Microbiotechnologies for steroid production
Marina DonovaLaboratory of Microbial Transformation of Organic Compounds, G.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Prospekt Nauki, h. 5, Puschino, Moscow region, Russian Federation, 142290a-Region University. Tel: +7 495 9563370, Email: donova@ibpm.pushchino.ru
Microbiology Australia 39(3) 126-129 https://doi.org/10.1071/MA18039
Published: 7 August 2018
Structural modification of steroids by microorganisms, known since the 1950s, is nowadays a base for industrial production of many steroid hormones and their high-value precursors. Phytosterols, renewable biomaterials of plant origin, are recognised now as most attractive, low-cost and available raw materials for the pharmaceutical industry.
Microbial technologies have been developed for production of value-added androstane steroids – androstenedione, androstadienedione, 9α-hydroxy androstenedione in a single biotech stage from phytosterol to yield over 30% (w/w) of the high-purity crystalline products. The bio-processes are based on the activity of selected mycobacterial strains (Mycobacterium neoaurum VKM Ac-2015D, 1816D, Mycobacterium sp. VKM Ac-1817D) capable of performing cascade reactions of the selective sterol side chain degradation and steroid core oxidation, which are the parts of sterol catabolic pathway.
New generation microbial technologies are based on the application of the engineered strains in combination with new approaches for the enhancement of hydrophobic steroid bioconversions. Heterologous expression of eukaryotic steroidogenesis genes in saprophyte mycobacteria allowed single-step biotechnological production of the valued steroid hormones such as testosterone, boldenone and progesterone from phytosterols. Novel manufacture schemes based on the one-pot two stage microbial conversions, or combined chemical-microbiological syntheses allow more key steroid intermediates to be produced from phytosterol. A range of the biotechnologies for production of hydroxylated steroids has been developed using selected filamentous fungi capable of regio- and stereospecific hydroxylation of androstane and pregnane steroids.
The results indicate great potential of actinobacteria and filamentous fungi for steroid production. Selection of the suitable strains, metabolic engineering of steroid catabolic pathways in combination with chemical derivatisation, fungal oxyfunctionalisation of steroids and effective down-stream procedures enable creation of improved bioprocesses and effective schemes to produce a large number of high-value steroids.
Steroids (Greek, stereos = solids) represent a specific class of terpenoid lipids that contain in their structure gonane core of four fused cycloalkane rings (A–D) (Figure 1). The steroid superfamily includes various structures such as sterols (e.g. cholesterol, sitosterol, ergosterol), bile acids, corticoids, cardiac aglycones, vitamin D, insect molting hormones, etc. These compounds fulfil essential vital functions in living organisms of the animal and plant kingdoms. They control various aspects of cell proliferation and differentiation, play a role as sex hormones in the reproduction of vertebrates, provide electrolyte and glucose homeostasis in higher organisms, regulate signal transduction pathways by the binding to the respective intracellular receptors and some of them serve also as signaling molecules in cell-cell interactions. Bile acids are extremely important for the vertebrate digestion: the so-called neurosteroids function as allosteric modulators of neurotransmitter receptors, etc.1–5. In eukaryotes, the hormones, bile acids and other essential steroids are produced from cholesterol, an important component of the cell membranes, playing a role in membrane fluidity, cell differentiation and proliferation.
High biological activities provide great importance to steroid preparations for medicine: more than 300 steroid therapeuticals are clinically approved6,7. Along with antibiotics, steroids represent the best-selling category, being a significant sector of the global pharmaceutical market and this trend is forecast to continue in the future6,7.
