What does microbiology have to do with the Hearing for Learning Initiative (HfLI)?
Amanda J. Leach A *A Menzies School of Health Research, PO Box 41096, Casuarina, NT 0811, Australia.

Professor Amanda Leach, AM, is leader of the Ear Health Research Program, Child Health Division at the Menzies School of Health Research, in Darwin, NT, Australia. Professor Leach led the NHMRC Centre of Research Excellence in Otitis Media and Hearing Loss in Aboriginal and Torres Strait Islander children. She also led the 2020 revision of the OM Guidelines including an OM app, now endorsed as a Guideline by the Royal Australian College of General Practitioners. Professor Leach is Joint Chair with Professor Kelvin Kong, for the Hearing for Learning Initiative – a funding partnership between The Balnaves Foundation, the Northern Territory Government, and the Australian Government. In 2021, Amanda was awarded a Member of the Order of Australia for her research. |
Microbiology Australia 43(3) 108-112 https://doi.org/10.1071/MA22035
Submitted: 26 July 2022 Accepted: 15 September 2022 Published: 3 October 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Where would we be without microbiology in tackling the high prevalence of otitis media (OM; middle ear infection) and disabling hearing loss that disadvantage Australian First Nations children living in remote communities? Understanding the microbiology of OM in this population has been critical in directing innovative clinical trials research and developing appropriate evidence-based practice guidelines. While these processes are critical to reducing disadvantage associated with OM and disabling hearing loss, a remaining seemingly insurmountable gap has remained, threatening progress in improving the lives of children with ear and hearing problems. That gap is created by the crisis in primary health care workforce in remote communities. Short stay health professionals and fly-in fly-out specialist services are under-resourced to manage the complex needs of the community, including prevention and treatment of otitis media and hearing loss rehabilitation. Hence the rationale for the Hearing for Learning Initiative – a workforce enhancement model to improve sustainability, cultural appropriateness, and effectiveness of evidence-based ear and hearing health care for young children in remote settings. This paper summarises the role of microbiology in the pathway to the Hearing for Learning Initiative.
Keywords: Aboriginal, antimicrobial resistance, child, clinical trial, guideline, hearing loss, non-typeable Haemophilus influenzae, otitis media, Streptococcus pneumoniae.
How does microbiology lead to a trial of an enhanced workforce model for ear and hearing services in remote community primary health care? This story follows a pathway from complex bacterial pathogen discovery, design of clinical trials that reveal the failure of standard therapies (based on trials in low-risk populations), and the need for innovative strategies to prevent or eradicate bacterial infections in high-risk populations. The story shows how understanding the underpinning microbiology has directed clinical trial design and evidence-based guidelines for management of otitis media (OM; middle ear infection) in high-risk Aboriginal children. These guidelines challenge national and international guidelines, and medical education. Yet in remote areas, services are largely delivered by practitioners trained in our major cities where the health profile is staggeringly different, leaving health practitioners who come to work in remote locations ill-prepared to diagnoses and manage major health problems of Aboriginal children. Local guidelines aim to assist management relevant to this unique context.1,2
The Hearing for Learning Initiative is evaluating a community-based workforce enhancement model designed to address ongoing high prevalence of persistent OM, disabling hearing loss and associated educational and social disadvantage.3 Almost every infant and child in remote communities has early and persistent OM, which is largely asymptomatic despite apparently painful appearance of the tympanic membrane (marked bulging or perforation).4 Failure to detect early acute OM (AOM) or middle ear effusions (OME) leads to chronic OM (all forms) which is the strongest predictor of developmental delay, high vulnerability on entering school, poor school attendance and performance.5–8 Children with hearing loss are at increased risk of substantiated maltreatment.9 OM must be prevented and appropriately managed to enable all children to develop their full potential.
