Could Australian ticks harbour emerging viral pathogens?
Caitlin A O’Brien A C , Roy A Hall A D and Ala Lew-Tabor B EA The University of Queensland, School of Chemistry and Molecular Biosciences, Building #76, Cooper Road, St Lucia, Qld 4072, Australia
B The University of Queensland, Queensland Alliance of Agriculture and Food Innovation, Building #80, 306 Carmody Road, St Lucia, Qld 4072, Australia
C Email: caitlin.obrien@uqconnect.edu.au
D Email: roy.hall@uq.edu.au
E Email: a.lewtabor@uq.edu.au
Microbiology Australia 39(4) 185-190 https://doi.org/10.1071/MA18060
Published: 24 October 2018
Tick-borne viruses contribute significantly to the disease burden in Europe, Asia and the US. Historically, some of the most well-known viruses from this group include the human pathogens, tick-borne encephalitis virus and Crimean-Congo haemorrhagic fever virus. More recently multiple emerging tick-borne viruses have been associated with severe disease in humans with Bourbon virus and Heartland virus isolated from patients in the US and severe fever with thrombocytopenia syndrome virus reported from China, Japan, and South Korea. Such examples highlight the need for broader approaches to survey arthropod pathogens, to encompass not only known but novel pathogens circulating in Australian tick populations.
There are currently 70 recognised species of ticks in Australia, with 22 reported infesting on humans1,2. Ixodes holocyclus is the most significant tick in terms of human and animal health in Australia, causing a myriad of problems from toxin-induced paralysis, mostly in domestic animals3, to allergic reactions in humans4. The feeding behaviour of I. holocyclus as a three-host tick, makes it a successful vector for Rickettsial pathogens that cause Queensland tick typhus, Flinders Island spotted fever, and Australian spotted fever5. Research into ‘Debilitating Symptom Complexes Attributed to Ticks’ has led to the discovery of several new bacterial species in I. holocyclus and other native Australian ticks from families containing known human pathogens6,7. Despite this, little is known of the viruses carried by Australian terrestrial ticks, with most isolations from seabird ticks collected on offshore islands8–10.
