Ticks in Australia: endemics; exotics; which ticks bite humans?
Stephen C Barker A C D and Dayana Barker BA Discipline of Parasitology, School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, Qld 4072, Australia
B School of Veterinary Science, The University of Queensland, Gatton, Qld 4343, Australia
C Tel: +61 7 3365 3303
D Email: s.barker@uq.edu.au
Microbiology Australia 39(4) 194-199 https://doi.org/10.1071/MA18062
Published: 2 November 2018
At least 71 species of ticks occur in Australia; a further 33 or so species are endemic to its neighbours, New Guinea and New Zealand. The ticks of Australia and other parts of Australasia are phylogenetically distinct. Indeed, there are at least two lineages of ticks that are unique to Australasia: the genus Bothriocroton Klompen, Dobson & Barker, 2002; and the new genus Archaeocroton Barker & Burger, 2018. Two species of ticks that are endemic to Australia are notorious for feeding on humans: (i) Ixodes holocyclus, the eastern paralysis tick, in eastern Australia; and (ii) Amblyomma triguttatum triguttatum, the ornate kangaroo tick, in Western Australia, at one place in South Australia, and in parts of Queensland. Three of the other endemic species of ticks that feed on humans in Australia are also noteworthy: (i) Bothriocroton hydrosauri, the southern reptile tick, which is a vector of Rickettsia honei (Flinders Island spotted fever); (ii) Haemaphysalis novaeguineae, the New Guinea haemaphysalid; and (iii) Ornithodoros capensis, the seabird soft tick. Here, we present images of female Ixodes holocyclus, Amblyomma t. triguttatum, Bothriocroton hydrosauri and Haemaphysalis novaeguineae and our latest maps of the geographic distributions of Ixodes holocyclus, Amblyomma t. triguttatum and Bothriocroton hydrosauri. None of the five exotic species of ticks in Australia typically feed on humans.
The Australian tick fauna
At least 71 species of ticks are known in Australia: 57 hard ticks (family Ixodidae) and 14 soft ticks (family Argasidae)1,2. Five of these 71 species of ticks were brought to Australia by humans and thus might be called exotic: (i) Argas persicus, the poultry tick; (ii) Otobius megnini, the spinose ear tick, a recent introduction, probably in the ears of horses; (iii) Haemaphysalis longicornis, the bush tick, which occurs in much of east Asia; (iv) Rhipicephalus sanguineus, the brown dog tick, a worldwide species; and (v) Rhipicephalus (Boophilus) australis, the Australian cattle tick. Barker and Walker3 has detailed species accounts for these five ticks.
Australia has a special place in the history of hard ticks (Ixodidae). Indeed, the hard ticks4–6, soft ticks and nutalliellid ticks1 may have first lived in Australia, or more accurately, that part of the super continent Gondwana that became Australia, as early as the Devonian era (362–409 million years ago). Accordingly, six of the eight subfamilies of ticks (Ixodida) are endemic to Australia: Argasinae, Bothriocrotinae, Amblyomminae, Haemaphysalinae, Ixodinae and the Ornithodorinae. Furthermore, there are at least three lineages of ticks that are unique to Australasia: (i) the sub-family Bothriocrotinae Klompen, Murrell & Barker, 2002 from Australia and New Guinea; (ii) the Australasian Ixodes lineage6; and (iii) the genus Archaeocroton Barker & Burger, 2018, which was made for the tick of the tuatara, a singular lizard, from New Zealand7.
Which ticks bite humans in Australia?
None of the five exotic species of ticks in Australia typically feed on humans. Two of the 66 species of ticks that are endemic to Australia are, however, notorious for feeding on humans or may often be found crawling on them: (i) Ixodes holocyclus, the eastern paralysis tick, in eastern Australia; and (ii) Amblyomma t. triguttatum, the ornate kangaroo tick, in Western Australia, at one place in South Australia, Innes National Park on Yorke Peninsula where it was recently introduced (refer to commentary and references3), and in parts of Queensland. These two ticks will be considered in detail in the present paper. Of the three other endemic species of ticks that may feed on humans in Australia: (i) Bothriocroton hydrosauri, the southern reptile tick, is a vector of Rickettsia honei (Flinders Island spotted fever); (ii) Haemaphysalis novaeguineae, the New Guinea haemaphysalid, which, although restricted to the far north of Australia and the Island of New Guinea is noteworthy because it is known to be a vector of R. honei strain marmionii8,9, which caused a fatality in a patient admitted to Townsville General Hospital in late 201610; and (iii) Ornithodoros capensis, the seabird soft tick, that will feed on humans and domestic poultry given the opportunity3,11. O. capensis is known to carry a large number of viruses although none of these have been confirmed to infect humans (see commentary3).
