Microbial conversion of waste gases into single-cell protein
Surbhi Jain A B * , James Heffernan B C , Jitendra Joshi B , Thomas Watts A , Esteban Marcellin B C * and Chris Greening A B *A Department of Microbiology, Biomedicine Discovery Institute, Monash University, Clayton, Vic., Australia.
B ARC Research Hub for Carbon Utilisation and Recycling, Monash University, Clayton, Vic., Australia.
C Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, Qld 4072, Australia.

Dr Surbhi Jain is a postdoctoral fellow at Monash University in the laboratory of Prof. Chris Greening. Her major interest includes physiology and genetic engineering of aerobic bacteria for conversion of greenhouse gases into valuable products. |

James Heffernan is a research officer at University of Queensland in the laboratory of Dr Esteban Marcellin. His major focus is scalable biological conversion of greenhouse gases into products by applying systems biology to gas fermentation technology. |

Dr Jitendra Joshi is the Chief Technologist for New Energy at Woodside Energy. His major interests include formulation of strategy for converting CO2 to value-added products, and integrating renewables in carbon transformation and hydrogen generation. |

Dr Thomas Watts is a postdoctoral fellow and team leader at Monash University in the laboratory of Professor Chris Greening. His major interest is the molecular biology of clostridia and mycobacteria. |

Dr Esteban Marcellin is an Associate Professor at University of Queensland. His interests include integrated systems and synthetic biology platforms to expand the product spectrum of greenhouse gas converting microbes. |

Prof. Chris Greening leads the One Health Microbiology Group at Monash University. His research investigates the biochemistry, physiology, ecology, biogeochemistry and applications of microbial gas metabolism. |
Microbiology Australia 44(1) 27-30 https://doi.org/10.1071/MA23007
Submitted: 10 January 2023 Accepted: 16 February 2023 Published: 9 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Climate change and food security are two of our most significant global challenges of our time. Conventional approaches for food production not only produce greenhouse gases but also require extensive land and water resources. An alternative is to use gas fermentation to convert greenhouse gases as feedstocks into microbial protein-rich biomass (single-cell protein). Aerobic methanotrophic (methane-oxidising) and hydrogenotrophic (hydrogen-oxidising) bacteria, which produce biomass using gases as their energy and carbon sources, are ideal candidates for single-cell protein production. However, multiple innovations are required for single-cell protein production to be economical and sustainable. Although current technologies rely on conversion of purified single gaseous substrates, the potential to directly use mixed gas streams from point sources remains reasonably unexplored. In addition, there is much potential to increase nutritional and commercial value of single-cell protein through synthetic biology. In this perspective, we discuss the principles, approaches, and outlook for gas fermentation technologies aiming to significantly reduce greenhouse gas emissions and enhance food security.
Background
The International Panel on Climate Change 2022 report has warned the world to limit global warming to 1.5°C within the next two decades.1 Global warming is a result of anthropogenic greenhouse gases (GHGs) such as carbon dioxide and methane, which are primarily produced by the energy, waste, transport, and agriculture and food industries. There is a paramount need to reduce and recycle these emissions given climate change is leading to environmental catastrophes and increasing human health risks. A circular economy would employ economically viable processes to convert emissions into products, thereby mitigating their impact. Gas fermentation technologies offer several advantages by producing various end-products such as high-value chemicals, biofuels and protein feed.2,3 In contrast to typical gas conversion processes by thermochemical catalysis (e.g. Fischer–Tropsch reactors), biological processes can occur at close to ambient temperatures and pressures. Toxic gases also poison chemical catalysts, whereas some bacteria tolerate and even utilise these compounds.4
The global population is expected to rise to 9.8 billion by 2050 (UN 2019 Revision of World Population Prospects5), increasing global food demand. Current practices for animal-derived protein production lead to significant release of GHGs, and may not be feasible for increasing consumption levels of a growing population. A recent study found that food production generated ~35% of the world’s anthropogenic GHG emissions from 2007 to 2013.6 There is much industry interest in transforming the food production system and, at the same time, minimising further climate impacts and biodiversity loss. Rising public awareness and ecological factors are encouraging consumers towards more sustainable products. Some microbes can convert GHGs into single-cell protein, reducing the climate’s adverse impacts on food production. Single-cell protein (SCP) is derived from bacteria, algae, yeast or fungi and has high protein content. SCP therefore has the potential to replace traditional protein sources such as fish and soybean products in human and animal feed.7 Currently, SCP occupies a reasonably small market for human nutrition, though SCP is likely to become a significant alternative amid rising global demand for protein and increasing need for sustainable food production.8 Advantages of SCP include reduced production time, water and carbon footprints, and biodiversity impacts, e.g. extensive use of Antarctic krill in aquaculture feed is driving concerns of Southern Ocean ecosystem collapse.9 Further, SCP can be produced at any time of the year, avoiding the risk of seasonal and climatic variations, as well as biotic factors such as pathogens or pests.10 Thus, SCP production can be a more efficient and sustainable solution than traditional agriculture.
