patients. Y. enterocolitica is heterogeneous and of the six biotypes 1A, 1B, 2, 3, 4 and 5, biotype 1A is non-pathogenic. Among pathogenic Y. enterocolitica, biotype 4 serotype O:3 is the predominant virulent bio-serotype worldwide.
Unlike Y. pestis and Y. pseudotuberculosis, Y. enterocolitica is known to produce two chromosomal or inherent β-lactamases, the enzymes that hydrolyse β-lactam antibiotics such as penicillin and more active agents of that group. The two β-lactamases are the broad spectrum enzyme A belonging to molecular class A and the enzyme B, an inducible cephalosporinase of class C1–4. The degree of enhancement of enzyme B by an inducer varies substantially with each biotype of Y. enterocolitica while it is consistent within each of the sub-types4–7. The induction of enzyme B in European Y. enterocolitica 4/O:3, all belonging to phage type VIII (4/O:3/VIII), the predominant virulent bio-serotype-phage type isolated in Europe and many parts of the world is low and negative in disc diffusion induction6,7. Canadian Y. enterocolitica 4/O:3/IXb strains of sub-type A, fail to show induction of β-lactamase. Their lack of induction is due to the absence of the inducible enzyme B6,7. Coincidentally, all Y. enterocolitica 4/O:3 isolated in Australia also fail to express enzyme B3–7.
The investigation
Over 200 Y. enterocolitica 4/O:3 isolated in Australia and Canada were checked for biotype, serotype and phage type. They were also examined for the β-lactamase expression by disc diffusion method using amoxicillin-clavulanate 3 μg (AMC 3) and ampicillin 25 μg (AMP 25) discs and induction of enzyme B by disc diffusion7,8.
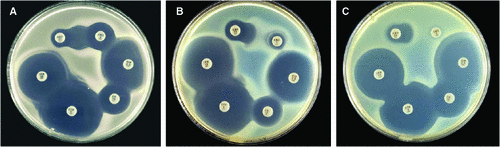
All but one strain of Y. enterocolitica 4/O:3 isolated in Australia were found to show synergy between AMC 3 and AMP 25 discs revealing the presence of enzyme A and an inhibitory zone around AMC 3 with a diameter of 16 mm ± 2 mm, consistent with the absence of enzyme B (Fig. 1A)6–8. The remaining Y. enterocolitica 4/O:3 showed the presence of both enzyme A and enzyme B (Fig. 1B). This organism was isolated from the blood culture of a thalassemic patient returning to Australia after visiting relatives in Greece for three months. The isolate was sent to WHO collaborating centre for Yersinia in Paris for phage typing (IP 24215), and was found to belong to phage type VIII, the European phage type known to produce both enzymes A and B6. The European sub-type 4/O:3/VIII of the isolate was consistent with the patient’s history of travelling to Europe and explained the presence of an European sub-type among the 87 strains of Y. enterocolitica 4/O:3 isolated in Australia. Australian Y. enterocolitica 4/O:3 had been previously phage typed, courtesy of WHO collaborating centre for Yersinia, Paris. They were found to belong to phage type IXb, the so-called Canadian phage type. Thus, without exception, all local Australian Y. enterocolitica 4/O:3 isolates examined were identical to Canadian Y. enterocolitica 4/O:3 phage type IXb that produced only enzyme A i.e. they were all of sub-type 4/O:3/IXb/A6.
|
Initial examination of 123 strains of Y. enterocolitica 4/O:3 isolated in Canada over a period of six years showed that all strains isolated in 2002 and 2003 and the majority of strains isolated in 2004–2007 belonged to phage type IXb, confirming that phage type IXb was typical of local Canadian Y. enterocolitica 4/O:3 (Table 1). The results of enzyme A and enzyme B detection showed that the majority of isolates failed to express enzyme B (Fig. 1A), thus belonged to subtype 4/O:3/IXb/A6. They were therefore identical to local Australian 4/O:3/IXb/A. There was also a small number of phage type IXb isolates that produced both enzyme A and enzyme B i.e. belonging to sub-type 4/O:3/IXb/AB (Table 1). These isolates had no zone around AMC 3 disc, therefore consistent with a high amoxi-clav MIC of 32 mg/L6. Canadian 4/O:3/IXb/AB isolates in this series were also positive in induction by disc diffusion test with the typical flattened zone of cetotaxime 5 µg (CTX 5) near imipenem 10 µg (IMP 10) disc, illustrating the presence of a highly inducible enzyme B (Fig. 1C)6,7. From 2004 to 2007, for each year, two to three isolates were of phage type VIII (Table 1). They produced both enzymes A and B and showed no flattened CTX 5 zone near IPM 10 disc i.e. negative in induction by disc diffusion test. The lack of the flattened CTX 5 zone near IPM 10 disc reflected the low induction of enzyme B, identical to European 4/O:3/VIII illustrated (Fig. 1B)6. These strains were therefore likely to have been brought to Canada by travellers from Europe, similar to the case of strain IP 24215 isolated in Australia.