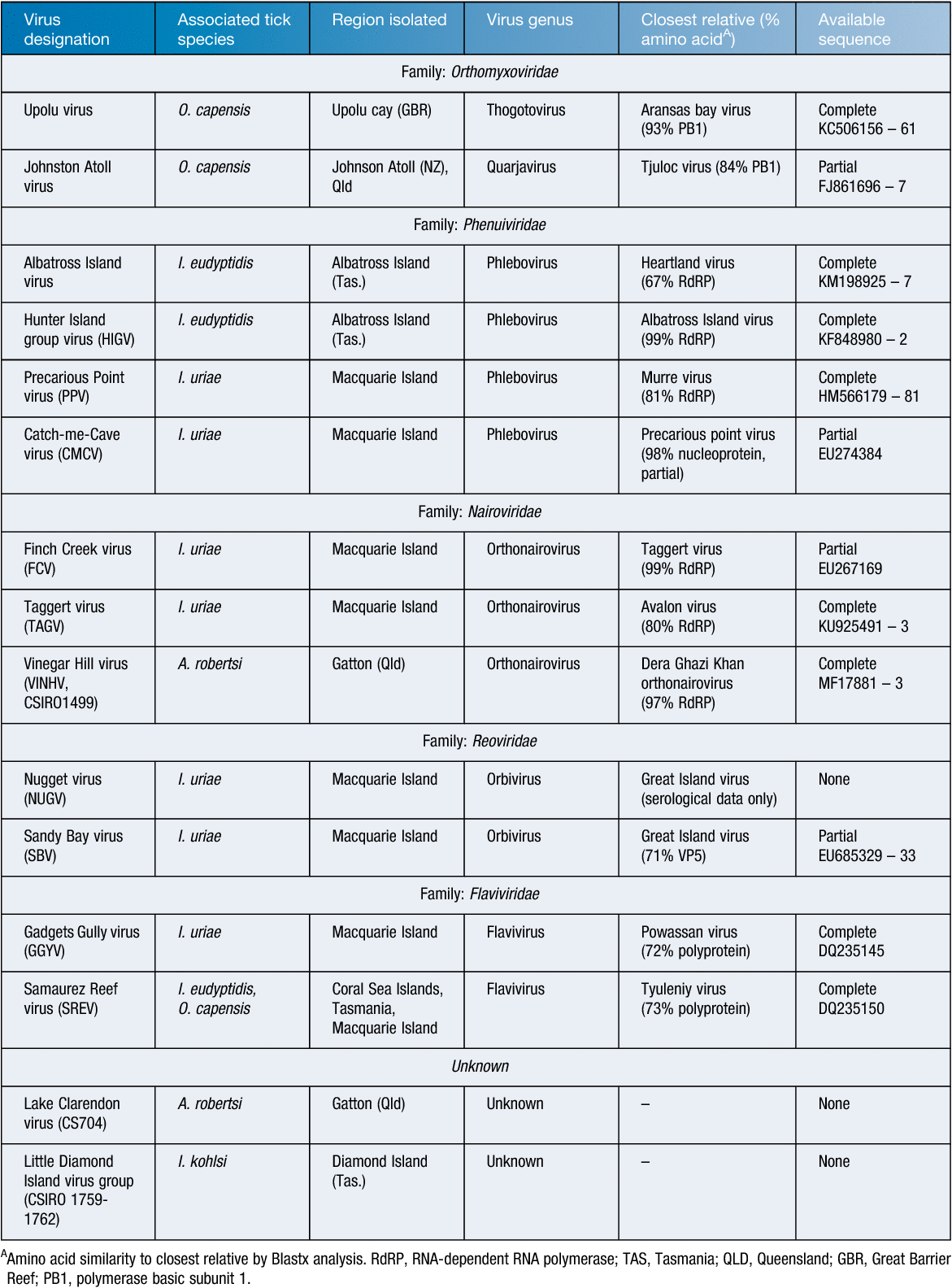
Viruses isolated from Australian seabird ticks show similarities with tick-borne pathogens in other parts of the world (Table 1). The flavivirus Gadgets Gully virus (GGYV), first isolated from Ixodes uriae ticks in 1985, clusters with the mammalian tick-borne flavivirus group including human pathogens Powassan virus and Tick-borne encephalitis virus9. While GGYV has not been associated with disease in humans, a serological survey demonstrated evidence for infection in inhabitants of a research station on Macquarie Island11. Similarly, a second flavivirus, Saumarez Reef virus (SREV), was isolated from Ixodes eudyptidis and Ornithodoros capensis ticks taken from nests of sooty terns (Onychoprion fuscatus) and silver gulls (Chroicocephalus novaehollandiae) after reports of illness and tick bites by technicians servicing weather stations on Saumarez reef (~330 km off the Central Queensland coast)10. Although no serological evidence of SREV infection was gathered, it was noted that the closely related Tyuleniy virus showed a 6% seroconversion rate in inhabitants of the Commodore Islands12.
|
In 2002, a disease outbreak in shy albatross (Thalassarche cauta) on Albatross Island (129 km north-west of Burnie, Tasmania) led to the isolation of Hunter Island Group virus. Next-generation sequencing identified the virus as a phlebovirus related to human pathogens severe fever with thrombocytopenia syndrome virus and Heartland virus13. Following this, sequencing of Albatross Island virus (ABIV), an isolate from I. eudyptidis ticks from the same location in 1983, showed that the two viruses were the same species14.
