Understanding the SARS-CoV-2 pandemic as evolution in action
Will CairnsMicrobiology Australia 41(4) 213-216 https://doi.org/10.1071/MA20057
Published: 22 October 2020
Abstract
In the midst of our pandemic, when we are up to our necks in a torrent of news, opinion and speculation, it is important to step back from our personal interests in SARS-CoV-2 to consider the broader biological and social evolutionary context of what we are experiencing.
First, we are not unique – many other organisms are also currently experiencing pandemics for which humans are at least partly responsible – rabbits, pigs, frogs, chestnut and other trees, and even bats1–7. Disease outbreaks in human populations are manifest simultaneously as biological, geopolitical, sociocultural and ecological evolutionary events. They have been shaped in part by the evolution of opportunities for disease transmission offered by our transformation from nomadic hunter-gatherers to urbanised world travellers.
It is important to remember that evolution is a journey without a destination or a goal – it is a consequence, not a response. In a world of unfathomably complex and interdependent ecosystems, the random chance of mutations and the vast complexity of interindividual interactions across and between species shape the probability that individuals and their social groupings will fail or succeed.
For social organisms, the viability of the complex emergent phenomenon of community is driven by the genetically driven behaviours of its members8,9. Human evolution, biological and cultural, is further complicated by our talents for overriding the effects of genes and learning novel behaviours. Most commonly, and in the absence of calamity, the social evolution of humans plays out over long time frames. For those interested in exploring these issues more broadly, I suggest the following references10–13.
So, how is this pandemic an evolutionary event? Coronaviruses evolve. The replication of viruses is an imperfect process and a random array of new variants emerge. Most new forms will be mutant duds and incapable of using any animal as a host. Occasionally, either in their original host and/or perhaps facilitated by recombination with viruses of or in another host, infected cells produce genetic variants that are more virulent.
Once in a while, a successful new variant coronavirus will latch on to a human cell, subvert its molecular machinery to replicate itself repeatedly, make us cough and sneeze through a runny, snotty, virus-laden nose to spread their countless viral offspring to other humans, disrupt our organ function and physiology in novel ways, and perhaps kill some of us.
Early research suggested that SARS-CoV-2 was transferred from bats to pangolins (a low likelihood event in a human-free world but plausible in an environment of exotic culinary tastes), and perhaps evolved further in their new host before infecting humans14. If so, this was a journey to human parasitism via an intermediate animal host similar to several other bat-derived viral infections: Hendra (horses), severe acute respiratory syndrome – SARS (palm civet cats) and Middle East respiratory syndrome – MERS (camels). Further molecular analyses indicate that SARS-CoV-2 emerged unnoticed in horseshoe bats about 40–70 years ago, and either passed via the pangolin, or jumped directly to humans15,16.
However, we are not passive in this relationship. While for pathogenic viruses we are simply a prospective substrate for reproduction, the challenges posed by parasites over countless millions of years has induced the consequence of the evolution of the attributes that comprise immune systems. Those that were more successful in sustaining their respective roles of host and parasites are our ancestors and those of our parasites.
Leaving aside our individual acquired personal susceptibilities, it is simply chance whether or not our genes render us non-susceptible or provide us with an immune system that might protect us when we meet a particular novel parasite. Sickle cell and similar diseases are examples of defences that emerged as random mutations that, although causing some morbidity and mortality, offer resistance to a parasite (malaria). These genes persisted in places where malaria is a risk because they have found a place of delicate balance in the probabilities of survival that characterises the evolution of a group of individuals17. There is of course no predicting the characteristics of the future novel parasites that jump from other species. We may all have attributes waiting to be exploited by an organism with the means to turn our trait into an opportunity18,19.
Contemporaneous records show that outbreaks of novel parasites have on occasions killed a huge proportion of human populations – over 50% mortality in some naïve communities20. Indeed, an epidemic in 1616–19 killed perhaps 90% of the native Americans of coastal Massachusetts who might have resisted the Pilgrims when they landed at Plymouth Rock in 162021,22.
Evolution can be rapid when selective mortality is very high: the genetics of a population of New Guinea highlanders who engaged in the mortuary ritual of endocannibalism was changed very significantly by the deaths of those who were genetically susceptible to Kuru, a transmissible prion disease related to Creutzfeldt Jacob disease (CJD)23.
