The 1918 Spanish influenza pandemic: plus ça change, plus c’est la même chose
Paul Selleck A C and Ross Barnard B DA CSIRO Australian Centre for Disease Preparedness, Private Bag 24, Geelong, Vic., 3220, Australia
B School of Chemistry and Molecular Biosciences, Australian Infectious Diseases Research Centre, and ARC Training Centre for Biopharmaceutical Innovation, The University of Queensland Brisbane, Qld 4072, Australia
C Tel.: +61 3 5227 5000 Fax.: +61 3 5227 5555 Email: Paul.Selleck@csiro.au
D Tel.: +61 4 1049 4472 Fax: +61 7 3365 4299 Email: rossbarnard@uq.edu.au
Microbiology Australia 41(4) 177-182 https://doi.org/10.1071/MA20049
Published: 7 October 2020
Abstract
Towards the end of world war one, the world faced a pandemic, caused not by smallpox or bubonic plague, but by an influenza A virus. The 1918–19 influenza pandemic was possibly the worst single natural disaster of all time, infecting an estimated 500 million people, or one third of the world population and killing between 20 and 100 million people in just over one year. The impact of the virus may have influenced the outcome of the first world war and killed more people than the war itself. The pandemic resulted in global economic disruption. It was a stimulus to establishment of local vaccine production in Australia. Those cities that removed public health restrictions too early experienced a second wave of infections. Unfortunately, it seems that the lessons of infection control and epidemic preparedness must be relearnt in every generation and for each new epidemic.
Introduction
In 1917 an influenza virus causing mild symptoms appeared in the USA. Due to the movement of soldiers from the USA to the European front in 1917 and 1918 the virus spread rapidly, causing numerous mild epidemics amongst the troops in March of 1918. The 1918–19 pandemic was subsequently called ‘Spanish influenza’, not because it originated in Spain, but because Spain was neutral during the war and allowed the press to report cases of infection, whereas such information was censored elsewhere. In September to October of 1918 a second wave of infection spread rapidly across the world causing a more severe clinical disease with symptoms including cyanosis, pulmonary oedema, pulmonary haemorrhage, aches, fever, coughing and an overwhelming weariness1. A third wave of infection occurred in the winter and spring of 1919, also with severe clinical signs. The disease in the second and third waves had an unusually high morbidity and mortality, with case fatality rates of more than 2.5 percent, compared with less than 0.1 percent in the first wave and normal seasonal influenza epidemics. The viruses of the second and third waves caused a higher case-fatality rate among 20–40-year-old people than in other age groups2–4. This is in stark contrast to normal seasonal influenza in which case-fatality rates are highest in the very young and very old. It is well documented that, in those pre-antibiotic times, many who died of ‘influenza’ died with, or because of secondary bacterial pneumonia2,4,5. So great was the impact of the virus that one German general blamed his country’s defeat in world war one, not on the 1917 influx of troops from the USA, but on the effects of the pandemic on his soldiers6. As a single cause of death, the pandemic may have been responsible for more deaths than the first world war (17 million military and civilian deaths), the second world war (60 million military and civilian deaths), and possibly the combined total of both4.
Origins of the virus
It was reported that influenza pneumonia mortality cases increased sharply in some US cities and states in December 1915 and January 1916, which may or may not have been related to the 1918–19 pandemic. One theory is that the 1918–19 pandemic influenza virus originated as early as December 1917 in the Midwest of the USA2. Although it is likely that the exact geographical source will never be established conclusively, Camp Funston, a US army training camp in Kansas, was the location of the first reported outbreak of influenza in the USA2. Outbreaks of influenza were also reported in several European countries before the first wave in the April and May of 1918 and later in June and July in Asia, before spreading to the Pacific and South America and then the rest of the world2. In less than one year a more virulent virus had emerged and was transported around the world by soldiers demobilised after the war4.