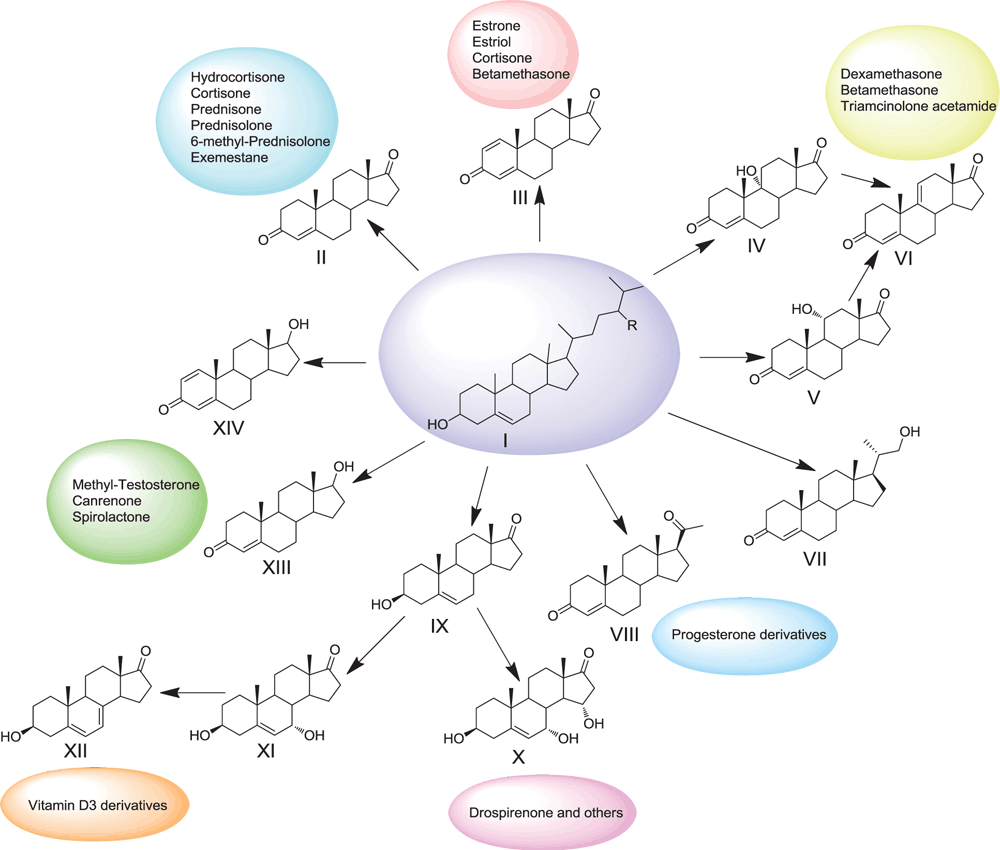
Nowadays, phytosterols (I) that are the mixtures of plant sterols such as sitosterol, stigmasterol, campesterol and others, are recognised as most cheap and available raw materials for steroid industry. Phytosterols are structurally close to cholesterol. They are produced in huge amounts from soya, pine, or wastes from cellulose production plants. Because all steroid hormones share several common precursors, production schemes for different kinds of steroid hormones (sex, adrenocortical, anabolic etc.) include phytosterol bioconversion to C19- steroids such as androstenedione (AD, II), androstadienedione (ADD, III), 9α-hydroxyandrostenedione (9-OH-AD, IV), testosterone (XIII) (Figure 1) and others.
Since their discovery in the early 1950s, microbial biotechnologies play a significant role in the steroid pharmaceutical industry thus replacing multistage, complicated and environmentally risky organic syntheses. Growing demand for steroid pharmaceuticals stimulates development of new cost-effective and ecologically friendly biotechnologies. Microbial steroid bioconversion may provide in one biotech stage single steroid modifications, or cascade reactions that are a part of catabolic pathways intrinsic to microorganisms, with cofactor regeneration and with mild conditions. It expands the toolbox of organic synthesis thus enabling production of both well-established and new steroid derivatives of potential biological and pharmacological activity that are otherwise inaccessible8.
There are some data showing that steroids originated hundreds millions years ago9, and microorganisms evolved, exposed to a variety of steroid substrates thus resulting in numerous metabolites and enzymatic activities. Natural steroid substrates serving as carbon sources have originated from plants (e.g. sitosterol, stigmasterol), or animal steroids excreted into the environment such as cholesterol, estrogens, androgens, or bile acids. Steroid microbial degradation plays a significant ecological role, being a key process for biomass decomposition, as well as removal/detoxification of steroid pollutants.
Most steroid transforming bacteria were isolated from soil, but recent metagenomic studies showed global distribution of microbial steroid degraders with the prevalence of Actinobacteria and Proteobacteria also in eukaryote hosts, aquatic environments and other habitats10.
Most effective phytosterol degraders have been found from mycolic acid rich actinobacteria of Corynebacterineae suborder such as representatives of Mycobacterium, Rhodococcus and Gordonia. It was assumed that the mycolic acid rich cell wall of these Actinobacteria may contribute to the effective transportation of lipophilic substances such as steroids.
Actinobacteria are known to catabolise phytosterol via the 9(10)-secosteroid pathway11. Along with degradation of the aliphatic side chain, different modifications of the steroid core occur during sterol bioconversion, such as 3β-hydroxy-5-ene to 3-keto-4-ene moiety transformation, Δ1-dehydrogenation, 9α-hydroxylation. Metabolic engineering allows overproduction of the valued steroids by exploiting the cascade reactions that are the part of the degradative pathway12.
We have developed microbial technologies based on phytosterol biotransformation by whole-cell actinobacteria, and especially, by the selected strains of Mycobacterium neoaurum VKM Ac-1815D, 1816D and Mycobacterium sp. VKM Ас-1817D, which provide effective production of AD (II), ADD (III) and 9-OH-AD (IV), respectively. These androstane steroids are the key intermediates in the synthesis of various steroid drugs. The microbial technologies developed enable full phytosterol conversion at the loadings over 12 g/L to yield 60–72% (of theor.) for 96–140 h fermentations. Effective down-stream procedures provide high purity (over 96%) crystalline products and the overall yield of the target steroids is over 30% (w/w).