More recently, a novel orbivirus and two bunyaviruses were reported from I. uriae collected during a survey of ticks on penguins at Macquarie Island15. The two bunyaviruses, tentatively named Catch-me-Cave virus and Finch Creek virus, show similarities to previously isolated Precarious Point and Taggert viruses9,16. However, full genome sequencing is required to confirm whether these viruses are contemporary strains of known viruses or new species. During this study, GGYV was also re-isolated from I. uriae suggesting that it is probable that the viruses first described between 1975 and 1985 are still circulating in the tick and bird populations on Macquarie Island15.
In contrast to the panel of viruses isolated from Australian seabird ticks, attempts to screen terrestrial tick populations have yielded only three viruses, all from Argas robertsi, a tick also found in Asia. Lake Clarendon virus (CSIRO704) was isolated from A. robertsi ticks collected at the Lake Clarendon cattle egret colony in Gatton, Queensland in 198017. The virus was not identified and showed no relatedness to known arboviruses by serum-neutralisation and complement-fixation tests. Neutralising antibodies in cattle egret sera suggested the virus was able to infect the birds with no apparent disease17. To our knowledge, this isolate remains unidentified. A second virus was isolated from A. robertsi ticks at the same colony following reports of death in nestling chicks. This virus, originally designated CSIRO1499, was shown to have no serological similarity to Lake Clarendon virus. Experimental infections subsequently showed that the isolate was able to infect and cause mortality in birds11. More recently, the full genome sequence of this virus revealed it to be closely related to Dera Ghazi Khan virus (family Nairoviridae) and the name Vinegar Hill virus (VINHV) was proposed8. Serological surveys found antibodies to VINHV in 3.4% of avian samples and 1% of human serum samples tested11. Finally, a virus isolated from A. robertsi in the Northern Territory (NT15470) was suggested to be a member of the Dera Ghazi Khan genogroup, thought to be either a strain of Kao Shuan virus (KSV) or a close relative18. As no genome sequence is available for this isolate, it remains to be seen whether it is in fact KSV or another isolate of VINHV.