Currently, for most children in Australia, OM is viral, acute, brief, and episodic. OM can be managed with pain relief and resolves spontaneously; judicious use of antibiotics can be applied, and watchful waiting is recommended.2 However, a major shift in understanding OM in Australian Aboriginal children was made in the early 1990s, at a time when Chlamydia trachomatis was a key suspect pathogen.10 Under leadership of Professor John Mathews, a birth cohort study established that major bacterial otopathogens were Moraxella catarrhalis (Mc), Streptococcus pneumoniae (Spn) and non-typeable Haemophilus influenzae (NTHi).11,12 Respiratory viruses and Chlamydia trachomatis were very rarely recovered.4 This study confirmed that OM in Aboriginal infants commenced soon after bacterial nasopharyngeal colonisation and within weeks of birth.4 The multiple bacterial pathogens involved and the high diversity of strains and serotypes in the population drove recurrent and sometimes multiple concurrent pathogen infections throughout early childhood. This revelation shifted the focus of OM prevention and treatment research to early detection, trials of appropriate use of antibiotics13–16 or watchful waiting,17 and subsequently to pneumococcal conjugate vaccine trials (Fig. 1).18,19
A series of clinical trials has been completed over several decades (some trials take 6–7 years to complete) that confirm a benefit of antibiotics for high-risk Aboriginal children.13–15 Importantly, the trials identified that longer courses and higher-dose antibiotics are needed compared with judicious use recommended from trials in low-risk populations.20 Current trials are evaluating the generalisability for non-high-risk Aboriginal children living in Australian urban jurisdictions.17,21
For each randomised controlled trial (RCT) of antibiotics or pneumococcal conjugate vaccines the nasopharyngeal microbiology, and microbiology of ear discharge from spontaneously perforated ear drums (acute otitis media with perforation) has been critically important in understanding the impact of each intervention on the complex underpinning biology (Fig. 1).4,12,22–24 Clinical outcomes alone tell us about clinical failure or success, but not why there is failure or success. Without microbiology we would be assuming clinical failure might be attributed to antimicrobial resistance (AMR), a higher virulence of strains in this population, viral interactions or high density bacterial load.25 These assumptions could potentially lead to misleading recommendations, or misguided research.
Examples include the Chronic Otitis Media Intervention Trial 1 (COMIT1) RCT, which demonstrated clinical superiority of amoxycillin over placebo with no increased AMR in the active compared with placebo recipients.14 The first trial of topical treatments for chronic suppurative otitis media (CSOM) found no difference in clinical outcomes; however, the microbiology identified superior eradication of Gram negatives in the ciprofloxacin arm, and persisting S. aureus in both arms.13 This led to a further CSOM trial which added oral co-trimoxazole (to standard topical ciprofloxacin) to target the residual S. aureus.16 The significant further clinical improvement (C. Wigger, pers. comm., 2019) has led to an OM Guideline recommendation for adjunct co-trimoxazole.2 The first trial of azithromycin for acute OM (AAATAC) compared single dose azithromycin with 7 days of twice daily amoxycillin.15 Clinical outcomes were not different; however, the microbiology and AMR prevalence confirmed the unexpected – that combined AMR was similar in both groups.15 Importantly, this led to a recommendation for single dose azithromycin for Aboriginal children where compliance was known to be difficult, or where refrigeration (essential for amoxycillin) was not possible (often the case for families in remote communities).2 The next RCT (azithromycin for asymptomatic acute OM (AAAOM)) went on to enrol children with asymptomatic AOM (almost all AOM is asymptomatic in this population) to receive either placebo or azithromycin. Fewer children in the azithromycin group had tympanic membrane perforations, and importantly again, the microbiology showed the unexpected – that pneumococcal resistance to azithromycin was similar in azithromycin and placebo groups (P. S. Morris, pers. comm., 2015; Fig. 1).