The 1957 H2N2 and 1968 H3N2 pandemic influenza viruses arose from reassortment between human and avian viruses2,3. The 2009 H1N1 pandemic virus was a reassortant between human, swine and avian influenza viruses2. Taubenberger et al.7 initially hypothesised that the 1918–19 H1N1 pandemic virus was introduced directly into the human population from an avian host (without reassortment) as the eight gene segments appeared to have evolved in the same, as yet unidentified, host. However, subsequent analyses by two independent groups8,9 contested this, arguing that the phylogenies were more characteristic of those that would result from the presence of an intermediate host. It is also possible that the 1918–19 pandemic virus was a reassortant between avian, swine and human viruses prior to 1918, although other evidence weighs against this. The gene sequences are distinct from other avian and mammalian influenza viruses, but without sequence data from before 1918 we may never identify the host. What is clear is that the viruses from the second and third waves were more virulent.
Seasonal influenza epidemics usually occur once per year, in late winter in temperate climates, or twice a year in the tropics, so the occurrence of three waves of the 1918–19 virus within one year is highly unusual. The first wave began in the northern hemisphere spring of 1918 and persisted into summer (March–August). The majority of morbidity and mortality associated with the first wave occurred in young adults between 15 and 34 years of age. The disease was typically mild and the fatality rate was 0.65 people per thousand. Fatalities in young soldiers may have been a consequence of overcrowding and poor general health on the European battle fields (dysentery was rife)10 and the lack of treatments for secondary bacterial infections associated with influenza cases. Many soldiers were affected by gas attacks, which may have exacerbated the effects of influenza infection. Infections were recorded throughout most of the world, although South America and Australia initially remained free1,2.
In summer of 1918 the number of reported cases of influenza began to decrease and it was hoped that the pandemic would be over by August. However, it is considered likely that a new strain of virus appeared, containing either mutations or a reassortment of genes, which could kill healthy young adults within days of infection. This virus was extremely virulent, with infected people showing a high fever, cyanosis and pulmonary oedema. In 5% of the cases death occurred within 3 days of clinical signs appearing although in most cases the time between symptoms and death was 7–10 days1,2. Between August and November large numbers of troops were being moved around the world, initially to the battlefields and later, home from the battlefields. During this period the second wave rapidly spread to the rest of the world and caused the majority of the recorded morbidities and mortalities. The third wave began in early 1919, but was generally less virulent and did not affect every country. It is thought that by this time much of the population was immune, hence reducing the transmission of the virus. In some countries the pandemic persisted, and was still claiming lives until 1920, before finally disappearing3,4,7.
Although most of those who died during the 1918–19 pandemic died from secondary bacterial infections, for which antibiotics were not available3,4, many others died in less than 5 days, showing clinical signs of cyanosis, pulmonary haemorrhage or pulmonary oedema. Necropsy findings showed that pathology was restricted to the respiratory tract with no evidence of systemic infection. These findings suggest that the virus was well adapted to replication in the human respiratory tract. An unusual feature of the 1918–19 pandemic virus was that the mortality rate in 15–34 year olds was more than 20 times higher than the usual mortality rate of seasonal influenza viruses in this age group4. Another unusual feature was that the mortality rate in people older than 65 was less than the mortality rate in people younger than 65. More than 99 percent of all influenza related deaths in 1918–19 were in people younger than 65 years old and almost 50% of all influenza related deaths were in the 20-40 age group1,2. Together these factors are responsible for the ‘W-shaped’ mortality curve characteristic of the 1918–19 pandemic1. The lower mortality in those older than 65 may have been due to immunity from previous exposure to influenza viruses of the same subtype but even allowing for this skewing, the high mortality in younger adults may have been be due to viral or host factors.
The influenza A virus from the 1918–19 outbreak was not reconstructed until July 1996, by Amy Krafft, working with Ann Reid and Jeffery Taubenberger11. The first recovered virus fragments came from formaldehyde fixed lung tissue, obtained at autopsy from an army private named Roscoe Vaughn, who was in an army camp in South Carolina. The virus was named Influenza A/South Carolina/1/18 (H1N1). He had died five days after admission to hospital, at the age of 2112. Subsequent RNA fragments were recovered from material collected by Johan Hultin from 1918 influenza victims buried in permafrost in the village of Brevig, Alaska. The haemagglutinin gene did not have the cleavage site mutation that is now known to make the H5 and H7 strains so deadly, so other factors were clearly at play in the exceptional virulence of this H1N1 virus.