Ixodes holocyclus, the eastern paralysis tick
I. holocyclus (Figure 1) is known as the eastern paralysis tick since most cases of tick paralysis in eastern Australia in domestic animals, wildlife and humans are caused by this tick. I. holocyclus is also known as the scrub tick in Queensland, particularly in North and Far North Queensland. The name scrub tick echoes the apparent predilection of I. holocyclus for the edges of wet-forests (‘scrub’).
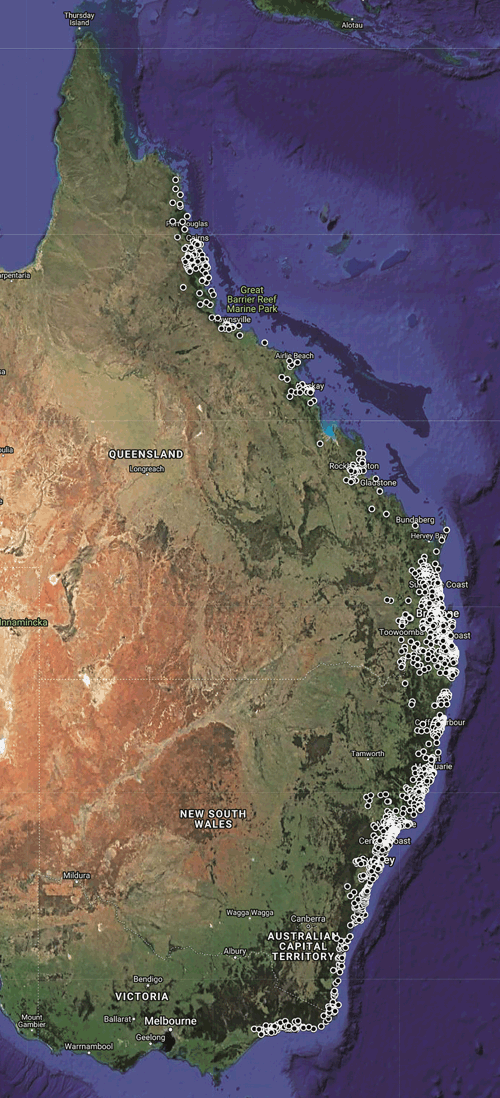
Geographic distribution and hosts: Ixodes holocyclus is strictly constrained to the east coast of Australia (Figure 2) despite the tick having been carried by domestic dogs, cattle, horses and people to many other parts of Australia that appear to be superficially suitable, such as Melbourne and Perth (SC Barker, E Teo and D Barker, unpublished data). I. holocyclus is considered to be catholic in its feeding habits. Indeed, I. holocyclus has been recorded from 34 species of mammals and seven species of birds3, but whether it feeds successfully on all of these species is another question. Where I. holocyclus is abundant, it will be found on most of the species of mammals present, but the bandicoots Isoodon macrourus and Perameles nasuta have been considered the principal hosts in southeastern Queensland since at least 197512. These bandicoots may carry many ticks. It seems that reasonable numbers of I. macrourus and P. nasuta are required for populations of I. holocyclus to persist from one tick season to another in southeastern Queensland12 but this is probably not the case in other parts of the geographic range of I. holocyclus where there seem to be large numbers of ticks but few if any bandicoots (SC Barker and D Barker, unpublished data).
|
Illnesses in humans associated with I. holocyclus: The toxins of this tick seem to be the most potent of all tick-toxins with at least 20 fatalities13: there have been comparable numbers of fatalities from red-back spiders (n = 18) and funnel-web spiders (n = 13)13. Thankfully, deaths from the bite of I. holocyclus are now rare due to the advent of intensive care-units in regional hospitals and expert medical treatment. The illnesses that I. holocyclus has been associated with include Australian multi-system disorder, post-infection fatigue, autoimmune disease, paralysis, allergies (particularly to the bites of larvae), Queensland Tick Typhus (Rickettsia australis), mammalian meat-allergy and tick anaphylaxis; Graves and Stenos14 reviewed these illnesses. Barker15 hypothesised that I. holocyclus may be a link in the transmission of Hendra virus from bats to horses to humans; this hypothesis has not yet been tested.