Pneumococcal conjugate vaccines (PCVs) have been highly effective in reducing the burden of invasive pneumococcal disease (IPD) globally, and also in Australian First Nations’ people.26 The Northern Territory was the first jurisdiction to introduce PCV7 for Aboriginal infants in 2001, and was the only jurisdiction to introduce PCV10, 2 years prior to national transition to PCV13 in 2011. Throughout these three PCV eras we continued ongoing clinical and microbiological surveillance of OM and nasopharyngeal carriage of otopathogens across multiple Northern Territory communities.27–29 One vaccine offered potential protection from NTHi-OM via inclusion of protein D of NTHi (HiD) as the conjugation protein.30 Perhaps not surprisingly our surveillance identified a reduction in AOM,31 reduced nasopharyngeal carriage of vaccine serotypes (but replacement by non-vaccine serotypes), and the microbiology of ear discharge confirmed reduced NTHi-OM32 (Fig. 2). These surveillance data provided the strong rationale for higher quality studies (RCTs) of novel mixed PHiD-CV10 and PCV13 schedules to maximise coverage of otopathogens.33 These RCTs found that: (1) early mixed PCVs are safe; (2) combined PCVs elicit strong immune responses to all vaccine targets; (3) responses to first dose of PHiD-CV10 given at age 1 or 2 months were superior to PCV13; (4) including at least one dose PCV13 is similar to three doses; (5) either vaccine can be the booster if priming included at least one dose PCV13; and (6) protein D immunity was not protective against NTHi infection in this population. These unique discoveries inform vaccine research and development, support flexibility of PCVs, allowing national programs to optimise sero-epidemiology and mitigate supply and cost risks, particularly for low- and middle-income countries. Unfortunately, in this population, the microbiology again revealed the challenge of preventing otitis media. Acquisition of nasopharyngeal NTHi and non-vaccine serotypes22 was associated with OM onset within weeks of birth in all PCV schedule groups.18,34 Follow up to 3 years of age showed that OM persists causing chronic disabling hearing loss throughout early years for an average of 80% children.35
This brings us back to the Hearing for Learning Initiative.3 An additional and parallel crisis has been highlighted in remote primary health care where average length of stay of health care practitioners is 4 months, and many are infrequent brief but costly fly-in fly-out stays.36,37 Lack of comprehensive primary ear health programs with scheduled surveillance and appropriate follow up is failing children and over-burdening specialist services (hearing and ENT surgical consultations), which have staggeringly long waitlists of several thousands of children.38
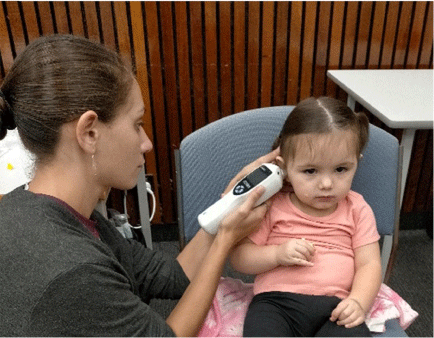
The Hearing for Learning Initiative is a 5-year stepped wedge cluster randomised controlled trial in 20 rural and remote communities in the Northern Territory. Each Community Reference Group selects community member trainees and supports the research team to deliver a 6-week course. Two Certificate II units of competency in Aboriginal Primary Health Care, and knowledge of ear and hearing and technical skills in otoscopy, tympanometry and hearScreen are learned and assessed. One or two successful graduates are then supported to join the health service workforce in new Ear Health Facilitator positions funded by the Hearing for Learning Initiative (Fig. 3). The Ear Health Facilitator residency, status in the community, cultural knowledge, local language, and communication skills are the foundation of a sustainable, culturally appropriate and skilled workforce to service the needs of children with undetected, untreated chronic ear infections, hearing loss, and social isolation. Opportunities for on-country training and appropriate job creation can be successful in remote regions through co-design and local community leadership.
|
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funding has been predominantly from the NHMRC, with contributions from Pfizer, GlaxoSmithKline, and Merck Sharp and Dohme. The Hearing for Learning Initiative is funded by The Balnaves Foundation, the Northern Territory and Federal Governments.
Acknowledgements
We acknowledge the First Nations families and thank them all for their participation in otitis media research over many years. We thank the many researchers, NT Health, and ACCHOs for their collaboration.