We do learn behaviours (cultural evolution) that reduce risk: not eating raw pigs (or polar bears or walruses!) because they may carry Trichinosis; cultural sanctions against eating sick marmots (in which plague bacteria – Yersinia pestis – are endemic) that are ignored at individual peril; and the public health measures of quarantine, masks and isolation24.
An outbreak of a species-jumping illness in a small and isolated community may burn out without spreading beyond the confines of that population. If numerous enough, the less susceptible or more robust survivors can endow their successors with genes that offer a diminished vulnerability to that disease.
The evolution of human culture – the increasing complexity of technology and farming, of travel and trading practices, and of our networks of larger settlements – created opportunities for a range of new microparasites.
Descriptions of smallpox and measles did not appear in literature prior to a few thousand years ago25. Measles outbreaks stopped depending on the parasite jumping repeatedly from another species and became endemic in humans only after our community size reached a minimum of perhaps 100 000. As confirmation, a recent study of the genetic clock of measles found that its antecedent rinderpest, a disease of cattle, became a separate endemic disease of humans about 2600 years ago26. Our short and meaningful relationship with smallpox seems to have been a far more complicated tale of mummies, Vikings and mutations27.
In 1492 Columbus, and the Spaniards who followed him, introduced smallpox and measles to the Americas. Having been isolated from the rest of the world for tens of thousands of years, before pandemics became possible, the indigenous populations were genetically naïve and died in droves from what were for them novel illnesses. Weakened, or even deconstructed, their civilisations were easily overwhelmed by small numbers of Spanish and Portuguese soldiers, and subsequently by northern Europeans. Other pandemic or epidemic diseases still require intermediate hosts that may be facilitated by human behaviour.
It was not until the development of extensive trade – along the Silk Road, across the Black Sea and throughout the Mediterranean – that humans were able to accelerate the spread of plague in our cohabiting and hitch-hiking fellow-traveller black rats and their fleas. Multiple outbreaks over many centuries killed over one-third of the population of Europe and shaped human social, cultural and political evolution13,20.
Plague arrived in North America and Australia28 around the end of the 19th century only because the invention of fast steam ships allowed its importation from China. In North America it caused short-lived epidemics and became endemic in native burrowing rodents with some help from ranchers seeking to control gophers. When my family and I arrived in Townsville in 1978, a new concrete slab on ground still required a 600 mm deep ratwall around the edge to exclude burrowing rodents. A friend and I shared ownership of a ratwall shovel!
African slaves imported to the Caribbean to grow sugar brought with them Yellow Fever and its vector mosquito Aedes aegypti. Together, Yellow Fever, to which Europeans were genetically naive, and rebellious slaves, who were resistant because they had evolved with the virus, eventually erased Napoleon’s hopes for empire in the Americas. Subsequently, Napoleon’s invasion of Russia was thwarted in large part by epidemics of Shigellosis and Typhus13.
Massive human mortality from recurring epidemics of microparasites only subsided with scientific understanding of diseases, their life cycles and their transmission, followed by effective public health measures and vaccination. A simple graph (Figure 1) shows how mortality in New York City from a wide range of microparasites has declined over the past ~217 years29. Notably, the peak of mortality in the 1918–19 global influenza pandemic was lower than in any year of the 19th century.
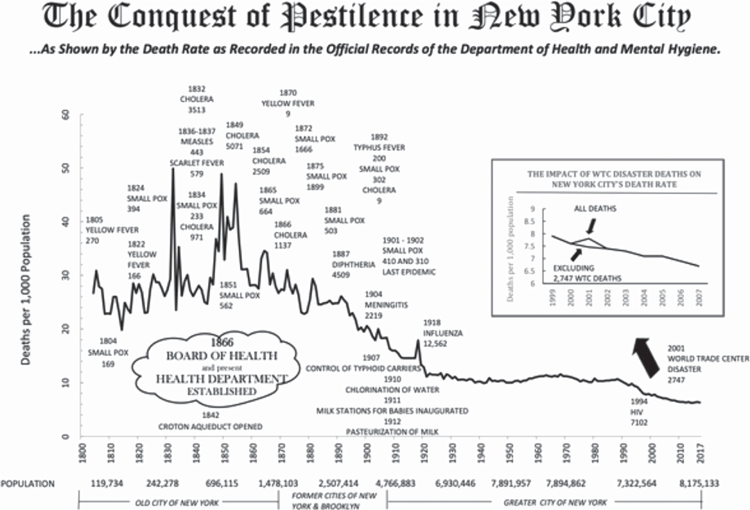
|
Where does all that leave us? SARS-CoV-2 is simply the latest of a stream of organisms that have chanced upon the means to take advantage of the molecular structure and function of our cells, our behaviour and/or our social organisation, and spread through our population.