It is of interest to note that influenza was first observed in swine in the autumn of 1918, corresponding with the second pandemic wave in humans. The clinical signs and pathological features of the disease in swine were remarkably similar to those in humans2. The absence of reports of any disease resembling swine influenza prior to 1918 suggests the possibility that influenza viruses had not infected swine prior to this time and that the virus spread from humans to pigs during the second wave of the 1918–19 pandemic. Outbreaks of influenza-like disease were also reported in swine in Europe and China in the autumn and winter of 1918 and 1919. Since 1918, influenza viruses of swine origin (for example A/California/04/2009 (H1N1)) have continued to circulate in North America, Europe and Asia2,3.
Spanish influenza in Australia
Reports of an epidemic of influenza circulating in Europe reached Australia in July of 1918, followed by reports of a highly virulent second wave in September. As the pandemic spread through Europe, Africa and Asia the newly created Australian Quarantine Service introduced strict quarantine measures at all Australian ports on 17 October 1918. Australia’s remoteness from Europe and North America, and the fact that the only way to reach Australia was by sea meant that the length of time taken to reach Australia by sea was longer than several incubation periods. This meant that most (but not all) troops on ships had either recovered or died before reaching Australia, delaying the introduction of the virus into Australia. However, some troop ships returning from Europe after the November armistice had large numbers of influenza infections. The first infected ship arrived in Australia on 18 October 1918 and over the next 6 months 174 of 323 vessels checked and 1102 of 81 510 people checked were diagnosed with influenza. Many deaths occurred at sea and in Australian quarantine stations before the virus first appeared in Melbourne on 9 or 10 January 19191,13,14.
In the six months following the introduction of the virus to Australia it is estimated that at least 15 000 people died of influenza and as many as two million people (40% of the population at the time) were infected. Almost one-third of deaths were in young adults 24–34 years of age, consistent with case-fatality patterns reported elsewhere1,13,14. Mortalities occurred in two waves, the first wave between mid-March and late May, affected twice as many males as females and caused approximately 31 per cent of total deaths over that time period8. The second wave peaked in June and July and was more virulent than the first; produced a higher mortality rate, affected a greater proportion of females and far more people over the age of 50 years. Mortality rates varied greatly between countries. In Australia the overall mortality rate was three deaths per thousand whereas in New Zealand it was almost double this. Indigenous people were particularly susceptible. In Western Samoa there were 8500 deaths from a population of only 38 000. In New Zealand, Maoris had a death rate of 42.3 per thousand, seven times that of European New Zealanders. Indigenous Australians were severely affected with some communities suffering mortality rates approaching 50%13,14.
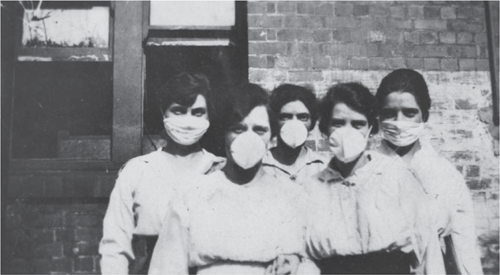
Australia was not prepared for a natural disaster of the scale of the 1918–19 pandemic. The whole of society and the economy had been disrupted by the war and the infection of key staff with the virus affected Australia’s ability to control the pandemic and to treat the sick. Quarantine was a key part of the Australian control program, with state borders closed and quarantine camps set up at border crossings. Places where people gathered in large numbers (schools, theatres, churches) were closed, roads were disinfected and the wearing of masks was made mandatory (Figure 1). People were asked to practice enhanced personal hygiene measures including hand washing, disinfection and cough protection. Hospital beds filled quickly and temporary hospitals were set up to cope with the overflow of patients. Medical staff were increasingly infected, putting further strain on health care services. Any hope of providing normal services and activities disappeared as more and more people became ill1,13,14.
|
The Commonwealth Serum Laboratories was established during world war one to produce vaccines locally. In 1918 it produced its first vaccine against pneumonic influenza. At the time, the cause of influenza was not known, and a vaccine was produced against the cause of the secondary bacterial infection1. At that time the aetiology of this disease was unclear; influenza was believed to be caused by a bacterium such as Bacillus influenza (Haemophilus influenzae) also known as Pfeiffer’s bacillus (Richard Pfeiffer described it during the 1889–1892 influenza epidemic). In the same period, the French microbiologists Charles Nicolle (1866–1936), Charles L. Bally and Ren. Dujarric de la Rivière (1885–1969) of the Pasteur Institute had shown that the influenza pathogen could pass through a fine filter15. However, despite their brilliant experiments, the virus hypothesis continued to be neglected until the virus was isolated from nasal secretions in 1932–33 by English scientists Wilson Smith (1897–1965), Christopher Andrewes (1896–1988) and Patrick Laidlaw (1881–1940), working at the Medical Research Council at Mill Hill, demonstrating the intranasal human transmission of the virus15.