Bacteria are particularly promising SCP producers, given they can use inorganic feedstocks and produce 50–80% protein by dry weight.11 Bacteria can produce SCP using a wide range of feedstocks, including waste gases. Waste gases are produced in abundance by various industries and released into the atmosphere. Methane is the second most abundant GHG after carbon dioxide and has contributed ~30% of global emissions to date.12 Aerobic methane-oxidising bacteria, also known as methanotrophs, consume significant amounts of methane before it is emitted into the atmosphere and also serve as the primary biological sink of atmospheric methane (~30 Tg year−1).13 There is much ongoing research and development on using methanotrophs to produce methane-derived SCP.14 Methane-derived SCP has a promising nutritional profile: it is reported to be rich in essential amino acids such as histidine, valine, phenylalanine, isoleucine, leucine, threonine, and lysine,15 with notable amounts of vitamins, minerals, and essential fatty acids.16 In addition to methanotrophs, aerobic hydrogen-oxidising bacteria (HOB), also known as Knallgas bacteria, have emerged as promising candidates for SCP production. HOB fix carbon dioxide using hydrogen as the electron donor and oxygen as the electron acceptor. Hydrogen is oxidised by hydrogenases and carbon dioxide is fixed for biomass production by the Calvin–Benson–Bassham cycle under aerobic conditions.17,18
Commercial advancements in SCP production
The need for sustainable feed solutions is increasing the demand for single-cell proteins. Though SCP production has gained increased attention in recent years, it has been under investigation since the 1960s. Pruteen is an example of SCP that was first commercialised by Imperial Chemical Industries using the methanotrophic bacterium Methylophilus methylotrophus.19 Despite the high protein content of Pruteen, the production was discontinued because of poor economics. Since then, SCP production is becoming more economically favourable and methane is gaining renewed attention as a cheap feedstock while reducing GHG emissions.20 Innovators include Calysta, an American company that uses methanotroph, Methylococcus capsulatus, to convert methane from natural gas into the protein feed, ‘FeedKind’. FeedKind is reported to use 100 times less water and 1000 times less land when compared to soy-based meal.21 Similarly, Unibio in Denmark produces methane-based SCP to feed pigs and pets.22 Other companies such as String Bio and Circe Biotechnologie GmbH are developing SCP focusing on aquaculture industry.23,24
In recent years, several companies have also explored the use of HOB to produce food and feed ingredients. Solar Foods, a Finnish company founded in 2017, has developed human-grade SCP, ‘Solein’, and has submitted to the European Commission for approval for safe human consumption.25 Similarly, other companies pioneering mass production of hydrogen-based SCP are Novonutrients, Kiverdi and Deep Branch Biotechnology.
It should be noted that SCP must not only have nutritional value, but should also be safe for human and animal nutrition. The limiting factor with bacterial SCP for human consumption is the presence of nucleic acids (~8–12%).26 This concentration of nucleic acids can cause human health issues such as kidney stones and gout.27 However, nucleic acids can be removed during food processing, for example by nuclease treatment.28 Another important aspect to consider in SCP production is from an economic point of view regarding feedstock availability, scalability and processing. Therefore, further advancements are important for producing cost-effective SCP.