Our biological and cultural evolution in a minimally changing environment generally unfolds so slowly as to be imperceptible in one lifespan. However, a major event that eliminates a subset of the population, disrupts the complex systems on which we all depend, unsettles our entrenched social order and exposes our failings may induce dramatic changes that are apparent in real time.
Two hundred and fifty years ago, SARS-CoV-2 would have been yet another evolutionary upheaval; those most vulnerable would die, and the genetic mix of the population as a whole would have moved on after it had swept around the world, perhaps eventually becoming another endemic disease. Over those 250 years, our world has changed a great deal. While for now we rely on traditional behavioural measures, it seems highly likely that science and the power and ingenuity of modern technology will eventually provide means for preventing and treating SARS-CoV-2.
Our communities have become far more complex30. This pandemic is exposing the extent of inequalities in social and health outcomes around the world. It will take some time for the consequences of the disruption of our social, political, economic and healthcare systems to play out; so far, there has been little discussion about remedial measures.
Pandemics also remind us of the reality that, while all lives are enmeshed in the biology of the natural world, too few of us consider the complexity of the consequences for the global environment in our pursuit of short-term personal gain31. In a destabilised world we all face an increasing risk of new microparasitic threats32,33. Sooner or later, another pandemic, perhaps promoted by global climate change, will emerge from the vast panoply of parasites circulating on our planet and, like SARS-CoV-2, it will be different from anything we have met before.
A basic requirement for life, and perhaps even intrinsic to its definition, is the unbroken sequence of its success34. On our unpredictably everchanging planet, this is only sustainable as an evolutionary process by which instability produces a collection of novel opportunities to be tested by probability and chance. The success of SARS-CoV-2, and its disruption of our lives, our cultures, our communities and our genomes, are simply manifestations of the benefits of mutability for the necessity of evolution of life on earth35.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
This research did not receive any specific funding. The author thanks his wife and the reviewers for their constructive comments.
References
[1] https://www.nytimes.com/2020/05/18/science/virus-outbreak-rabbits.html?algo=identity&fellback=false&imp_id=396956005&imp_id=355015288&action=click&module=Science%20%20Technology&pgtype=Homepage[2] https://www.oie.int/en/animal-health-in-the-world/animal-diseases/african-swine-fever/
[3] https://www.newscientist.com/article/2222501-a-quarter-of-all-pigs-have-died-this-year-due-to-african-swine-fever/
[4] https://www.the-scientist.com/daily-news/origin-of-frog-killing-chytrid-fungus-found-36616
[5] https://www.acf.org/the-american-chestnut/history-american-chestnut/
[6] https://www.theguardian.com/environment/2019/feb/09/blight-fight-the-story-of-americas-chestnuts-offers-hope-for-british-trees
[7] https://www.daf.qld.gov.au/business-priorities/biosecurity/animal-biosecurity-welfare/animal-health-pests-diseases/a-z-list-of-significant-animal-pests-and-diseases/white-nose-syndrome/white-nose-syndrome
[8] Wilson, E.O. (2020) Genesis: The Deep Origins of Societies. Penguin.
[9] Hölldobler, B. and Wilson, E.O. (2009) The Superorganism: The Beauty, Elegance and Strangeness of Insect Societies. W. W. Norton and Co.
[10] McNeill, W.H. (1976) Plagues and Peoples. Anchor Books.
[11] Lederberg, J. (1988) Pandemic as a natural evolutionary phenomenon. Social Research 55, 343–359.
[12] Kucharski, A. (2020) The Rules of Contagion: Why Things Spread and Why They Stop. Profile Books.
[13] Snowden, F. (2019) Epidemics and Society: From the Black Death to the Present. Yale University Press.
