Hendra virus – a ‘One Health’ success story.
Hume Field and Brad McCall BA Queensland Centre for Emerging Infectious Diseases
39 Kessels Road, Coopers Plains 4108 Brisbane.
Tel: 07 32766054
Fax: 07 32166591
Email: hume.field@qld.gov.au
B Brad McCall
Brisbane Southside Public Health Unit
PO Box 333 Archerfield Qld 4108
Tel: 07 3000 9148
Fax: 07 3000 9130
Microbiology Australia 33(4) 167-169 https://doi.org/10.1071/MA12167
Published: 1 November 2012
Abstract
Zoonoses account for 60% of emerging diseases threatening humans. Wildlife are the origin of an increasing proportion of zoonoses over recent decades to a point where they now account for 75% of all zoonoses1. Concurrently and/or consequentially, there has been an increasing recognition of the inter-connectedness of wildlife, livestock and human health, and increasing momentum of an ecosystem-level approach (most commonly termed ‘One Health’) to complex emerging disease scenarios2. This paper describes the evolution and application of such an approach to periodic Hendra virus incidents in horses and humans in Australia.
Hendra virus, a novel member of the virus family Paramyxoviridae, was first described in 1994 in the Brisbane suburb of Hendra. It appeared suddenly and dramatically in a Thoroughbred racing stables in suburban Hendra as an acute equine respiratory disease outbreak, resulting in the deaths of 13 of 20 infected horses over a 16 day period3. But the subsequent infection of the trainer and the stable-hand, who had tended the sick and dying horses, caused even greater consternation in both animal health and public health authorities. Industry and public alarm was magnified when the trainer succumbed to the infection after a short illness4.
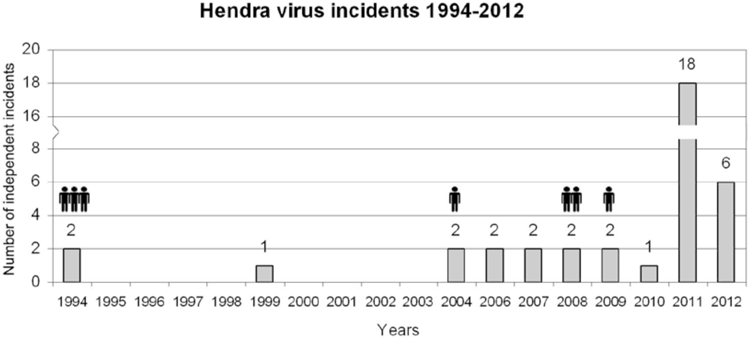
The subsequent identification of species of fruit bats (Pteropus spp.) as the natural reservoir of the virus in 1996 provided the complex wildlife-livestock-human continuum illustrated by Daszak et al, 20005, and thus invited a cross-disciplinary approach. A succession of equine incidents (some single cases, some involving horse to horse transmission) has occurred since 1994, several involving horse to human transmissions6 (Figure 1).
|
In 2012, the response to diagnosis of an equine case invokes a coordinated multi-agency threat abatement team approach. Animal health authorities report a positive diagnosis to public health, wildlife, and workplace health and safety authorities. There is cross-agency coordination not only at the policy and operational level, but also more broadly in communication with industry, community, and media. However, the current unambiguous ‘one health’ approach reflects an evolutionary process rather than a proscriptive process, and is in strong contrast to the situation at the time of the first identified Hendra virus incident in 1994. This evolution is elaborated in Hayman et al, 20127, and summarized here by way of illustration.
In early September 1994, a gravely ill horse named ‘Drama Series’ was moved from a spelling paddock to a training stable in the Brisbane suburb of Hendra for veterinary care. This action inadvertently precipitated the first recognised and single largest Hendra virus outbreak to date. A cascade of horses succumbed to a highly pathogenic and novel syndrome over the ensuing days, culminating in a total 20 equine and two human cases. Thirteen horses died or were euthanased terminally after an acute clinical course, a case fatality rate of 65%. Hendra virus had emerged3,8,9. The outbreak prompted quarantine declarations, horse movement restrictions, and the cancellation of race meetings in south-east Queensland as investigation of the aetiology proceeded. Within a week, both the Queensland Animal Research Institute and the CSIRO Australian Animal Health Laboratory had isolated a virus, initially named equine morbillivirus, and subsequently re-named Hendra virus8.
Within a week of Drama Series death, both the trainer and a stable-hand became ill, presenting with an influenza-like illness. The trainer in particular had direct and repeated contact with oro-nasal secretions as he endeavoured to force-feed by hand the gravely ill horse. The stable-hand recovered with symptomatic treatment, but the trainer’s condition deteriorated. As the equine outbreak peaked at his stables, the trainer was in an intensive care unit. The differential diagnoses were legionnaire’s disease, viral pneumonitis, melioidosis or glanders, and toxic pneumonitis4. The trainer subsequently died; Hendra virus was isolated from kidney tissue.
While the animal and human health agencies successfully coordinated and managed this initial outbreak response, the equine and human components of the response were primarily conducted as discrete parallel activities. Subsequent retrospective investigations would show that the Brisbane outbreak did not mark the first emergence of Hendra virus. A month earlier and 800 kilometres north, two horses in adjoining paddocks on a Thoroughbred stud near Mackay in north Queensland died after acute illness characterised respectively by respiratory and neurological symptoms3. The veterinarian owner, assisted by her husband, performed necropsies on both horses. The husband became ill shortly after, and was diagnosed with a mild meningo-encephalitis. After a short illness, he recovered10. The following year, he developed severe encephalitis which resulted in his hospitalisation and subsequent death, 14 months after assisting with the horse necropsies11. Matching Hendra virus genome sequence was subsequently detected in the human and (archived) horse samples.