Future directions
To improve the economic viability of SCP production, mixtures of waste gases should be used as substrates. So far, SCP production relies on single gaseous substrates typically derived from fossil fuels. The advantages of using waste gases are that they are cheap, available and have a lower environmental footprint. One notable example is Lanzatech, a key technology developer in gas fermentation, which uses steel mill off-gas or syngas to produce carbon-negative products by anaerobic acetogenic bacteria.29
We now have evidence that most aerobic bacteria are mixotrophs and can use multiple substrates to enhance growth and survival.30,31 The metabolic versatility of aerobic bacteria can provide a platform to exploit mixed waste gases as a substrate to produce protein-rich biomass. Our current work aims to assess whether metabolic versatility will enable mixotrophic bacteria to convert mixed waste gases from different sources, such as steel mill gas, biogas and syngas to maximise the benefits offered by mixotrophic bacteria for the production of animal feed or human food (Fig. 1). To do this, we are assessing growth, gas conversion and metabolite production of a panel of hydrogenotrophs and methanotrophs. For example, we have discovered that the verrucomicrobial methanotroph Methylacidiphilum sp. RTK17.1 optimally grows using a combination of hydrogen and methane simultaneously as energy sources, carbon dioxide as the carbon source and oxygen as the final electron acceptor.30 This depends on the activities of its uptake [NiFe]-hydrogenase, particulate methane monooxygenases, and RuBisCO-dependent Calvin–Benson–Bassham cycle. It is therefore ideal to use a range of flue gas and biogas streams. Hydrogenophaga pseudoflava is a promising candidate for aerobic syngas conversion. This bacterium grows by simultaneously oxidising hydrogen and carbon monoxide to support aerobic respiration and carbon fixation, a process that depends on the activities of oxygen- and carbon monoxide-tolerant hydrogenases and carbon monoxide dehydrogenase.32 Another hydrogenotrophic model organism, Cupriavidus necator H16, has been intensively studied and grows rapidly in the presence of hydrogen and carbon dioxide, while accumulating high titres of biomass.33
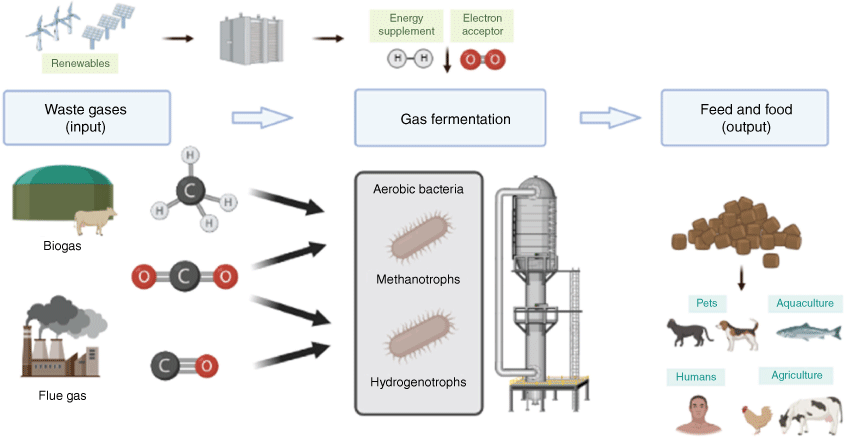
|
The ultimate aim is to produce high-value SCP suitable for pets, livestock and aquaculture feed and eventually food-grade protein by lowering nucleic acid content. With the rapid development of genetic engineering, SCP can be leveraged in the food and feed industry. The process can be optimised using systems engineering (e.g. changing substrate inputs, bioreactor design), adaptive laboratory evolution and synthetic biology. Gas-consuming bacteria can be genetically engineered to produce desired products such as carotenoid colourants and omega-3 fatty acids, potentially assisting the replacement of wild-catch fishmeal with sustainable fish feed. Astaxanthin, a keto-carotenoid, improves salmon colouration and omega-3 fatty acids are a nutritional supplement for consumers. The well-characterised biosynthesis genes for these metabolites can be synthesised, transformed and induced in the bacterium of interest. Equivalent approaches could achieve the opportunity for producing other high-value chemicals.
Summary
Industrial processes such as fuel refining and waste treatment produce large quantities of mixed waste gases. At the same time, the demand for feed for agriculture, aqua-culture and pets is growing. Sustainable feed production is needed to alleviate land use change, biodiversity loss and climate change impacts. Therefore, a solution is to use mixotrophic bacteria to produce nutritionally rich animal feed from waste gases. Mixotrophic bacteria provide an opportunity to use mixed waste gases as feedstock, given their mixotrophic capability for utilising diverse gases contributing to alleviating climate change. Although such approaches are scientifically sound, efficient bioprocesses are needed for economic implementation and scalability.