Amblyomma triguttatum triguttatum, the ornate kangaroo tick
Although known as a kangaroo tick, A. t. triguttatum (Figure 1) will feed on humans; it is one of four subspecies of A. triguttatum (see Barker et al.1).
Geographic distribution and hosts: The geographic distribution of A. t. triguttatum has two parts: eastern Australia and western Australia (Figure 3). A. t. triguttatum is primarily a tick of kangaroos (genus Macropus). A. t. triguttatum is also common on wild (feral) pigs in Australia16. Of a sample of 88 grey kangaroos, M. giganteus, in southeast Queensland, 84% were infested with A. t. triguttatum; 97% of all these ticks were found in the ears16. McCarthy17 also found that A. triguttatum prefers to attach in the ears of kangaroos, sheep and cattle, sheep and cattle. No other species of ticks were found on these kangaroos16.

|
Illnesses in humans associated with A. t. triguttatum: A. t. triguttatum is vector of the spotted fever organism, Rickettsia gravesii18. So far this Rickettsia has been found only in A. triguttatum taken from feral pigs (Sus scrofa)19 and humans20 in Western Australia. Owen et al.21 suggested that the geographic distribution of R. gravesii may coincide with that of A. triguttatum (possibly the subspecies A. t. triguttatum – inferred by us from the known geographic distribution of A. t. triguttatum (Figure 3)). A. t. triguttatum is also a vector of Coxiella burnetii, the aetiological agent of Q fever14. Pearce and Grove22 described the local skin reactions of 175 soldiers who were bitten by A. triguttatum (probably A. t. triguttatum). Moorhouse23 also reported local skin reactions, which he described as allergic dermatitis, in humans bitten by A. triguttatum (possibly the subspecies A. t. triguttatum – inferred by us from the known geographic distribution of A. t. triguttatum (Figure 3)).
Bothriocroton hydrosauri, the southern reptile tick
Although known as the southern reptile tick, B. hydrosauri (Figure 1) will, however, feed on humans.
Geographic distribution and hosts: The geographic distribution of B. hydrosauri (Figure 3) is very well known. In South Australia, at Bundey Bore Station, north-east of Adelaide, the distribution of B. hydrosauri has been mapped to a scale of metres24,25. B. hydrosauri was aptly named the southern reptile tick, since it may be found on all of the main types of reptiles in southern Australia: lizards, snakes and even a terrestrial turtle3. The main host of B. hydrosauri, in much of South Australia at least, is Tiliqua rugosa (sleepy lizard). Nonetheless, B. hydrosauri will, given the opportunity, attach to and feed on humans, cattle and horses.
Illnesses in humans associated with B. hydrosauri: B. hydrosauri is the arthropod-host of R. honei on Flinders Island, Tasmania26 and mainland-Tasmania27. R. honei causes Flinders Island spotted fever in humans9,28. Flinders Island spotted fever is typically a relatively mild disease; no deaths have been reported29 although a patient in Nepal had severe illness30. R. honei has been isolated from the blood of patients with chronic illness, including fatigue, from Melbourne, Victoria, and Adelaide, South Australia, but it is not known whether or not R. honei was causally related to the illness31. R. honei was not detected by PCR nor cell culture in the blood of Tiliqua nigrolutea (southern blue-tongue lizard), Austrelaps superbus (copperhead snakes) nor Notechis scutatus (tiger snake), but more than 60% of B. hydrosauri from those lizards and snakes were PCR-positive or cell culture-positive for R. honei26. So, R. honei is apparently sustained in populations of B. hydrosauri on Flinders Island by vertical, trans-ovarial transmission. That is, R. honei infects the eggs of B. hydrosauri in situ and thus the next generation of B. hydrosauri become infected with R. honei, without feeding on an infected vertebrate. So apparently, vertebrates are not needed for the survival of R. honei on Flinders Island, and probably elsewhere. Furthermore, horizontal transmission, that is transmission between the arthropod-host (tick) and the vertebrate-host (lizards and snakes), has not yet been demonstrated experimentally for R. honei although there are confirmed cases of infection with R. honei in people who had been bitten by ticks from Iron Range, Cape York Peninsula, Queensland (H. novaeguineae)9; and Nepal (species of tick unknown)30.