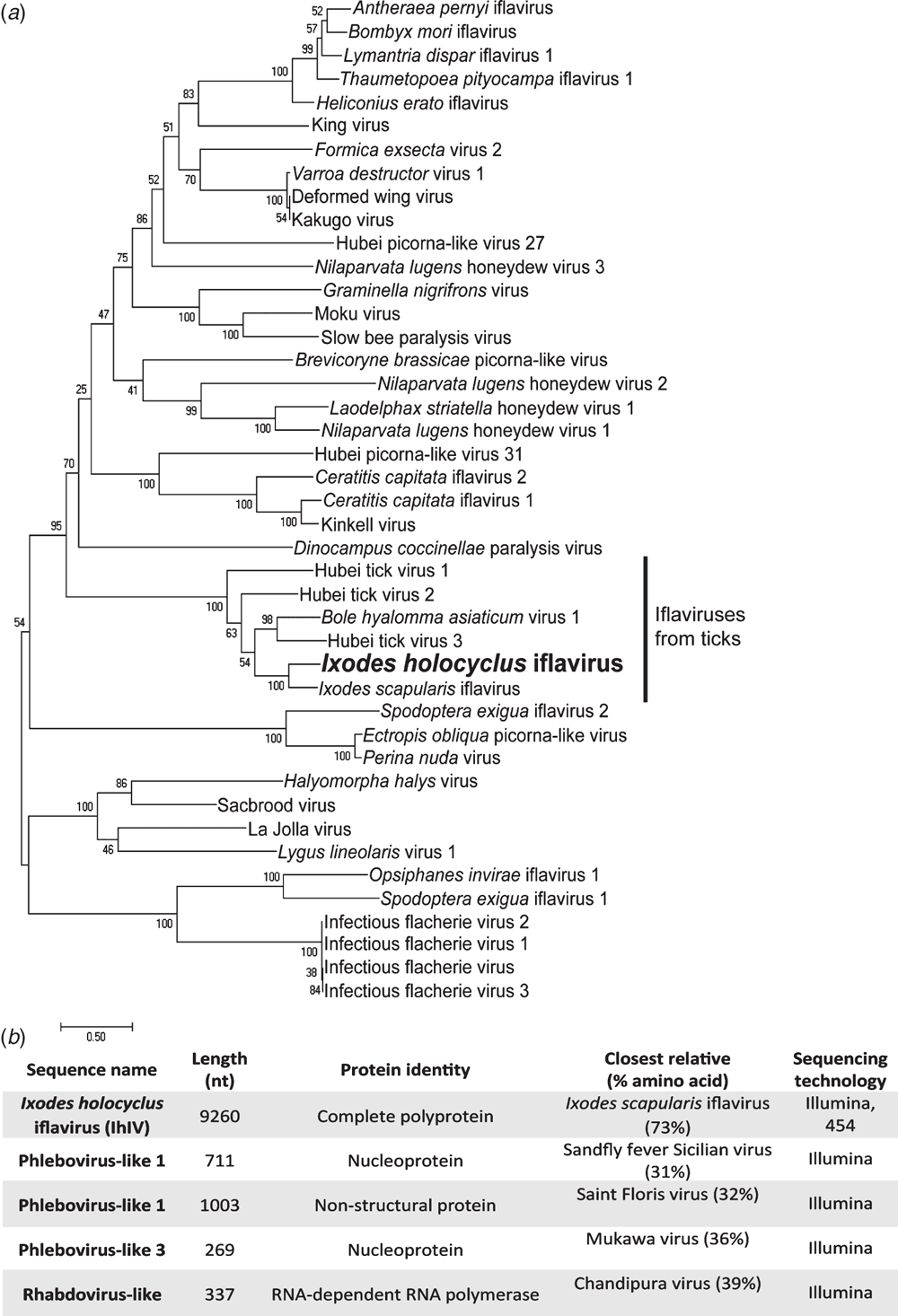
To our knowledge, there have been no virus isolations from an Australian terrestrial hard tick species. Next generation sequencing performed on I. holocyclus ticks collected in New South Wales and Queensland have allowed some insights into their potential virome19. Recently we published the genome of a novel iflavirus assembled from the transcriptome of I. holocyclus salivary glands (Figure 1a)20. Interestingly, related iflaviruses have also been identified in the Ixodes scapularis cell line (ISE6) and in ticks collected from China21,22. Several bunyavirus nucleocapsid sequences have also been identified in I. holocyclus transcriptome sequence data but whether these sequences belong to a virus or an integration in the host genome is yet to be elucidated (Figure 1b). This data indicates that the virome of Australian terrestrial ticks may mirror that of terrestrial ticks found in the northern hemisphere.
|
We have developed a broad-spectrum screening system that detects viral isolates in cell cultures inoculated with mosquito homogenates. The MAVRIC system (Monoclonal antibodies against viral RNA intermediates in cells) targets long (>30 bp) double-stranded RNA molecules, produced during replication of viruses, in a sequence-independent manner23. MAVRIC led to the isolation of at least 9 new viral species from 7 different families allowing the identification of numerous viruses previously not known to exist in Australian mosquito populations24. Based on the success of MAVRIC in mosquito screening, we aim to apply this system to screen Australian terrestrial ticks. One barrier is the lack of suitable cell lines derived from Australian tick species and their hosts. While the use of vertebrate cell cultures (generally BHK-21 and Vero cell lines) has proven successful for the isolation of mosquito and seabird tick viruses in Australia9,25, alternative cell lines reflecting the common hosts of terrestrial ticks (i.e. marsupials), may need to be considered for tick virus isolation on the mainland. Furthermore, while mosquito cell culture has been well established, tick cell culture has proven more difficult requiring a complex mix of vitamins and minerals which must be formulated in-house26.