References
[1] Central Australian Rural Practitioners Association (CARPA) (2017) Central Australian Rural Practitioners Association (CARPA) Standard Treatment Manual, 7th edn. Central Australian Rural Practitioners Association (CARPA), Alice Springs, NT, Australia.[2] Leach, AJ et al.. (2021) Otitis media guidelines for Australian Aboriginal and Torres Strait Islander children: summary of recommendations. Med J Aust 214, 228–33.
| Otitis media guidelines for Australian Aboriginal and Torres Strait Islander children: summary of recommendations.Crossref | GoogleScholarGoogle Scholar |
[3] Kong, K et al.. (2021) A community-based service enhancement model of training and employing Ear Health Facilitators to address the crisis in ear and hearing health of Aboriginal children in the Northern Territory, the Hearing for Learning Initiative (the HfLI): study protocol for a stepped-wedge cluster randomised trial. Trials 22, 403.
| A community-based service enhancement model of training and employing Ear Health Facilitators to address the crisis in ear and hearing health of Aboriginal children in the Northern Territory, the Hearing for Learning Initiative (the HfLI): study protocol for a stepped-wedge cluster randomised trial.Crossref | GoogleScholarGoogle Scholar |
[4] Leach, AJ et al.. (1994) Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian Aboriginal infants. Pediatr Infect Dis J 13, 983–9.
| Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian Aboriginal infants.Crossref | GoogleScholarGoogle Scholar |
[5] Bell, MF et al.. (2016) Chronic Illness and Developmental Vulnerability at School Entry. Pediatrics 137, e20152475.
| Chronic Illness and Developmental Vulnerability at School Entry.Crossref | GoogleScholarGoogle Scholar |
[6] Su, J-Y et al.. (2019) The impact of hearing impairment on Aboriginal children’s school attendance in remote Northern Territory: a data linkage study. Aust N Z J Public Health 43, 544–50.
| The impact of hearing impairment on Aboriginal children’s school attendance in remote Northern Territory: a data linkage study.Crossref | GoogleScholarGoogle Scholar |
[7] Su, J-Y et al.. (2020) Impact of hearing impairment on early childhood development in Australian Aboriginal children: a data linkage study. J Paediatr Child Health 56, 1597–606.
| Impact of hearing impairment on early childhood development in Australian Aboriginal children: a data linkage study.Crossref | GoogleScholarGoogle Scholar |
[8] Su, J-Y et al.. (2020) The impact of hearing impairment on early academic achievement in Aboriginal children living in remote Australia: a data linkage study. BMC Public Health 20, 1521.
| The impact of hearing impairment on early academic achievement in Aboriginal children living in remote Australia: a data linkage study.Crossref | GoogleScholarGoogle Scholar |
[9] He, VY et al.. (2020) The link between hearing impairment and child maltreatment among Aboriginal children in the Northern Territory of Australia: is there an opportunity for a public health approach in child protection? BMC Public Health 20, 449.
| The link between hearing impairment and child maltreatment among Aboriginal children in the Northern Territory of Australia: is there an opportunity for a public health approach in child protection?Crossref | GoogleScholarGoogle Scholar |
[10] Dawson, VM et al.. (1985) Microbiology of chronic otitis media with effusion among Australian Aboriginal children: role of Chlamydia trachomatis. Aust J Exp Biol Med Sci 63, 99–107.
| Microbiology of chronic otitis media with effusion among Australian Aboriginal children: role of Chlamydia trachomatis.Crossref | GoogleScholarGoogle Scholar |
[11] Leach, AJ et al.. (2006) Microbiology of acute otitis media with perforation (AOMwiP) in Aboriginal children living in remote communities—monitoring the impact of 7-valent pneumococcal conjugate vaccine (7vPCV). Int Congr Ser 1289, 89–92.