Flinders Island spotted fever is now known from three continents: (i) Australia (Flinders Island, Tasmania; mainland Tasmania; and South Australia); (ii) Asia (Thailand; Orchid Island, Taiwan; Nepal); and (iii) North America (Texas)30,32–34. Confirmed tick-hosts of R. honei are: (i) B. hydrosauri from Flinders Island and mainland Tasmania; Cooma, New South Wales; and Bundy Bore Station, South Australia; (ii) Haemaphysalis novaeguineae (R. honei strain marmionii) from Iron Range, Queensland; (iii) Ixodes granulatus from Rattus rattus (black rat) from Thailand (adult ticks but not yet from larval or nymphal I. granulatus35); and (iv) Amblyomma cajennense from cattle from Texas, USA26,27,32.
Where to next?
We still need cytochrome c oxidase subunit I (COX1) and Internal Transcribed Spacer (ITS2) rRNA nucleotide sequences for most of the Australian ticks. These sequences will help non-experts to identify quickly ticks, particularly larvae and nymphs. We also need to know where exactly H. novaeguineae, the New Guinea haemaphysalid, lives in northern Australia, and how abundant it is there.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We sincerely thank Julianne Waldock (Western Australian Museum) for identifying many Amblyomma triguttatum to sub-species for our map. We also thank Brodie Foster and Wil McGuire for expert and creative images of ticks, our Honours student Melani Vial who pioneered map-making in our laboratory, our undergraduate research students, Samuel Kelava, Ernest Teo, Semira Hailu and Truc Le, for help with the geographic distributions of Australian ticks, and Jordan Clough for expert work on a troublesome tick image. This research did not receive any specific funding.
References
[1] Barker, S.C. et al. (2014) A list of the 70 species of Australian ticks; diagnostic guides to and species accounts of Ixodes holocyclus (paralysis tick), Ixodes cornuatus (southern paralysis tick) and Rhipicephalus australis (Australian cattle tick); and consideration of the place of Australia in the evolution of ticks with comments on four controversial ideas. Int. J. Parasitol. 44, 941–953.| A list of the 70 species of Australian ticks; diagnostic guides to and species accounts of Ixodes holocyclus (paralysis tick), Ixodes cornuatus (southern paralysis tick) and Rhipicephalus australis (Australian cattle tick); and consideration of the place of Australia in the evolution of ticks with comments on four controversial ideas.Crossref | GoogleScholarGoogle Scholar |
[2] Ash, A. et al. (2017) Morphological and molecular description of Ixodes woyliei n. sp. (Ixodidae) with consideration for co-extinction with its critically endangered marsupial host. Parasit. Vectors 10, 70.
| Morphological and molecular description of Ixodes woyliei n. sp. (Ixodidae) with consideration for co-extinction with its critically endangered marsupial host.Crossref | GoogleScholarGoogle Scholar |
[3] Barker, S.C. and Walker, A.R. (2014) Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 3816, 1–144.
| Ticks of Australia. The species that infest domestic animals and humans.Crossref | GoogleScholarGoogle Scholar |
[4] Dobson, S.J. and Barker, S.C. (1999) Phylogeny of the hard ticks (Ixodidae) inferred from 18S rRNA indicates that the genus Aponomma is paraphyletic. Mol. Phylogenet. Evol. 11, 288–295.
| Phylogeny of the hard ticks (Ixodidae) inferred from 18S rRNA indicates that the genus Aponomma is paraphyletic.Crossref | GoogleScholarGoogle Scholar |
[5] Barker, S.C. and Murrell, A. (2002) Phylogeny, evolution and historical zoogeography of ticks: a review of recent progress. Exp. Appl. Acarol. 28, 55–68.
| Phylogeny, evolution and historical zoogeography of ticks: a review of recent progress.Crossref | GoogleScholarGoogle Scholar |
[6] Barker, S.C. and Murrell, A. (2004) Systematics and evolution of ticks with a list of valid genus and species names. Parasitology 129, S15–S36.
| Systematics and evolution of ticks with a list of valid genus and species names.Crossref | GoogleScholarGoogle Scholar |
[7] Barker, S.C. and Burger, T.D. (2018) Two new genera of hard ticks, Robertsicus n. gen. and Archaeocroton n. gen., and the solution to the mystery of Hoogstraal and Kaufman’s ‘primitive’ tick from the Carpathian Mountains. Zootaxa 4500, 543–552.