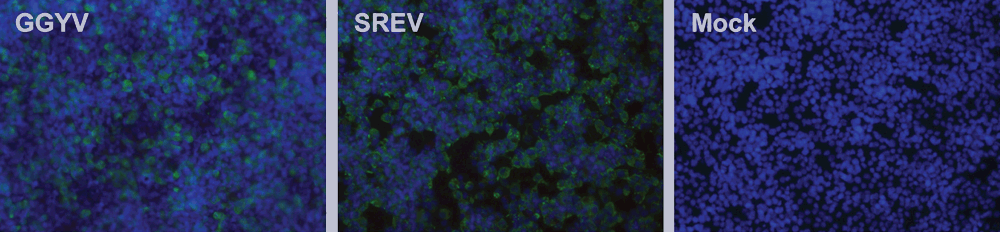
Ixodes holocyclus iflavirus was unable to replicate in the I. scapularis cell line (ISE6), but appeared to replicate in the host tick raising the question of the suitability of cell lines derived from ticks of the northern hemisphere to isolate Australian tick-borne viruses20,27. Phylogenetic analyses have demonstrated that I. holocyclus and I. uriae are divergent from ‘other’ Ixodes species28. Preliminary analysis from our group has suggested that some of the vertebrate-infecting viruses of I. uriae and I. eudyptidis are able to replicate in the ISE6 cell line (Figure 2), however this remains to be demonstrated for viruses of terrestrial ticks. Bell-Sakyi and Attoui recently discussed the role of tick cell culture in virus discovery, particularly in relation to tick-specific viruses29. In this instance, the development of cell lines from Australian native ticks may be necessary.
|
Finally, the risk that Australian seabird-associated tick viruses pose to human health should be considered. A study undertaken to investigate the health risk posed to residents and tourists on the islands in the Great Barrier Reef and Coral Sea by seabird-associated arboviruses identified two isolates in O. capensis collected on Masthead Island, which appeared closely related to VINHV30. This may demonstrate the potential for incursion of seabird-associated viruses to the mainland and vice versa. Serological surveys undertaken after the initial isolation of ABIV identified a black noddy (Anous minutus) from Heron Island off the coast of Queensland with neutralising antibodies to the virus30. Finally, SREV was isolated from O. capensis ticks found on Saumarez reef off the coast of Queensland and 2000 km away in Tasmania10.
While our knowledge of the viruses harboured by Australian ticks is still limited, the data thus far suggests that our tick viromes may mirror those seen in the northern hemisphere. Next generation sequencing of terrestrial ticks performed by our group and others will greatly contribute to the characterisation of tick-viruses in Australia. In this context, a recent deep sequencing study of Australian ticks by Eddie Holmes’ group at the University of Sydney, has identified a plethora of novel viral sequences that will provide a useful reference for further studies (unpublished data available online: https://www.biorxiv.org/content/early/2018/08/07/386573). Complementary to next generation sequencing, a system for efficient isolation of newly discovered viruses will allow for complete characterisation. Finally, comprehensive characterisation of the current tick-borne virus isolates held in archive is required to avoid re-discovery of these viruses in future studies.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Toby St George for reviewing this manuscript, for helpful discussions and providing information on Australian tick isolates. We also thank Steve Davis for providing information on Australian tick isolates. We are grateful to Dr Sonja Hall-Mendelin, Professor Steve Barker, Dr Dayana Barker and Ms Greta Busch for tick collections. Professor Ulrike Munderloh for providing us with the ISE6 cell line and advice on culture. Professor Lesley Bell-Sakyi and Dr Jeff Grabowski for helpful discussions and advice on tick cell culture. Dr Andy Allen and Dr Georgia Deliyannis for the original IhIV sequence. Ixodes holocyclus transcriptome sequence data was funded by the Australian Research Council Linkage project LP120200836. We are grateful to the staff and students from this ARC project associated with collating the transcriptome data including Dr Manuel Rodriguez Valle, Dr Roberto Barrero, Dr Paula Moolhuijzen, Ms Chian Teng Ong and Mr Mitchell Booth.