While the outbreak in Brisbane required significant professional interaction between public health and animal health authorities, it was arguably the ‘reinforcement’ provided by the Mackay incident that marked the beginning of an enduring change in the relationship between animal health and public health authorities. Both continued to operate as discrete agencies, but communication and collaboration increasingly occurred at the senior management level, the research level, and the operational level. Cooperation was strengthened by the emergence of two other zoonoses in the mid-1990s – Japanese encephalitis virus and Australian bat lyssavirus - which both precipitated a series of inter-agency assessments. Thus, when subsequent Hendra incidents occurred, strong cross-agency linkages already existed, facilitating ready communication and coordination of response activities. Another milestone in the ‘One Health’ evolution was the 2008 Hendra virus incident, which involved multiple equine and human cases at a veterinary clinic12. Subsequent to this incident, an inter-agency technical working group was formed to provide current, science-based, best practice recommendations to limit Hendra virus transmission, informing both animal health and public health policy. External review of inter-agency cooperation in the form of commissioned Incident Reviews and Ombudsman’s Reports have further strengthened and indeed championed the ‘One Health’ approach.
The identification of species of fruit bats as the natural host of Hendra virus brought a third perspective that invited a broader ‘One Health’ approach. However, the involvement of environmental agencies was slower to occur, perhaps reflecting a fundamental wildlife management focus and a limited infectious disease focus. Thus, an emerging zoonosis involving horse to human transmission (notwithstanding the virus’s wildlife reservoir) may not initially have been viewed as a priority. It is now recognised that awareness and consideration of ecological factors that contribute to Hendra virus spillover events are fundamental to effective risk mitigation, and that this perspective informs and complements the risk management and response perspectives of the other agencies.
The evolution of a ‘One Health’ approach to Hendra virus in Australia has resulted in more informed, efficient and effective management of what is a complex personal, political, social and biological system. However, the transition to ‘One Health’ has involved something of a cultural shift for animal health and public health practitioners in Australia. Historically, veterinarians have tended to believe that they have a greater awareness of threatening zoonoses than their medical counterparts - a scenario with a tendency to foster professional tension. A ‘One Health’ approach, like all effective collaboration, requires mutual respect, trust and acknowledgement of the complementary skills of all parties. It also requires dynamic expert leadership, practice in complex incident management structures, and the necessary skill-sets. These aspects need to be underpinned by formal Memoranda of Understanding and agreements between the agencies which facilitate good outcomes by defining the roles and expectations of each agency, and the communication pathways which support the management of the incident.
References
[1] Jones, K. E. et al.. (2008) Global trends in emerging infectious diseases. Nature 451, 990–993.| Global trends in emerging infectious diseases.Crossref | GoogleScholarGoogle Scholar |
[2] Anon (2010) The FAO-OIE-WHO Collaboration. A Tripartite Concept Note. http://web.oie.int/downld/FINAL_CONCEPT_NOTE_Hanoi.pdf.
[3] Baldock, F. C. et al.. (1996) Epidemiological investigations into the 1994 Equine Morbillivirus outbreaks in Queensland, Australia. Singapore Veterinary J. 20, 57–61.
[4] Selvey, L. A. et al.. (1995) Infection of humans and horses by a newly described morbillivirus. Med. J. Aust. 162, 642–645.
[5] Daszak, P. et al.. (2000) Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science 287, 443–448.
| Emerging infectious diseases of wildlife - threats to biodiversity and human health.Crossref | GoogleScholarGoogle Scholar |
[6] Mahalingam, S. et al.. (2012) Hendra virus: an emerging paramyxovirus in Australia. The Lancet 12, .
[7] Hayman, D. T. et al.. (2012) The development of One Health approaches in henipavirus research. Curr. Top. Microbiol. Immunol. , .
| The development of One Health approaches in henipavirus research.Crossref | GoogleScholarGoogle Scholar |
[8] Murray, K. et al.. (1995) A Morbillivirus that caused fatal disease in horses and humans. Science 268, 94–97.
| A Morbillivirus that caused fatal disease in horses and humans.Crossref | GoogleScholarGoogle Scholar |
[9] I. C. Douglas, et al., (1997) Outbreak investigation of an emerging disease (Equine Morbillivirus). In Epidemiologie et Sante Animale Proceedings of the 8th ISVEE conference, 04.08.01-04.08.03, International society for veterinary epidemiology and economics.
[10] Allworth, A. et al.. (1995) Equine morbillivirus in Queensland. Commun. Dis. Intell. 19, 575.
[11] Osullivan, J. D. et al.. (1997) Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet 349, 93–95.
| Fatal encephalitis due to novel paramyxovirus transmitted from horses.Crossref | GoogleScholarGoogle Scholar |
[12] Field, H. et al.. (2010) Hendra virus outbreak with novel clinical features, Australia. Emerg. Infect. Dis. 16, 338–340.
| Hendra virus outbreak with novel clinical features, Australia.Crossref | GoogleScholarGoogle Scholar |