[8] Lane, A.M. et al. (2005) Evidence of a spotted fever-like rickettsia and a potential new vector from northeastern Australia. J. Med. Entomol. 42, 918–921.
| Evidence of a spotted fever-like rickettsia and a potential new vector from northeastern Australia.Crossref | GoogleScholarGoogle Scholar |
[9] Unsworth, N.B. et al. (2007) Flinders Island spotted fever rickettsioses caused by ‘marmionii strain’ of Rickettsia honei, eastern Australia. Emerg. Infect. Dis. 13, 566–573.
| Flinders Island spotted fever rickettsioses caused by ‘marmionii strain’ of Rickettsia honei, eastern Australia.Crossref | GoogleScholarGoogle Scholar |
[10] Graham, R.M.A. et al. (2017) Detection of spotted fever group Rickettsia DNA by deep sequencing. Emerg. Infect. Dis. 23, 1911–1913.
| Detection of spotted fever group Rickettsia DNA by deep sequencing.Crossref | GoogleScholarGoogle Scholar |
[11] Kohls, G.M. et al. (1965) The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions of three new species. Ann. Entomol. Soc. Am. 58, 331–364.
| The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions of three new species.Crossref | GoogleScholarGoogle Scholar |
[12] Doube, B.M. (1975) Cattle and the paralysis tick Ixodes holocyclus. Aust. Vet. J. 51, 511–515.
| Cattle and the paralysis tick Ixodes holocyclus.Crossref | GoogleScholarGoogle Scholar |
[13] Sutherland, S.K. and Tibballs, J. (2001) Australian animal toxins: the creatures, their toxins, and care of the poisoned patient (second edition). Oxford University Press.
[14] Graves, S.R. and Stenos, J. (2017) Tick-borne infectious diseases in Australia. Med. J. Aust. 206, 320–324.
| Tick-borne infectious diseases in Australia.Crossref | GoogleScholarGoogle Scholar |
[15] Barker, S.C. (2003) The Australian paralysis tick may be the missing link in the transmission of Hendra virus from bats to horses to humans. Med. Hypotheses 60, 481–483.
| The Australian paralysis tick may be the missing link in the transmission of Hendra virus from bats to horses to humans.Crossref | GoogleScholarGoogle Scholar |
[16] Guglielmone, A.A. (1990) Sites of attachment in Amblyomma triguttatum triguttatum Koch (Acari: Ixodidae) on natural hosts. Ann. Parasitol. Hum. Comp. 65, 145–148.
| Sites of attachment in Amblyomma triguttatum triguttatum Koch (Acari: Ixodidae) on natural hosts.Crossref | GoogleScholarGoogle Scholar |
[17] McCarthy, P.H. (1960) Observations on the infestation of the larger domestic animals and the kangaroo, by the ornate kangaroo tick (Amblyomma triguttatum). Aust. Vet. J. 36, 436–437.
| Observations on the infestation of the larger domestic animals and the kangaroo, by the ornate kangaroo tick (Amblyomma triguttatum).Crossref | GoogleScholarGoogle Scholar |
[18] Abdad, M.Y. et al. (2017) Rickettsia gravesii sp. nov.: a novel spotted fever group rickettsia in Western Australian Amblyomma triguttatum triguttatum ticks. Int. J. Syst. Evol. Microbiol. 67, 3156–3161.
| Rickettsia gravesii sp. nov.: a novel spotted fever group rickettsia in Western Australian Amblyomma triguttatum triguttatum ticks.Crossref | GoogleScholarGoogle Scholar |
[19] Li, A.Y. et al. (2010) High prevalence of Rickettsia gravesii sp. nov. in Amblyomma triguttatum collected from feral pigs. Vet. Microbiol. 146, 59–62.
| High prevalence of Rickettsia gravesii sp. nov. in Amblyomma triguttatum collected from feral pigs.Crossref | GoogleScholarGoogle Scholar |
[20] Owen, H. et al. (2006) Detection and identification of a novel spotted fever group rickettsia in Western Australia. Ann. N. Y. Acad. Sci. 1078, 197–199.
| Detection and identification of a novel spotted fever group rickettsia in Western Australia.Crossref | GoogleScholarGoogle Scholar |
[21] Owen, H. et al. (2006) Potentially pathogenic spotted fever group rickettsiae present in Western Australia. Aust. J. Rural Health 14, 284–285.
| Potentially pathogenic spotted fever group rickettsiae present in Western Australia.Crossref | GoogleScholarGoogle Scholar |
[22] Pearce, R.L. and Grove, D.I. (1987) Tick infestation in soldiers who were bivouacked in the Perth region. Med. J. Aust. 146, 238–240.
[23] Moorhouse, D.E. (1981) Animal toxins and man. Human poisoning by toxic Australian venomous creatures (Pearn J., ed.). pp. 63–69. Division of Health Education and Information, Queensland Health Department.
