Biodegradation of emerging pollutants: focus on pharmaceuticals
Irina Ivshina A B D , Elena Tyumina B and Elena Vikhareva CA Institute of Ecology and Genetics of Microorganisms, Ural Branch of the Russian Academy of Sciences, Perm, Russia
B Perm State University, Perm, Russia
C Perm State Pharmaceutical Academy, Perm, Russia
D Tel: + 7 342 280 81 14, Email: ivshina@iegm.ru
Microbiology Australia 39(3) 117-122 https://doi.org/10.1071/MA18037
Published: 7 August 2018
A priority environmental problem is pollution and disturbance of natural environments by emerging pollutants ‒ substances of various origins and structures and with known and/or potential ecotoxic effects. One of the most dangerous groups of emerging pollutants is pharmaceutical substances due to their highly stable chemical structure and pronounced biological activity. They are found in soil, bottom sediments, surface, sewage, groundwater and drinking water. Uncontrolled release of pharmaceuticals in open ecosystems is potentially dangerous, entailing environmental consequences. Their negative impacts on living organisms are evident. This has driven the search for effective ways to neutralise persistent pollutants. In Russia, pharmaceutical pollution of the environment has commenced recently and is still presented as research with a local focus. In particular, the dynamics and metabolic mechanisms of pharma pollutants by Rhodococcus actinobacteria, outstanding among other microorganisms for their capacity to degrade a great diversity of degradable pollutants, are most intensively investigated. These studies are implemented at the junction of organic chemistry, molecular biology, biotechnology, and pharmacology. They include a set of interrelated fundamental tasks, such as developing drug detection methods in the cultivation media of microorganisms, elucidating the relationships between the systematic affiliation of microorganisms and their ability to degrade chemically different drug substances, as well as studying the degree of biodegradability and toxic effects of new compounds on the degrading microorganisms, and also the features of their decomposition and co-metabolism. Solving these tasks is important to enable understanding of the environmental fate of pharmaceuticals and to create prerequisites for innovative technical solutions in the advanced treatment of pharmaceutical wastewater. It is also essential for the development of environmentally safe approaches to hazardous pharmaceutical waste management.
Emerging pollutants are a new global environmental concern. The term emerging pollutants refers to natural or synthetic substances found in ecosystems. Their ecotoxic impacts on the environment and humans are already known, whereas the occurrence and environmental fate are uncontrolled or unregulated1,2. Emerging contaminants are not necessarily new substances. Such ecotoxicants comprise compounds long present in the environment, but now detected due to improved analytical methods. In 2016, the Norman network listed more than 1,000 most frequently reported emerging pollutants3. This list includes pharmaceuticals, personal care products, pesticides, industrial and household chemicals, metals, surfactants, industrial additives and solvents.
Of particular concern are the release and accumulation of highly persistent and bioactive pharmaceutical pollutants in the environment. According to aus der Beek et al.4, about 700 pharmaceuticals were found in the aquatic ecosystems of 71 countries. Pharmaceutical substances and their metabolites have been detected in soil, sediments, surface, sewage, ground and even drinking water4–8. Trace amounts of drugs found in bottled water is an unprecedented case9. Stumm-Zollinger and Fair10, and Hignite and Azarmoff11 first reported on pharmaceuticals in wastewater. The toxicity and biodegradability of these compounds was first discussed in the 1980s by Richardson and Bowron12.
Since the late 1990s, pharmaceutical pollutants present in natural ecosystems have been seen as an emerging environmental problem13. The limited knowledge about their negative impacts on animals and humans remains the weak link. Despite the relatively low concentrations (ng/L to µg/L) of pharmaceutical pollutants in nature, their constant replenishment can lead to high permanent concentrations and stimulate negative effects on humans and the environment. The best-known case is the decline in vulture populations (Gyps bengalensis, G. indicus, and G. tenuirostris) in the Indian subcontinent. Toxic exposure was caused by veterinary-used diclofenac in South Asia. Birds fed on cattle carcasses medicated with the anti-inflammatory drug diclofenac died of intoxication and kidney failure14. Pharmaceutical pollutants can move across food chains. British scientists have found diclofenac in otter wool, indicating diclofenac contamination of aquatic ecosystems, fish and fauna, the habitat and food for these animals15. Several studies reported on feminisation of male fish caused by a synthetic hormone 17-α-ethinyl-estradiol in their habitat16,17.
For most drugs detected environmentally, potential acute and chronic effects on ecosystem components have not yet been studied. Though drug influence on animals and humans is intensively studied, and reports describing impacts of widely used antipyretics and analgesics on plants have appeared18,19, they are not sufficiently studied in ecologically relevant microorganisms. Upon contact with these xenobiotics, microorganisms detoxify them, and as principal biosphere constituents, they are sensitive to changes in the habitat. The relevant studies have recently commenced.
Pharma pollutants in ecosystems of Russia
According to aus der Beek et al.4, the prevalence of antibiotics and analgesics in the environment, including non-steroidal anti-inflammatory drugs, is a typical situation for the eastern European countries and Russia.