| Microbiology of acute otitis media with perforation (AOMwiP) in Aboriginal children living in remote communities—monitoring the impact of 7-valent pneumococcal conjugate vaccine (7vPCV).Crossref | GoogleScholarGoogle Scholar |
[12] Smith-Vaughan, HC et al.. (2013) Dominance of Haemophilus influenzae in ear discharge from Indigenous Australian children with acute otitis media with tympanic membrane perforation. BMC Ear Nose Throat Disord 13, 12.
| Dominance of Haemophilus influenzae in ear discharge from Indigenous Australian children with acute otitis media with tympanic membrane perforation.Crossref | GoogleScholarGoogle Scholar |
[13] Leach, AJ et al.. (2008) Topical ciprofloxin versus topical framycetin-gramicidin-dexamethasone in Australian Aboriginal children with recently treated chronic suppurative otitis media: a randomized controlled trial. Pediatr Infect Dis J 27, 692–8.
| Topical ciprofloxin versus topical framycetin-gramicidin-dexamethasone in Australian Aboriginal children with recently treated chronic suppurative otitis media: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[14] Leach, AJ et al.. (2008) Compared to placebo, long-term antibiotics resolve otitis media with effusion (OME) and prevent acute otitis media with perforation (AOMwiP) in a high-risk population: a randomized controlled trial. BMC Pediatr 8, 23.
| Compared to placebo, long-term antibiotics resolve otitis media with effusion (OME) and prevent acute otitis media with perforation (AOMwiP) in a high-risk population: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar |
[15] Morris, PS et al.. (2010) Single-dose azithromycin versus seven days of amoxycillin in the treatment of acute otitis media in Aboriginal children (AATAAC): a double blind, randomised controlled trial. Med J Aust 192, 24–9.
| Single-dose azithromycin versus seven days of amoxycillin in the treatment of acute otitis media in Aboriginal children (AATAAC): a double blind, randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[16] Wigger, C et al.. (2019) Povidone-iodine ear wash and oral cotrimoxazole for chronic suppurative otitis media in Australian aboriginal children: study protocol for factorial design randomised controlled trial. BMC Pharmacol Toxicol 20, 46.
| Povidone-iodine ear wash and oral cotrimoxazole for chronic suppurative otitis media in Australian aboriginal children: study protocol for factorial design randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[17] Abbott, P et al.. (2016) A multi-centre open-label randomised non-inferiority trial comparing watchful waiting to antibiotic treatment for acute otitis media without perforation in low-risk urban Aboriginal and Torres Strait Islander children (the WATCH trial): study protocol for a randomised controlled trial. Trials 17, 119.
| A multi-centre open-label randomised non-inferiority trial comparing watchful waiting to antibiotic treatment for acute otitis media without perforation in low-risk urban Aboriginal and Torres Strait Islander children (the WATCH trial): study protocol for a randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[18] Leach, AJ et al.. (2021) Interchangeability, immunogenicity and safety of a combined 10-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (Synflorix) and 13-valent-PCV (Prevenar13) schedule at 1-2-4-6 months: PREVIX_COMBO, a 3-arm randomised controlled trial. Vaccine X 7, 100086.
| Interchangeability, immunogenicity and safety of a combined 10-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (Synflorix) and 13-valent-PCV (Prevenar13) schedule at 1-2-4-6 months: PREVIX_COMBO, a 3-arm randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[19] Oguoma, VM et al.. (2020) 10-Valent pneumococcal non-typeable H. influenzae protein D conjugate vaccine (PHiD-CV10) versus 13-valent pneumococcal conjugate vaccine (PCV13) as a booster dose to broaden and strengthen protection from otitis media (PREVIX_BOOST) in Australian Aboriginal children: study protocol for a randomised controlled trial. BMJ Open 10, e033511.
| 10-Valent pneumococcal non-typeable H. influenzae protein D conjugate vaccine (PHiD-CV10) versus 13-valent pneumococcal conjugate vaccine (PCV13) as a booster dose to broaden and strengthen protection from otitis media (PREVIX_BOOST) in Australian Aboriginal children: study protocol for a randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[20] Suzuki, HG et al.. (2020) Clinical practice guidelines for acute otitis media in children: a systematic review and appraisal of European national guidelines. BMJ Open 10, e035343.