References
[1] Barker, S.C. et al. (2014) A list of the 70 species of Australian ticks; diagnostic guides to and species accounts of Ixodes holocyclus (paralysis tick), Ixodes cornuatus (southern paralysis tick) and Rhipicephalus australis (Australian cattle tick); and consideration of the place of Australia in the evolution of ticks with comments on four controversial ideas. Int. J. Parasitol. 44, 941–953.| A list of the 70 species of Australian ticks; diagnostic guides to and species accounts of Ixodes holocyclus (paralysis tick), Ixodes cornuatus (southern paralysis tick) and Rhipicephalus australis (Australian cattle tick); and consideration of the place of Australia in the evolution of ticks with comments on four controversial ideas.Crossref | GoogleScholarGoogle Scholar |
[2] Kwak, M.L. (2018) The first records of human infestation by the hard tick Ixodes (Endopalpiger) australiensis (Acari: Ixodidae), with a review of human infestation by ticks in Australia. Exp. Appl. Acarol. 74, 185–190.
| The first records of human infestation by the hard tick Ixodes (Endopalpiger) australiensis (Acari: Ixodidae), with a review of human infestation by ticks in Australia.Crossref | GoogleScholarGoogle Scholar |
[3] Hall-Mendelin, S. et al. (2011) Tick paralysis in Australia caused by Ixodes holocyclus Neumann. Ann. Trop. Med. Parasitol. 105, 95–106.
| Tick paralysis in Australia caused by Ixodes holocyclus Neumann.Crossref | GoogleScholarGoogle Scholar |
[4] van Nunen, S. (2015) Tick-induced allergies: mammalian meat allergy, tick anaphylaxis and their significance. Asia Pac. Allergy 5, 3–16.
| Tick-induced allergies: mammalian meat allergy, tick anaphylaxis and their significance.Crossref | GoogleScholarGoogle Scholar |
[5] Graves, S.R. et al. (2016) Ixodes holocyclus tick-transmitted human pathogens in North-Eastern New South Wales, Australia. Trop. Med. Infect. Dis 1, 4.
| Ixodes holocyclus tick-transmitted human pathogens in North-Eastern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
[6] Gofton, A.W. et al. (2015) Bacterial profiling reveals novel ‘Ca. Neoehrlichia’, Ehrlichia, and Anaplasma species in Australian human-biting ticks. PLoS One 10, e0145449.
| Bacterial profiling reveals novel ‘Ca. Neoehrlichia’, Ehrlichia, and Anaplasma species in Australian human-biting ticks.Crossref | GoogleScholarGoogle Scholar |
[7] Gofton, A.W. et al. (2015) Inhibition of the endosymbiont ‘Candidatus Midichloria mitochondrii’ during 16S rRNA gene profiling reveals potential pathogens in Ixodes ticks from Australia. Parasit. Vectors 8, 345.
| Inhibition of the endosymbiont ‘Candidatus Midichloria mitochondrii’ during 16S rRNA gene profiling reveals potential pathogens in Ixodes ticks from Australia.Crossref | GoogleScholarGoogle Scholar |
[8] Gauci, P.J. et al. (2017) Genomic characterisation of Vinegar Hill Virus, an Australian nairovirus isolated in 1983 from Argas Robertsi ticks collected from cattle egrets. Viruses 9, 373.
| Genomic characterisation of Vinegar Hill Virus, an Australian nairovirus isolated in 1983 from Argas Robertsi ticks collected from cattle egrets.Crossref | GoogleScholarGoogle Scholar |
[9] St George, T.D. et al. (1985) The isolation of arboviruses including a new flavivirus and a new Bunyavirus from Ixodes (Ceratixodes) uriae (Ixodoidea: Ixodidae) collected at Macquarie Island, Australia, 1975–1979. Am. J. Trop. Med. Hyg. 34, 406–412.