In 1935, Frank Macfarlane Burnet published the first of his 114 papers on influenza virus, showing that it could be grown on the chorioallantoic membrane of embryonated hens’ eggs16. This was also discovered independently by Wilson Smith. It was subsequently demonstrated that the formalin inactivated virus was immunogenic in humans, that the influenza virus grew easily in fertilized hen eggs, and that the virus could be purified by means of high-speed centrifugation, a procedure that is still used today to manufacture most influenza vaccines17.
Many lessons from Australia’s experience during with the 1918–19 influenza pandemic are applicable to the current COVID-19 pandemic; lessons on people’s reactions to a pandemic, the importance of inter-governmental co-operation, the importance of maintaining a well-resourced health system and epidemic response capacity during non-pandemic times. As we have seen with the present COVID-19 pandemic, in the 1918–19 influenza pandemic many people attempted to ‘run’ the state border quarantine, refused to obey movement restrictions or wear masks1,2,14. It is also of interest to note that the second wave occurred in Australia after social restrictions were relaxed following a decrease in cases in the Australian autumn. This paralleled the experience in several US cities when relaxation of social restrictions was quickly followed by a resurgence of infections18–21.
Australia’s experience in the 1918–19 pandemic demonstrated that cooperation between various governments and government authorities during such crises cannot be taken for granted. In late November 1918, state ministers of health, medical authorities and the Commonwealth Government met for a national influenza planning conference. The meeting adopted a 13-point plan for dealing with the spread of the virus, with the federal government taking responsibility for proclaiming which states were infected and organising maritime and land quarantine. The states would arrange emergency hospitals, vaccination depots, ambulance services, medical staff and public awareness campaigns. Under the agreement, state authorities were required to promptly report any cases to the Commonwealth, which would then close that state’s borders to protect its neighbours. Victoria did not report positive influenza cases until 28 January 1919, the day after New South Wales confirmed an outbreak. Other states viewed Victoria’s delay in reporting as a breach of the November agreement and the agreement collapsed, with each state imposing its own conditions and organising its own containment policies14.
The Experience in the USA
Strochlic and Champine18 elegantly summarised the findings of three studies on the effects of public health measures on the spread of 1918 influenza in the United States. A study published in 2007 in the Journal of the American Medical Association analysed data from the US census taken during the 1918 pandemic19. Death rates in 43 US cities were charted. Two other studies published in the same year examined how public health responses influenced the spread of the disease in cities across the United States20,21. By comparing fatality rates and timing of public health interventions, they discovered that death rates were approximately 50 percent lower in cities that implemented preventative measures early, versus those that did so late, or not at all. The most effective actions were those that simultaneously closed schools, churches, and cinemas, and banned public gatherings; measures intended to lessen the strain on health care systems.
Those authors reached the important conclusion that relaxing intervention measures too early could cause an otherwise stabilised city to experience another spike in case numbers. The city of St. Louis, for example, encouraged by its initially low death rate, lifted restrictions on public gatherings less than two months after the outbreak began. A wave of new cases followed19, with the surge particularly evident after restrictions on public gatherings were lifted. Of the cities that kept interventions in place, none experienced a second wave of high death rates. In 1918, the key to ‘flattening the curve’ was social distancing. This remains true a century later, in the current battle against coronavirus. Unfortunately, it seems that the lessons of infection control and epidemic preparedness must be relearned in every generation and for each new epidemic. We still face major delays and bottlenecks in vaccine production 100 years on from the ‘Spanish’ flu22.