| Clinical practice guidelines for acute otitis media in children: a systematic review and appraisal of European national guidelines.Crossref | GoogleScholarGoogle Scholar |
[21] Walsh, R et al.. (2022) INFLATE: a protocol for a randomised controlled trial comparing nasal balloon autoinflation to no nasal balloon autoinflation for otitis media with effusion in Aboriginal and Torres Strait Islander children. Trials 23, 309.
| INFLATE: a protocol for a randomised controlled trial comparing nasal balloon autoinflation to no nasal balloon autoinflation for otitis media with effusion in Aboriginal and Torres Strait Islander children.Crossref | GoogleScholarGoogle Scholar |
[22] Beissbarth, J et al.. (2021) Nasopharyngeal carriage of otitis media pathogens in infants receiving 10-valent non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10), 13-valent pneumococcal conjugate vaccine (PCV13) or a mixed primary schedule of both vaccines: a randomised controlled trial. Vaccine 39, 2264–73.
| Nasopharyngeal carriage of otitis media pathogens in infants receiving 10-valent non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10), 13-valent pneumococcal conjugate vaccine (PCV13) or a mixed primary schedule of both vaccines: a randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[23] Coleman, A et al.. (2018) The unsolved problem of otitis media in indigenous populations: a systematic review of upper respiratory and middle ear microbiology in indigenous children with otitis media. Microbiome 6, 199.
| The unsolved problem of otitis media in indigenous populations: a systematic review of upper respiratory and middle ear microbiology in indigenous children with otitis media.Crossref | GoogleScholarGoogle Scholar |
[24] Jervis-Bardy, J et al.. (2015) The microbiome of otitis media with effusion in Indigenous Australian children. Int J Pediatr Otorhinolaryngol 79, 1548–55.
| The microbiome of otitis media with effusion in Indigenous Australian children.Crossref | GoogleScholarGoogle Scholar |
[25] Binks, MJ et al.. (2011) Viral-bacterial co-infection in Australian Indigenous children with acute otitis media. BMC Infect Dis 11, 161.
| Viral-bacterial co-infection in Australian Indigenous children with acute otitis media.Crossref | GoogleScholarGoogle Scholar |
[26] Meder, KN et al.. (2020) Long-term impact of pneumococcal conjugate vaccines on invasive disease and pneumonia hospitalizations in Indigenous and non-Indigenous Australians. Clin Infect Dis 70, 2607–15.
| Long-term impact of pneumococcal conjugate vaccines on invasive disease and pneumonia hospitalizations in Indigenous and non-Indigenous Australians.Crossref | GoogleScholarGoogle Scholar |
[27] Stubbs, E et al.. (2005) Streptococcus pneumoniae and noncapsular Haemophilus influenzae nasal carriage and hand contamination in children: a comparison of two populations at risk of otitis media. Pediatr Infect Dis J 24, 423–8.
| Streptococcus pneumoniae and noncapsular Haemophilus influenzae nasal carriage and hand contamination in children: a comparison of two populations at risk of otitis media.Crossref | GoogleScholarGoogle Scholar |
[28] Leach, AJ et al.. (2009) Emerging pneumococcal carriage serotypes in a high-risk population receiving universal 7-valent pneumococcal conjugate vaccine and 23-valent polysaccharide vaccine since 2001. BMC Infect Dis 9, 121.
| Emerging pneumococcal carriage serotypes in a high-risk population receiving universal 7-valent pneumococcal conjugate vaccine and 23-valent polysaccharide vaccine since 2001.Crossref | GoogleScholarGoogle Scholar |
[29] Leach, AJ et al.. (2016) General health, otitis media, nasopharyngeal carriage and middle ear microbiology in Northern Territory Aboriginal children vaccinated during consecutive periods of 10-valent or 13-valent pneumococcal conjugate vaccines. Int J Pediatr Otorhinolaryngol 86, 224–32.