Data availability
The data that support this study are available in the article.
Conflicts of interest
Woodside Energy has interest in commercialising waste gases into SCP in feed or food industry. J. Joshi is an employee of Woodside Energy.
Declaration of funding
This work was supported by the Australian Research Council (ARC) Research Hub for Carbon Utilisation and Recycling and a Woodside Monash Energy Partnership project grant to J. Joshi, E. Marcellin and C. Greening. C. Greening is supported by a National Health and Medical Research Council EL2 Fellowship (APP1178715).
References
[1] Pörtner H-O et al. (2022) Climate Change 2022: Impacts, Adaptation and Vulnerability. Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press.| Crossref |
[2] Liew, F et al. (2016) Gas fermentation—a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front Microbiol 7, 694.
| Gas fermentation—a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks.Crossref | GoogleScholarGoogle Scholar |
[3] Marcellin, E et al. (2022) Recycling carbon for sustainable protein production using gas fermentation. Curr Opin Biotechnol 76, 102723.
| Recycling carbon for sustainable protein production using gas fermentation.Crossref | GoogleScholarGoogle Scholar |
[4] Mendes, SS et al. (2021) Hydrogen sulfide and carbon monoxide tolerance in bacteria. Antioxidants 10, 729.
| Hydrogen sulfide and carbon monoxide tolerance in bacteria.Crossref | GoogleScholarGoogle Scholar |
[5] United Nations Department of Economic and Social Affairs Population Division (2019) World Population Prospects 2019: Highlights. ST/ESA/SER.A/423. UN, New York, NY, USA. https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf
[6] Xu, X et al. (2021) Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods. Nat Food 2, 724–732.
| Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods.Crossref | GoogleScholarGoogle Scholar |
[7] Bratosin, BC et al. (2021) Single Cell protein: a potential substitute in human and animal nutrition. Sustainability 13, 9284.
| Single Cell protein: a potential substitute in human and animal nutrition.Crossref | GoogleScholarGoogle Scholar |
[8] Boland, MJ et al. (2013) The future supply of animal-derived protein for human consumption. Trends Food Sci Technol 29, 62–73.
| The future supply of animal-derived protein for human consumption.Crossref | GoogleScholarGoogle Scholar |
[9] Atkinson, A et al. (2019) Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat Clim Change 9, 142–147.
| Krill (Euphausia superba) distribution contracts southward during rapid regional warming.Crossref | GoogleScholarGoogle Scholar |
[10] Tzachor, A et al. (2021) Future foods for risk-resilient diets. Nat Food 2, 326–329.
| Future foods for risk-resilient diets.Crossref | GoogleScholarGoogle Scholar |
[11] Anupama, and Ravindra, P (2000) Value-added food: single cell protein. Biotechnol Adv 18, 459–479.
| Value-added food: single cell protein.Crossref | GoogleScholarGoogle Scholar |
[12] International Energy Agency (2022) Global Methane Tracker 2022. IEA, Paris, France. https://www.iea.org/reports/global-methane-tracker-2022
[13] Hanson, RS and Hanson, TE (1996) Methanotrophic bacteria. Microbiol Rev 60, 439–471.
| Methanotrophic bacteria.Crossref | GoogleScholarGoogle Scholar |
[14] Ritala, A et al. (2017) Single cell protein—state-of-the-art, industrial landscape and patents 2001–2016. Front Microbiol 8, 2009.
| Single cell protein—state-of-the-art, industrial landscape and patents 2001–2016.Crossref | GoogleScholarGoogle Scholar |
[15] Zha, X et al. (2021) Bioconversion of wastewater to single cell protein by methanotrophic bacteria. Bioresour Technol 320, 124351.
| Bioconversion of wastewater to single cell protein by methanotrophic bacteria.Crossref | GoogleScholarGoogle Scholar |
[16] Øverland, M et al. (2010) Evaluation of methane-utilising bacteria products as feed ingredients for monogastric animals. Arch Anim Nutr 64, 171–189.
| Evaluation of methane-utilising bacteria products as feed ingredients for monogastric animals.Crossref | GoogleScholarGoogle Scholar |
[17] Volova, TG and Barashkov, VA (2010) Characteristics of proteins synthesized by hydrogen- oxidizing microorganisms. Appl Biochem Microbiol 46, 574–579.