In 1957, although the US government was aware that H2N2 was on the way, the government would not commit to a huge public health vaccination program. On the other hand, pharmaceutical companies would not risk producing large quantities of vaccine without financial guarantees, until they were certain that it was needed. The trouble with influenza A is that by the time the need for a vaccine becomes clear it is too late to start production. Hence the introduction of the advance warning systems and advance vaccine production systems now in place for influenza. The WHO Collaborating Centre for Reference and Research on Influenza at the Victorian Infectious Diseases Reference Laboratory in Melbourne is part of the World Health Organization Global Influenza Surveillance and Response System. The network was established in 1952 to monitor the seasonal changes in influenza viruses, and to anticipate emerging subtypes, with the aim of reducing the impact of influenza through enabling the early preparation of vaccines containing currently circulating subtypes23.
Most of the evidence indicates that the economic effects of the 1918 influenza pandemic were short-term24; however, many businesses, especially those in the service and entertainment industries, suffered double-digit losses in revenue. Other businesses that specialized in health care products experienced an increase in revenues24. The Great Influenza Pandemic is estimated to have caused an average reduction in real per capita GDP of 6.2 percent. Given the cross-country range of experience with flu intensity, this result accords with the observation that the pandemic could have caused a substantial number of macroeconomic disasters in the sense of declines in real per capita GDP by 10 percent or more25.
Australian medical authorities dealing with the present COVID-19 pandemic would do well to carefully study the 1918–19 influenza pandemic for, in the words of George Santayana, ‘Those who do not remember the past are condemned to repeat it’.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding
References
[1] Huf, B. and Mclean, H. (2000) Epidemics and pandemics in Victoria: historical perspectives. Research Paper No. 1, May 2020. Research & Inquiries Unit, Parliamentary Library & Information Service.[2] Short, K.R. et al. (2018) Back to the future: lessons learned from the 1918 influenza pandemic. Front. Cell. Infect. Microbiol. 8, 343.
| Back to the future: lessons learned from the 1918 influenza pandemic.Crossref | GoogleScholarGoogle Scholar | 30349811PubMed |
[3] Taubenberger, J.K. (2006) The origin and virulence of the 1918 ‘Spanish’ influenza virus. Proc. Am. Philos. Soc. 150, 86–112.
| 17526158PubMed |
[4] Taubenberger, J.K. and Morens, D.M. (2006) 1918 influenza: the mother of all pandemics. Emerg. Infect. Dis. 12, 15–22.
| 1918 influenza: the mother of all pandemics.Crossref | GoogleScholarGoogle Scholar | 16494711PubMed |
[5] Kilbourne, E.D. (2009) Commentary on fatalities in the 1918–1919 influenza pandemic. J. Infect. Dis. 199, 913.
| Commentary on fatalities in the 1918–1919 influenza pandemic.Crossref | GoogleScholarGoogle Scholar | 19239341PubMed |
[6] Ludendorff, E. (1919) Meine Kriegserinnerungen 1914–1918. Berlin: Ernst Siegfried Mittler und Sohn Verlagsbuchhandlung; p. 514.
[7] Taubenberger, J.K. et al. (2005) Characterization of the 1918 influenza virus polymerase genes. Nature 437, 889–893.
| Characterization of the 1918 influenza virus polymerase genes.Crossref | GoogleScholarGoogle Scholar | 16208372PubMed |
[8] Gibbs, M.J. and Gibbs, A.J. (2006) Was the 1918 pandemic caused by a bird flu? Nature 440, E8.
| Was the 1918 pandemic caused by a bird flu?Crossref | GoogleScholarGoogle Scholar | 16641948PubMed |
[9] Antonovics, J. et al. (2006) Was the 1918 flu avian in origin? Nature 440, E9.
| Was the 1918 flu avian in origin?Crossref | GoogleScholarGoogle Scholar | 16641950PubMed |
[10] Morison, P. (2019) The Martin Spirit. Halstead Press, Braddon, ACT, Australia.
[11] Taubenberger, J.K. et al. (1997) Initial genetic characterization of the 1918 “Spanish” influenza virus. Science 275, 1793–1796.
| Initial genetic characterization of the 1918 “Spanish” influenza virus.Crossref | GoogleScholarGoogle Scholar | 9065404PubMed |
[12] Davies, P. (1999) Catching Cold. Michael Joseph, Penguin Books Ltd, Harmondsworth, Middlesex, UK.