Based on the results of the full genome sequencing and genome-wide transcriptomic profiling the specific genes and gene clusters, which are essential for steroid modifications have been revealed13,14. The data were used for the generation of engineered strains with improved biocatalytic capabilities for production of AD (II), 20-hydroxymethyl pregn-4-ene-3-one (VII) and other value-added steroids (Figure 1). The recombinant strains capable of single-step converting of phytosterol to testosterone (XIII), 1-dehydrotestosterone (XIV), progesterone (VIII) have been generated using heterologous expression of eukaryotic steroidogenic systems in mycobacterial hosts15,16. Effective production of dehydroepiandrosterone (DHEA, IX) from phytosterol (I) has been provided by the combination of the chemical protection-deprotection of the oxygen functionality at C3 with selective side chain degradation of the 3-substituted sterols using M. neoaurum VKM Ac-1815D (Figure 1).
Unlike diverse bacteria, most fungi are not capable of full degradation of the steroid skeleton, but detoxify steroids as fungitoxic molecules by oxyfunctionalisation of the steroid core, or other reactions. These features are very important for biotechnological application since they provide effective production of clinically important hydroxylated steroids. One-pot two-stage microbial conversion of phytosterol using M. neoaurum VKM Ac-1815D and Aspergillus ochraceus VKM F-830 enable effective production of 11α-hydroxy-AD (V), which is a key precursor in the syntheses of halogenated corticoids17. Regio- and stereospecific hydroxylations of DHEA at positions 7α, 7β as well as 7α,15α-dihydroxylation using selected fungal strains enabled effective production of the valued 3β-ol-5-ene derivatives18 (X, XII), thus allowing further effective schemes to drospirenone and its derivatives (Figure 1). Combination of chemical derivatisation with steroid hydroxylation by selective fungal strains allows production of the Δ5,7-steroids19 (XII), which can be used in the syntheses of vitamin D3 derivatives. Effective procedures for the isolation and purification have been developed which provide high (>96%) purity of the final crystalline products. The number of valued products that can be produced from phytosterol using our biotechnologies and active pharmaceutical ingredients (APIs) that can be synthesised from these steroidal products are presented in Figure 1.
Thus, selection of the suitable strains, metabolic engineering of the mycobacterial phytosterol catabolic pathway in combination with chemical derivatisation, fungal oxyfunctionalisation of steroids and effective down-stream procedures enable creation of improved bioprocesses and effective production schemes for obtaining of a vast number of the high-value steroids.
Acknowledgements
The support from Russian Science Foundation (Project no. 18-14-00361) is greatly appreciated. Dr G. Sukhodolskaya, Mr D. Dovbnya, Dr N. Strizhov, Mr M. Karpov, Dr V. Nikolayeva, Dr T. Lobastova, Dr V. Kollerov, Dr S. Khomutov and Mr E. Bragin from the Laboratory MTOC at G.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms RAS are highly acknowledged for their impact.
References
[1] Baker, M.E. (2011) Origin and diversification of steroids: Co-evolution of enzymes and nuclear receptors. Mol. Cell. Endocrinol. 334, 14–20.| Origin and diversification of steroids: Co-evolution of enzymes and nuclear receptors.Crossref | GoogleScholarGoogle Scholar |
[2] Waters, C.M. and Bonnie, L. (2005) Bassler Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346.
| Bassler Quorum sensing: cell-to-cell communication in bacteria.Crossref | GoogleScholarGoogle Scholar |
[3] Wollam, J. and Antebi, A. (2011) Sterol regulation of metabolism, homeostasis, and development. Annu. Rev. Biochem. 80, 885–916.
| Sterol regulation of metabolism, homeostasis, and development.Crossref | GoogleScholarGoogle Scholar |
[4] Melcangi, R.C. et al. (2011) Neuroactive steroids: focus on human brain. Neuroscience 191, 1–5.
| Neuroactive steroids: focus on human brain.Crossref | GoogleScholarGoogle Scholar |
[5] Craigie, E. et al. (2009) Glucocorticoids and mineralocorticoids. In Cardiovascular hormone systems: from molecular mechanisms to novel therapeutics (Bader, M., ed.), pp. 1–64. Wiley-VCH Verlag GmbH & Co, Weinheim.