| General health, otitis media, nasopharyngeal carriage and middle ear microbiology in Northern Territory Aboriginal children vaccinated during consecutive periods of 10-valent or 13-valent pneumococcal conjugate vaccines.Crossref | GoogleScholarGoogle Scholar |
[30] Prymula, R et al.. (2006) Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367, 740–8.
| Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study.Crossref | GoogleScholarGoogle Scholar |
[31] Leach, AJ et al.. (2014) Otitis media in children vaccinated during consecutive 7-valent or 10-valent pneumococcal conjugate vaccination schedules. BMC Pediatr 14, 200.
| Otitis media in children vaccinated during consecutive 7-valent or 10-valent pneumococcal conjugate vaccination schedules.Crossref | GoogleScholarGoogle Scholar |
[32] Leach, AJ et al.. (2015) Reduced middle ear infection with non-typeable Haemophilus influenzae, but not Streptococcus pneumoniae, after transition to 10-valent pneumococcal non-typeable H. influenzae protein D conjugate vaccine. BMC Pediatr 15, 162.
| Reduced middle ear infection with non-typeable Haemophilus influenzae, but not Streptococcus pneumoniae, after transition to 10-valent pneumococcal non-typeable H. influenzae protein D conjugate vaccine.Crossref | GoogleScholarGoogle Scholar |
[33] Leach, AJ et al.. (2015) Pneumococcal conjugate vaccines PREVenar13 and SynflorIX in sequence or alone in high-risk Indigenous infants (PREV-IX_COMBO): protocol of a randomised controlled trial. BMJ Open 5, e007247.
| Pneumococcal conjugate vaccines PREVenar13 and SynflorIX in sequence or alone in high-risk Indigenous infants (PREV-IX_COMBO): protocol of a randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[34] Leach, AJ et al.. (2021) Otitis media outcomes of a combined 10-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine and 13-valent pneumococcal conjugate vaccine schedule at 1-2-4-6 months: PREVIX_COMBO, a 3-arm randomised controlled trial. BMC Pediatr 21, 117.
| Otitis media outcomes of a combined 10-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine and 13-valent pneumococcal conjugate vaccine schedule at 1-2-4-6 months: PREVIX_COMBO, a 3-arm randomised controlled trial.Crossref | GoogleScholarGoogle Scholar |
[35] Leach, AJ et al.. (2022) Immunogenicity, otitis media, hearing impairment, and nasopharyngeal carriage 6-months after 13-valent or ten-valent booster pneumococcal conjugate vaccines, stratified by mixed priming schedules: PREVIX_COMBO and PREVIX_BOOST randomised controlled trials. Lancet Infect Dis 22, 1374–87.
| Immunogenicity, otitis media, hearing impairment, and nasopharyngeal carriage 6-months after 13-valent or ten-valent booster pneumococcal conjugate vaccines, stratified by mixed priming schedules: PREVIX_COMBO and PREVIX_BOOST randomised controlled trials.Crossref | GoogleScholarGoogle Scholar |
[36] Russell, DJ et al.. (2017) Patterns of resident health workforce turnover and retention in remote communities of the Northern Territory of Australia, 2013–2015. Hum Resour Health 15, 52.
| Patterns of resident health workforce turnover and retention in remote communities of the Northern Territory of Australia, 2013–2015.Crossref | GoogleScholarGoogle Scholar |
[37] Zhao, Y et al.. (2017) Long-term trends in supply and sustainability of the health workforce in remote Aboriginal communities in the Northern Territory of Australia. BMC Health Serv Res 17, 836.
| Long-term trends in supply and sustainability of the health workforce in remote Aboriginal communities in the Northern Territory of Australia.Crossref | GoogleScholarGoogle Scholar |
[38] Australian Institute of Health and Welfare (2021) Hearing health outreach services for Aboriginal and Torres Strait Islander children in the Northern Territory: July 2012 to December 2020. Cat no. IHW 260.