[13] Curson, P. and McCracken, K. (2006) An Australian perspective of the 1918–1919 influenza pandemic. N S W Public Health Bull. 17, 103–107.
| An Australian perspective of the 1918–1919 influenza pandemic.Crossref | GoogleScholarGoogle Scholar | 17136138PubMed |
[14] McQueen, H. (1976) The ‘Spanish’ influenza pandemic in Australia, 1918–19. In Social Policy in Australia – Some Perspectives 1901–1975 (J. Roe, ed.). Cassell, Sydney, NSW, Australia.
[15] Barberis, I. et al. (2016) History and evolution of influenza control through vaccination: from the first monovalent to universal vaccines. J. Prev. Med. Hyg. 57, E115–E120.
| 27980374PubMed |
[16] Fenner, F. (ed.) (1990) History of Microbiology in Australia. Brolga Press, Curtin, ACT, Australia.
[17] Burnett, F.M. (1940) Influenza virus infection of the chick embryo lung. Br. J. Exp. Pathol. 21, 147–153.
[18] Strochlic, N. and Champine, R.D. (2020) How some cities ‘flattened the curve’ during the 1918 pandemic. National Geographic 27 March.
[19] Markel, H. et al. (2007) Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic. JAMA 298, 644–654.
| Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic.Crossref | GoogleScholarGoogle Scholar | 17684187PubMed |
[20] Hatchett, R.J. et al. (2007) Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc. Natl. Acad. Sci. USA 104, 7582–7587.
| Public health interventions and epidemic intensity during the 1918 influenza pandemic.Crossref | GoogleScholarGoogle Scholar | 17416679PubMed |
[21] Bootsma, M.C.J. and Ferguson, N.M. (2007) The effect of public health measures on the 1918 influenza pandemic in U.S. cities. Proc. Natl. Acad. Sci. USA 104, 7588–7593.
| The effect of public health measures on the 1918 influenza pandemic in U.S. cities.Crossref | GoogleScholarGoogle Scholar |
[22] Pregelj, L. et al. (2020) Working hard or hardly working? Regulatory bottlenecks in developing a COVID-19 vaccine. Trends Biotechnol. , .
| Working hard or hardly working? Regulatory bottlenecks in developing a COVID-19 vaccine.Crossref | GoogleScholarGoogle Scholar | 32600777PubMed |
[23] http://www.influenzacentre.org (accessed 12 August 2020).
[24] Garrett, T.A. (2008) Pandemic economics: the 1918 influenza and its modern-day implications . Federal Reserve Bank of St Louis Review 90, 75–93.
[25] Barro, R.J. et al. (2020) The coronavirus and the great influenza pandemic: lessons from the “Spanish flu” for the coronavirus’s potential effects on mortality and economic activity. NBER working paper no. 26866. National Bureau of Economic Research, Cambridge, MA, USA. http://www.nber.org/papers/26866
Biographies
Paul Selleck has been at the Australian Animal Health Laboratory, now the Australian Centre for Disease Preparedness, since 1983. In this time, he was head of the Avian Disease Diagnostic Laboratory, incorporating the National, OIE and FAO Reference Laboratory for Avian Influenza and Newcastle Disease and an OIE Reference Expert for Avian Influenza and Newcastle Disease. He was also involved in the Australian equine and swine influenza outbreaks in 2007 and 2009 respectively and has worked with Hendra, Nipah and SARS at physical containment level 4. Paul now works extensively in Asia, running training courses on biosafety and biosecurity and laboratory diagnosis. He also audits laboratories and runs training courses on quality systems and ISO laboratory accreditation.
Emeritus Professor Ross Barnard, FASM, was director of the biotechnology program at the University of Queensland from 2000 to 2019. Prior to that he was at Panbio Ltd (as program leader for nucleic acid diagnostics development) and the Cooperative Research Centre for Diagnostic Technologies. He has worked on diagnostics development for a variety of infectious agents. In 2005 he undertook a sabbatical at the Australian Animal Health Laboratory (Geelong), now the Australian Centre for Disease Preparedness, during which time he collaborated on the development of a broad spectrum, RT-PCR based Influenza A diagnostic. He was an NHMRC C.J. Martin Fellow at the University of Queensland and the University of California, Santa Cruz.