|
The similarity between the predominant Y. enterocolitica 4/O:3 IXb/A isolated in Australia and Canada, currently two geographically distant parts of the earth is supported by other evidence. Not only do they belonged to the same phage type IXb and failed to express enzyme B due to an inactive ampC gene10, their behaviour at molecular level was also identical and in the same token, differed from that of European Y. enterocolitica 4/O:3/VIII. When de la Prieta and colleagues investigated the defective ampC gene in Y. enterocolitica, they examined four unusual European Y. enterocolitica 4/O:3/VIII strains that failed to express the enzyme B along with two Australian 4/O:3/XIb/A and two Canadian 4/O:3/XIb/A. In all four unusual European 4/O:3/VIII strains examined, they found a mutation at position 2298, which introduced an A to G change at that position, leading to the substitution of a threonine with an alanine at position 252, causing a misreading of the protein, thus the failure to produce enzyme B11. On the other hand, with the two Canadian and two Australian strains of Y. enterocolitica 4/O:3/IXb/A, they found that the lack of expression of enzyme B was due to the same 2 base pair (bp) deletion, CG at position 755-756 of ampC gene. The deletion caused a change in the reading frame resulting in the misreading of the protein after amino-acid 252 and a premature stop thus the failure to express enzyme B.
The insight
When an unusual European 4/O:3/VIII strains fails to express enzyme B, it is invariably due a mutation at position 2298 on ampC gene. By contrast, all four Australian and Canadian 4/O:3/IXb/A examined have a 2 base pair deletion, CG at position 755-756 causing the misreading of the protein after amino-acid 252 and the failure of enzyme B expression. These findings prove that Australian and Canadian Y. enterocolitica 4/O:3/XIb/A are not Y. enterocolitica 4/O:3/VIII that had undergone a mutation leading to the lack of expression of enzyme B and at the same time had become susceptible to bacterio-phage IXb instead of VIII. Such distinctive difference at molecular level for the failure to express enzyme B between European 4/O:3/VIII and Canadian and Australian 4/O:3/IXb/A demonstrates that Y. enterocolitica 4/O:3/IXb/A are not of European origin12.
That insight raises the interesting question concerning the origin of the unique and predominant Y. enterocolitica of subtype Y. enterocolitica 4/O:3/IXb/A isolated in Canada and Australia, two areas far apart on the earth. The likely and perhaps only possible explanation is that they share the same geographic origin. That origin would date at a period before the drifting of the continents13. The presence of a unique and identical micro-organism found only in Canada and Australia supports the hypothesis that the Pacific Margin of Laurentia and East Antartica-Australia is a conjugate rift pair13.
The presence of a low number of Y. enterocolitica 4/O:3 phage type XIb producing an enzyme A and a highly inducible enzyme B .i.e. Y. enterocolitica 4/O:3/XIb/AB amongst the 123 Canadian 4/O:3/XIb strains adds another layer to the origin and the evolution of Canadian strains of Y. enterocolitica 4/O:3/IXb. These highly inducible Canadian 4/O:3/XIb/AB (Fig. 1C) have never been reported elsewhere except in Canada. The presence of a low number of Y. enterocolitica sub-type 4/O:3/IXb/AB with a highly inducible enzyme B, isolated only in Canada and nowhere else in the world, suggests that in fact, they are most likely descendants of an enzyme B producer mutant with no defect in ampC gene. The mutant must have arisen from the original and predominant Y. enterocolitica sub-type 4/O:3/XIb/A after the drifting of the continents. These observations present another argument against the presumptive European origin for Canadian and Australian Y. enterocolitica 4/O:3/IXb12.
The observations compiled in this investigation provide a new insight to both Microbiology and Geology along with a new perspective to the age and origin of micro-organisms which inhabited the earth long before the drifting of the continents13–15.
Author’s note
This article is an extract from the book of the same title, The Yersinia story: A proof of the Laurentia and Australia continents link (Lambert Academic Publishing) available at Amazon.com.
Acknowledgements
Many sincere thanks to The Department of Age and Health Care, Ontario, Canada for having kindly shared their collection of Y. enterocilitica 4/O:3 for the investigation and to Dr Elisabeth Carniel and her staff at WHO Yersinia Centre, Institut Pasteur, Paris for the bioptyping, serotyping and phage typing of Y. enterocilitica 4/:O3.