| The isolation of arboviruses including a new flavivirus and a new Bunyavirus from Ixodes (Ceratixodes) uriae (Ixodoidea: Ixodidae) collected at Macquarie Island, Australia, 1975–1979.Crossref | GoogleScholarGoogle Scholar |
[10] St George, T.D. et al. (1977) The isolation of Saumarez Reef virus, a new flavivirus, from bird ticks Ornithodoros capensis and Ixodes eudyptidis in Australia. Aust. J. Exp. Biol. Med. Sci. 55, 493–499.
| The isolation of Saumarez Reef virus, a new flavivirus, from bird ticks Ornithodoros capensis and Ixodes eudyptidis in Australia.Crossref | GoogleScholarGoogle Scholar |
[11] Humphery-Smith, I. et al. (1991) Seroepidemiology of arboviruses among seabirds and island residents of the Great Barrier Reef and Coral Sea. Epidemiol. Infect. 107, 435–440.
| Seroepidemiology of arboviruses among seabirds and island residents of the Great Barrier Reef and Coral Sea.Crossref | GoogleScholarGoogle Scholar |
[12] Lvov, D.K. et al. (1972) Isolation of Tyuleniy virus from ticks Ixodes (Ceratixodes) putus Pick.-Camb. 1878 collected on Commodore Islands. Arch. Gesamte Virusforsch. 38, 139–142.
| Isolation of Tyuleniy virus from ticks Ixodes (Ceratixodes) putus Pick.-Camb. 1878 collected on Commodore Islands.Crossref | GoogleScholarGoogle Scholar |
[13] Wang, J. et al. (2014) Novel phlebovirus with zoonotic potential isolated from ticks, Australia. Emerg. Infect. Dis. 20, 1040–1043.
| Novel phlebovirus with zoonotic potential isolated from ticks, Australia.Crossref | GoogleScholarGoogle Scholar |
[14] Gauci, P.J. et al. (2015) Hunter Island group phlebovirus in ticks, Australia. Emerg. Infect. Dis. 21, 2246–2248.
| Hunter Island group phlebovirus in ticks, Australia.Crossref | GoogleScholarGoogle Scholar |
[15] Major, L. et al. (2009) Ticks associated with Macquarie Island penguins carry arboviruses from four Genera. PLoS One 4, e4375.
| Ticks associated with Macquarie Island penguins carry arboviruses from four Genera.Crossref | GoogleScholarGoogle Scholar |
[16] Doherty, R.L. et al. (1975) Isolation of arboviruses (Kemerovo group, Sakhalin group) from Ixodes uriae collected at Macquarie Island, Southern ocean. Am. J. Trop. Med. Hyg. 24, 521–526.
| Isolation of arboviruses (Kemerovo group, Sakhalin group) from Ixodes uriae collected at Macquarie Island, Southern ocean.Crossref | GoogleScholarGoogle Scholar |
[17] St George, T.D. et al. (1984) Isolation of a new arbovirus from the tick Argas robertsi from a cattle egret (Bubulcus ibis coromandus) colony in Australia. Aust. J. Biol. Sci. 37, 85–89.
| Isolation of a new arbovirus from the tick Argas robertsi from a cattle egret (Bubulcus ibis coromandus) colony in Australia.Crossref | GoogleScholarGoogle Scholar |
[18] Doherty, R.L. et al. (1976) Isolation of virus strains related to kao shuan virus from Argas robertsi in Northern Territory, Australia. Search 7, 484–486.
[19] Rodriguez-Valle, M. et al. (2018) Transcriptome and toxin family analysis of the paralysis tick, Ixodes holocyclus. Int. J. Parasitol. 48, 71–82.
| Transcriptome and toxin family analysis of the paralysis tick, Ixodes holocyclus.Crossref | GoogleScholarGoogle Scholar |
[20] O’Brien, C.A. et al. (2018) Discovery of a novel iflavirus sequence in the eastern paralysis tick Ixodes holocyclus. Arch. Virol. 163, 2451–2457.