| Characteristics of proteins synthesized by hydrogen- oxidizing microorganisms.Crossref | GoogleScholarGoogle Scholar |
[18] Pohlmann, A et al. (2006) Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat Biotechnol 24, 1257–1262.
| Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16.Crossref | GoogleScholarGoogle Scholar |
[19] Bungard, SJ (1992) Bugs in the works—biology at ICI-Billingham. J Biol Educ 26, 252–256.
| Bugs in the works—biology at ICI-Billingham.Crossref | GoogleScholarGoogle Scholar |
[20] Guerrero-Cruz, S et al. (2021) Methanotrophs: discoveries, environmental relevance, and a perspective on current and future applications. Front Microbiol 12, 678057.
| Methanotrophs: discoveries, environmental relevance, and a perspective on current and future applications.Crossref | GoogleScholarGoogle Scholar |
[21] Cumberlege T et al. (2016) Assessment of environmental impact of FeedKind protein. Carbon Trust. https://ctprodstorageaccountp.blob.core.windows.net/prod-drupal-files/documents/resource/public/Assessment%20of%20environmental%20footprint%20of%20FeedKind%20protein%20-%20REPORT.pdf
[22] Unibio Group (2020) Chemical composition of uniprotein. https://www.unibio.dk/end-product/chemical-composition-1
[23] Lee A (2021) Barriers to adopting alternative protein are decreasing, post-Covid-19: String Bio co-founder Ezhil Subbian. https://www.eco-business.com/news/barriers-to-adopting-alternative-protein-are-decreasing-post-covid-19-string-bio-co-founder-ezhil-subbian/
[24] Circe Biotech (2021) Protein: feed & food. https://www.circe.at/products/protein
[25] Solar Foods (2021) Solein submitted to the European Commission for novel food approval. Solar Foods. https://solarfoods.fi/our-news/solein-submitted-to-the-european-commission-for-novel-food-approval
[26] Miller BM, Litsky W (1976) Single cell protein in industrial microbiology. McGraw-Hill Book Co., New York, NY, USA.
[27] Erdman, MD et al. (1977) Amino acid profiles and presumptive nutritional assessment of single-cell protein from certain lactobacilli. Appl Environ Microbiol 33, 901–905.
| Amino acid profiles and presumptive nutritional assessment of single-cell protein from certain lactobacilli.Crossref | GoogleScholarGoogle Scholar |
[28] Skrede, A et al. (2009) Effects of growth substrate and partial removal of nucleic acids in the production of bacterial protein meal on amino acid profile and digestibility in mink. J Anim Feed Sci 18, 689–698.
| Effects of growth substrate and partial removal of nucleic acids in the production of bacterial protein meal on amino acid profile and digestibility in mink.Crossref | GoogleScholarGoogle Scholar |
[29] Liew, FE et al. (2022) Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat Biotechnol 40, 335–344.
| Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale.Crossref | GoogleScholarGoogle Scholar |
[30] Carere, CR et al. (2017) Mixotrophy drives niche expansion of verrucomicrobial methanotrophs. ISME J 11, 2599–2610.
| Mixotrophy drives niche expansion of verrucomicrobial methanotrophs.Crossref | GoogleScholarGoogle Scholar |
[31] Greening, C and Grinter, R (2022) Microbial oxidation of atmospheric trace gases. Nat Rev Microbiol 20, 513–528.
| Microbial oxidation of atmospheric trace gases.Crossref | GoogleScholarGoogle Scholar |
[32] Grenz, S et al. (2019) Exploiting Hydrogenophaga pseudoflava for aerobic syngas-based production of chemicals. Metab Eng 55, 220–230.
| Exploiting Hydrogenophaga pseudoflava for aerobic syngas-based production of chemicals.Crossref | GoogleScholarGoogle Scholar |
[33] Poladyan, A et al. (2019) Growth of the facultative chemolithoautotroph Ralstonia eutropha on organic waste materials: growth characteristics, redox regulation and hydrogenase activity. Microb Cell Fact 18, 201.
| Growth of the facultative chemolithoautotroph Ralstonia eutropha on organic waste materials: growth characteristics, redox regulation and hydrogenase activity.Crossref | GoogleScholarGoogle Scholar |