[24] Smyth, M. (1973) The distribution of three species of reptile ticks, Aponomma hydrosauri (Denny), Amblyomma albolimbatum Neumann, and Amb. limbatum Neumann. I. Distribution and hosts. Aust. J. Zool. 21, 91–101.
| The distribution of three species of reptile ticks, Aponomma hydrosauri (Denny), Amblyomma albolimbatum Neumann, and Amb. limbatum Neumann. I. Distribution and hosts.Crossref | GoogleScholarGoogle Scholar |
[25] Bull, C. M. and King, D. R. (1981) A parapatric boundary between two species of reptile ticks in the Albany area, Western Australia. Trans. R. Soc. S. Aust. 105, 205–208.
[26] Stenos, J. et al. (2003) Aponomma hydrosauri, the reptile-associated tick reservoir of Rickettsia honei on Flinders Island, Australia. Am. J. Trop. Med. Hyg. 69, 314–317.
| Aponomma hydrosauri, the reptile-associated tick reservoir of Rickettsia honei on Flinders Island, Australia.Crossref | GoogleScholarGoogle Scholar |
[27] Whitworth, T. et al. (2003) Ultrastructural and genetic evidence of a reptilian tick, Aponomma hydrosauri, as a host of Rickettsia honei in Australia: possible transovarial transmission. Ann. N. Y. Acad. Sci. 990, 67–74.
| Ultrastructural and genetic evidence of a reptilian tick, Aponomma hydrosauri, as a host of Rickettsia honei in Australia: possible transovarial transmission.Crossref | GoogleScholarGoogle Scholar |
[28] Stenos, J. et al. (1998) Rickettsia honei sp. nov., the aetiological agent of Flinders Island spotted fever in Australia. Int. J. Syst. Bacteriol. 48, 1399–1404.
[29] Graves, S. and Stenos, J. (2009) Rickettsioses in Australia. Ann. N. Y. Acad. Sci. 1166, 151–155.
| Rickettsioses in Australia.Crossref | GoogleScholarGoogle Scholar |
[30] Murphy, H. et al. (2011) Rickettsia honei infection in human, Nepal, 2009. Emerg. Infect. Dis. 17, 1865–1867.
| Rickettsia honei infection in human, Nepal, 2009.Crossref | GoogleScholarGoogle Scholar |
[31] Unsworth, N. et al. (2008) Markers of exposure to spotted fever rickettsiae in patients with chronic illness, including fatigue, in two Australian populations. QJM 101, 269–274.
| Markers of exposure to spotted fever rickettsiae in patients with chronic illness, including fatigue, in two Australian populations.Crossref | GoogleScholarGoogle Scholar |
[32] Graves, S. and Stenos, J. (2003) Rickettsia honei: a spotted fever group Rickettsia on three continents. Ann. N. Y. Acad. Sci. 990, 62–66.
| Rickettsia honei: a spotted fever group Rickettsia on three continents.Crossref | GoogleScholarGoogle Scholar |
[33] Dyer, J. R. et al. (2005) A new focus of Rickettsia honei spotted fever in South Australia. Med. J. Aust. 182, 231–234.
[34] Unsworth, N.B. et al. (2005) Not only ‘Flinders Island’ spotted fever. Pathology 37, 242–245.
| Not only ‘Flinders Island’ spotted fever.Crossref | GoogleScholarGoogle Scholar |
[35] Kollars, T.M. et al. (2001) Short report: Thai tick typhus, Rickettsia honei, and a unique rickettsia detected in Ixodes granulatus (Ixodidae: Acari) from Thailand. Am. J. Trop. Med. Hyg. 65, 535–537.
| Short report: Thai tick typhus, Rickettsia honei, and a unique rickettsia detected in Ixodes granulatus (Ixodidae: Acari) from Thailand.Crossref | GoogleScholarGoogle Scholar |
Biographies
Stephen Barker is a Professor of Parasitology, in the School of Chemistry and Molecular Biosciences, at the University of Queensland, Australia. He is a specialist in ticks and other ectoparasites; he has worked on ticks and other ectoparasites at the University of Queensland for 27 years.
Dayana Barker has a Bachelor of Biological Science and a Masters in Animal Science (major in Animal Health) from the Federal University of Mato Grosso do Sul, Brazil. She has a particular interest in the taxonomy and biology of Australasian ticks. Dayana is a PhD candidate (Veterinary Parasitology) at the School of Veterinary Sciences, University of Queensland, Australia.