| Discovery of a novel iflavirus sequence in the eastern paralysis tick Ixodes holocyclus.Crossref | GoogleScholarGoogle Scholar |
[21] Nakao, R. et al. (2017) Putative RNA viral sequences detected in an Ixodes scapularis-derived cell line. Ticks Tick Borne Dis. 8, 103–111.
| Putative RNA viral sequences detected in an Ixodes scapularis-derived cell line.Crossref | GoogleScholarGoogle Scholar |
[22] Shi, M. et al. (2016) Redefining the invertebrate RNA virosphere. Nature 540, 539–543.
| Redefining the invertebrate RNA virosphere.Crossref | GoogleScholarGoogle Scholar |
[23] O’Brien, C.A. et al. (2015) Viral RNA intermediates as targets for detection and discovery of novel and emerging mosquito-borne viruses. PLoS Negl. Trop. Dis. 9, e0003629.
| Viral RNA intermediates as targets for detection and discovery of novel and emerging mosquito-borne viruses.Crossref | GoogleScholarGoogle Scholar |
[24] Hall, R.A. et al. (2016) Commensal viruses of mosquitoes: host restriction, transmission, and interaction with arboviral pathogens. Evol. Bioinform 12, 35–44.
[25] Standfast, H.A. et al. (1984) Isolation of arboviruses from insects collected at Beatrice Hill, Northern Territory of Australia, 1974-1976. Aust. J. Biol. Sci. 37, 351–366.
| Isolation of arboviruses from insects collected at Beatrice Hill, Northern Territory of Australia, 1974-1976.Crossref | GoogleScholarGoogle Scholar |
[26] Munderloh, U.G. and Kurtti, T.J. (1989) Formulation of medium for tick cell culture. Exp. Appl. Acarol. 7, 219–229.
| Formulation of medium for tick cell culture.Crossref | GoogleScholarGoogle Scholar |
[27] Munderloh, U.G. et al. (1994) Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J. Parasitol. 80, 533–543.
| Establishment, maintenance and description of cell lines from the tick Ixodes scapularis.Crossref | GoogleScholarGoogle Scholar |
[28] Barker, S.C. and Murrell, A. (2008) Systematics and evolution of ticks with a list of valid genus and species names. In Ticks: biology, disease and control (Bowman, A.S. and Nuttall, P.A., eds), pp. 1–39. Cambridge University Press.
[29] Bell-Sakyi, L. and Attoui, H. (2016) Virus discovery using tick cell lines. Evol. Bioinform 12, 31–34.
[30] Humphery-Smith, I. et al. (1986) Arboviruses and zoonotic infections on the Great barrier Reef and in the Coral Sea, Arbovirus Research in Australia: Proceedings 4th Symposium, pp. 209–217. CSIRO Division of Tropical Animal Science, Brisbane, Queensland.
Biographies
Caitlin O’Brien is a final year PhD student focusing on the optimisation of novel culture-based methods for virus discovery in Australian arthropods. Her most recent work includes the identification and characterisation of novel virus species in Australian mosquitoes and ticks. Her six years in research have led to the discovery of novel biological control mechanisms for pathogenic arboviruses, development of monoclonal antibodies to flaviviruses and insect specific viruses and the establishment of the ISE6 cell line for use in Australian tick virus work.
Professor Roy Hall is a specialist in vector-borne virology at the University of Queensland. His research explores emerging arthropod-borne viruses with a focus on their pathogenesis and the development of novel vaccine and diagnostic platforms. The work of his group has led to the design and development of novel diagnostic assays and vaccine candidates and the discovery of several new mosquito- and tick-borne viruses.
Professor Ala Lew-Tabor is a molecular biologist and ‘research focused’ academic at the University of Queensland. Research highlights include the developing novel vaccines and molecular assays for ticks and tick-borne diseases, respectively. Her group produces translational outputs for cattle and pets including patented vaccines for commercial uptake (cattle tick and paralysis tick), and laboratory assays for government-based diagnostic facilities.