[14] https://www.nytimes.com/2020/06/19/science/coronavirus-rats-vietnam.html?referringSource=articleShare
[15] Boni, M.F. et al. (2020) Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. , .
| Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic.Crossref | GoogleScholarGoogle Scholar | 32724171PubMed |
[16] Cyranowski, D. (2020) Profile of a killer: the complex biology powering the coronavirus pandemic. Nature, News feature, 4 May. https://www.nature.com/articles/d41586-020-01315-7
[17] Piel, F.B. et al. (2017) Sickle cell disease. N. Engl. J. Med. 376, 1561–1573.
| Sickle cell disease.Crossref | GoogleScholarGoogle Scholar | 28423290PubMed |
[18] https://www.nytimes.com/2020/06/03/health/coronavirus-blood-type-genetics.html?action=click&algo=top_conversion&block=trending_recirc&fellback=false&imp_id=839560899&impression_id=276097808&index=1&pgtype=Article®ion=footer
[19] Bunyavanich, S. et al. (2020) Racial/ethnic variation in nasal gene expression of transmembrane serine protease 2 (TMPRSS2). JAMA. , .
| Racial/ethnic variation in nasal gene expression of transmembrane serine protease 2 (TMPRSS2).Crossref | GoogleScholarGoogle Scholar | 32910146PubMed |
[20] McNeill, W.H. (1976) Appendix: epidemics in China [BCE 243BC to CE 1911]. In Plagues and Peoples, p. 297. Anchor Books. https://www.penguinrandomhouse.com/books/112492/plagues-and-peoples-by-william-h-mcneill/
[21] Marr, J.S. and Cathey, J.T. (2010) New hypothesis for cause of epidemic among Native Americans, New England, 1616–1619. Emerg. Infect. Dis. 16, 281–286.
| New hypothesis for cause of epidemic among Native Americans, New England, 1616–1619.Crossref | GoogleScholarGoogle Scholar | 20113559PubMed |
[22] Mann, C.C. (2005) Native intelligence. Smithsonian Magazine, December. https://www.smithsonianmag.com/history/native-intelligence-109314481/
[23] Mead, S. et al. (2009) A novel protective prion protein variant that co-localizes with kuru exposure. N. Engl. J. Med. 361, 2056–2065.
| A novel protective prion protein variant that co-localizes with kuru exposure.Crossref | GoogleScholarGoogle Scholar | 19923577PubMed |
[24] https://www.theguardian.com/world/2020/jul/15/teenage-boy-dies-plague-mongolia-after-eating-marmot?CMP=Share_iOSApp_Other
[25] McNeill, W.H1976 Chapter III: Confluence of civilized disease. In Plagues and Peoples. pp. 94-159. Anchor Books.
[26] Düx, A. et al. (2020) Measles virus and rinderpest virus divergence dated to the sixth century BCE. Science 368, 1367–1370.
| Measles virus and rinderpest virus divergence dated to the sixth century BCE.Crossref | GoogleScholarGoogle Scholar | 32554594PubMed |
[27] Mühlemann, B. et al. (2020) Diverse variola virus (smallpox) strains were widespread in northern Europe in the Viking Age. Science 369, eaaw897724.
| 32703849PubMed |
[28] https://www.nma.gov.au/defining-moments/resources/bubonic-plague
[29] https://www.reddit.com/r/nyc/comments/cg2v4b/the_conquest_of_pestilence_in_nyc_18002017/
[30] Cairns, W. (2020) Disaster of system failure: challenges for healthcare in 21st century. MJA InSight+ 13 January. https://insightplus.mja.com.au/2020/1/disaster-of-system-failure-challenges-for-health-care-in-2020/
[31] https://www.nytimes.com/2020/05/19/opinion/trump-coronavirus.html?action=click&module=Opinion&pgtype=Homepage
[32] Gibb, R. et al. (2020) Zoonotic host diversity increases in human-dominated ecosystems. Nature 584, 398–402.
| Zoonotic host diversity increases in human-dominated ecosystems.Crossref | GoogleScholarGoogle Scholar | 32759999PubMed |
[33] https://www.theguardian.com/us-news/2020/aug/11/ticks-lyme-disease-climate-change-us?CMP=Share_iOSApp_Other
[34] Nurse, P. (2020) What is Life?: Understand Biology in Five Steps. Scribe Publications.
[35] Cairns, W. (2014) Death Rules: How Death Shapes Life on Earth, and What it Means for Us. Vivid Publishing (e-book).
Biography
Dr Will Cairns OAM FRACGP FAChPM was educated in Australia, the US, and the UK before settling in Townsville in 1978. After working as a GP for a decade he moved into specialist palliative care and was deeply involved in the creation of the specialty of Palliative Medicine. He has a long-standing interest in the provision of palliative care in disasters and has written extensively on the matter. He is an active member of the Australian COVID-19 Palliative Care Working Group.