References
[1] Cornelis, G. (1975) Distribution of β-lactamase A and B in some groups of Yersinia enterocolitica and their role in resistance. J. Gen. Microbiol. 91, 391–402.| Distribution of β-lactamase A and B in some groups of Yersinia enterocolitica and their role in resistance.Crossref | GoogleScholarGoogle Scholar |
[2] Pham, J.N. et al. (1991) Biotype and antibiotic sensitivity of 100 clinical isolates of Yersinia enterocolitica. J. Antimicrob. Chemother. 28, 13–18.
| Biotype and antibiotic sensitivity of 100 clinical isolates of Yersinia enterocolitica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXlsFCrtbg%3D&md5=ec216610dc7ab38e4b35d2fc949204a0CAS |
[3] Pham, J.N. et al. (1991) A study of the β-lactamases of 100 clinical isolates of Yersinia enterocolitica. J. Antimicrob. Chemother. 28, 19–24.
| A study of the β-lactamases of 100 clinical isolates of Yersinia enterocolitica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXlsFCrtbk%3D&md5=c071ae4d5d9934ab647cd2d3ac7dca57CAS |
[4] Pham, J.N. et al. (1992) β-lactamase induction by imipenem in Yersinia enterocolitica. Path. 24, 201–204.
| β-lactamase induction by imipenem in Yersinia enterocolitica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXhsVehtrc%3D&md5=f806ab268ddb14311346c3a21a9165aaCAS |
[5] Pham, J.N. et al. (2000) The β-lactamases and β-lactam antibiotic susceptibility of Yersinia enterocolitica. J. Antimicrob. Chemother. 46, 951–957.
| The β-lactamases and β-lactam antibiotic susceptibility of Yersinia enterocolitica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotlGq&md5=40979443c510467a70f0ac333636340fCAS |
[6] Pham, J.N. et al. (1995) Susceptibility to β-lactam agents of Yersinia enterocolitica biotype 4, serotype O:3 isolated in various parts of the world. J. Med. Microbiol. 43, 9–13.
| Susceptibility to β-lactam agents of Yersinia enterocolitica biotype 4, serotype O:3 isolated in various parts of the world.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXns1Clt7c%3D&md5=a6d79e1cee05d4eb3f3b9cc50661ee2cCAS |
[7] Pham, J.N. et al. (1993) The detection by a disc diffusion technique of inducible β-lactamase in Yersinia enterocolitica. J. Antimicrob. Chemother. 31, 1004–1005.
| The detection by a disc diffusion technique of inducible β-lactamase in Yersinia enterocolitica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXisF2rtA%3D%3D&md5=b6dc0e7707b4e20ba9382f381a543328CAS |
[8] Pham, J.N. et al. (1999) The detection of enzyme A of Yersinia enterocolitica by a disc diffusion method. Path. 31, 268–270.
| The detection of enzyme A of Yersinia enterocolitica by a disc diffusion method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmsVCqt74%3D&md5=3126ad9aab700bf5dc2e4d59c5e8b1e8CAS |
[9] Bell, S.M. et al. (2012) Antibiotic susceptibility testing by the CDS method. A Manual for Medical and Veterinary laboratories, Modified Sixth Edition, Sept 2012. Link for web site: http://web.med.unsw.edu.au/cdstest.
[10] de la Prieta, M.C. et al. (1995) β-lactamase genes and β-lactam antibiotic susceptibility in Yersinia enterocolitica. Contrib. Microbiol. Immunol. 13, 184–187.
| 1:CAS:528:DyaK28Xit1Crs7w%3D&md5=d52c791d2eb840e0f6e5b586ba62448fCAS |
[11] de la Prieta, M.C. et al. (2006) Characterisation of defective β-lactamase genes in Yersinia enterocolitica. J. Antimicrob. Chemother. 58, 661–664.
| Characterisation of defective β-lactamase genes in Yersinia enterocolitica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xotl2ltb8%3D&md5=4da7ab6453a690d5f8c6d7a2740073feCAS |
[12] Neubauer, H. et al. (2000) Yersinia enterocolitica 16S rRNA gene types belong to the same genospecies but form three homology groups. Int. J. Med. Microbiol. 290, 61–64.
| Yersinia enterocolitica 16S rRNA gene types belong to the same genospecies but form three homology groups.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXisVKitbk%3D&md5=54fe68ad026b270d9e232fa7933f570bCAS |
[13] Burrett, C. et al. (2000) Proterozoic Australia-Western United States (AUSWUS) fit between Laurentia and Australia. Geol. 28, 103–106.
| Proterozoic Australia-Western United States (AUSWUS) fit between Laurentia and Australia.Crossref | GoogleScholarGoogle Scholar |
[14] Thornkelson, D.J. et al., (2001) Early Proterozoic magmatism in Yukon, Canada: constraints on the evolution of northwestern Laurentia. Can J Earth Sci/Rev Can Sci Terre. 38, 1479–1494.
[15] Betts, P.J. et al. (2008) Comparing 1800–1600 Ma accretionary and basin processes in Australia and Laurentia: possible geographic connections in Columbia. Precambrian Res. 166, 81–92.
| Comparing 1800–1600 Ma accretionary and basin processes in Australia and Laurentia: possible geographic connections in Columbia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1yqs73K&md5=7e6f77b45b1676d91101f62471634483CAS |
Biography
Jeanette Pham is the Senior Scientist in charge of the CDS method of Antibiotic Susceptibility Testing Reference Laboratory, Sydney, Australia. Her research interests are mechanisms of resistance to β-lactam antibiotics and the β-lactamases of Gram-negative bacteria especially those of Y. enterocolitica.