[6] Tong, W.-Y. and Dong, X. (2009) Microbial biotransformation: recent developments on steroid drugs. Recent Pat. Biotechnol. 3, 141–153.
| Microbial biotransformation: recent developments on steroid drugs.Crossref | GoogleScholarGoogle Scholar |
[7] Donova, M.V. and Egorova, O.V. (2012) Microbial steroid transformations: current state and prospects. Appl. Microbiol. Biotechnol. 94, 1423–1447.
| Microbial steroid transformations: current state and prospects.Crossref | GoogleScholarGoogle Scholar |
[8] Świzdor, A. et al. (2014) Microbial Baeyer–Villiger oxidation of 5α-steroids using Beauveria bassiana. A stereochemical requirement for the 11α-hydroxylation and the lactonization pathway. Steroids 82, 44–52.
| Microbial Baeyer–Villiger oxidation of 5α-steroids using Beauveria bassiana. A stereochemical requirement for the 11α-hydroxylation and the lactonization pathway.Crossref | GoogleScholarGoogle Scholar |
[9] Maser, E. and Lanisnik Rizner, T. (2012) Steroids and microorganisms. J. Steroid Biochem. Mol. Biol. 129, 1–3.
| Steroids and microorganisms.Crossref | GoogleScholarGoogle Scholar |
[10] Holert, J. et al. (2018) Metagenomes reveal global distribution of bacterial steroid catabolism in natural, engineered, and host environments. MBio 9, e02345-17.
| Metagenomes reveal global distribution of bacterial steroid catabolism in natural, engineered, and host environments.Crossref | GoogleScholarGoogle Scholar |
[11] Uhía, I. et al. (2012) Cholesterol metabolism in Mycobacterium smegmatis. Environ. Microbiol. Rep. 4, 168–182.
| Cholesterol metabolism in Mycobacterium smegmatis.Crossref | GoogleScholarGoogle Scholar |
[12] Donova, M. (2017) Steroid bioconversions. In Methods in Molecular Biology, Book: Microbial Steroids (Barredo, J.L., ed.), pp. 1–13.
[13] Bragin, E.Y. et al. (2013) Comparative analysis of genes encoding key steroid core oxidation enzymes in fast-growing Mycobacterium spp. strains. J. Steroid Biochem. Mol. Biol. 138, 41–53.
| Comparative analysis of genes encoding key steroid core oxidation enzymes in fast-growing Mycobacterium spp. strains.Crossref | GoogleScholarGoogle Scholar |
[14] Shtratnikova, V.Y. et al. (2017) Effect of methyl-β-cyclodextrin on gene expression in microbial conversion of phytosterol. Appl. Microbiol. Biotechnol. 101, 4659–4667.
| Effect of methyl-β-cyclodextrin on gene expression in microbial conversion of phytosterol.Crossref | GoogleScholarGoogle Scholar |
[15] Karpov, M. et al. (2016) Bio-based testosterone production from phytosterol. Proc. Nat. Acad. Sci. Belarus. Chemical Series 3, 78–79.
[16] Strizhov, N. et al. (2014) Progesterone biosynthesis by combined action of adrenal steroidogenic and mycobacterial enzymes in fast growing mycobacteria. N. Biotechnol. 31, S67.
| Progesterone biosynthesis by combined action of adrenal steroidogenic and mycobacterial enzymes in fast growing mycobacteria.Crossref | GoogleScholarGoogle Scholar |
[17] Dovbnya, D. et al. (2017) Obtaining of 11α-Hydroxyandrost-4-ene-3,17-dione from Natural Sterols. In: Methods in Molecular Biology, Book: Microbial Steroids (Barredo, J.L., ed.), pp. 259–269.
[18] Lobastova, T.G. et al. (2009) Dihydroxylation of dehydroepiandrosterone in positions 7α and 15α by mycelial fungi. Appl. Biochem. Microbiol. 45, 617–622.
| Dihydroxylation of dehydroepiandrosterone in positions 7α and 15α by mycelial fungi.Crossref | GoogleScholarGoogle Scholar |
[19] Lobastova, T.G. et al. (2009) Synthesis of 3β-hydroxy-androsta-5,7-dien-17-one from 3β-hydroxyandrost-5-en-17-one via microbial 7α-hydroxylation. Steroids 74, 233–237.
| Synthesis of 3β-hydroxy-androsta-5,7-dien-17-one from 3β-hydroxyandrost-5-en-17-one via microbial 7α-hydroxylation.Crossref | GoogleScholarGoogle Scholar |
Biography
Dr Marina Donova is the Head of Laboratory of Microbiological Transformation of Organic Compounds at the G.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Pushchino, Russia; a member of Presidium of Russian Microbiological Society. She got her DSci (Biology) level in 2007. Her fields of research interests comprise all aspects of steroid conversions by microorganisms including discovery of novel biocatalysts capable of performing known and rare reactions, isolation and identification of novel metabolites with potent therapeutic effects, investigation of metabolic pathways, physical chemical aspects of steroid bioconversions, product recovery processing and the development and scaling up of the biotechnologies.