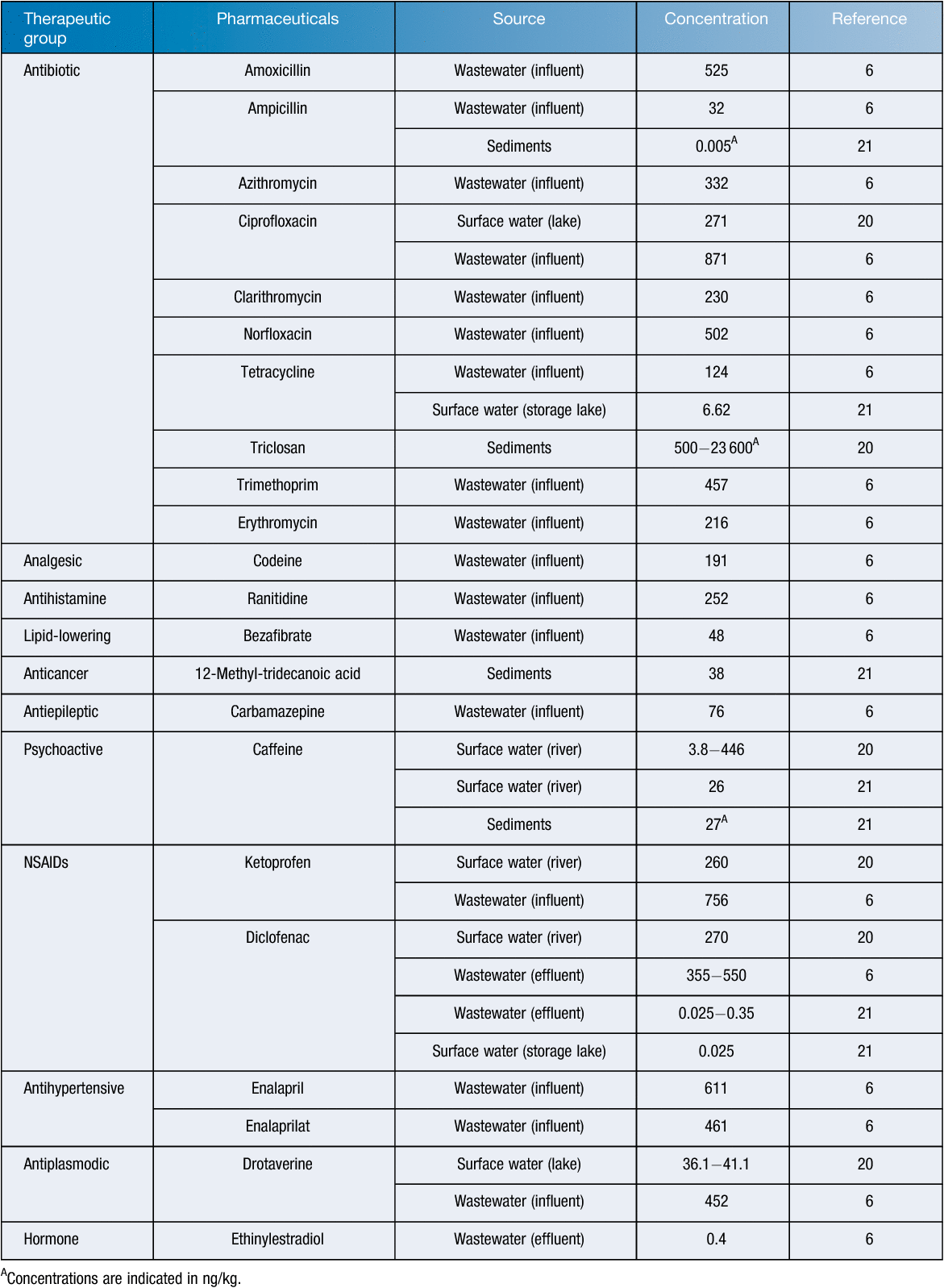
Investigations on pharmaceuticals and their metabolites in wastewater and surface waters in Russia are few and concentrated mainly in the Central and North-Western regions. Table 1 summarises data on pharmaceutical occurrences and concentrations detected in aqueous samples. The average concentrations of pharmaceuticals found in surface water, untreated wastewater, and treated wastewater were 136, 360 and 181ng/L, respectively.
|
Pharmaceuticals-related studies of aquatic ecosystems and bottom sediments in the Northwest region detected a number of over-the-counter medications, including the psychostimulant caffeine, anti-inflammatories ketoprofen and diclofenac, and antispasmodic drotaverine hydrochloride20.
Recent studies under the project ‘Implementation of the Baltic Sea Action Plan in Russia’ (BASE) detected 20 pharmaceuticals in the wastewater of St. Petersburg, though the initial targets were only diclofenac and ethinylestradiol as the most cosmopolitan pharma pollutants6. The effluent contained diclofenac ranging from 355 ng/L in summer to 550 ng/L in winter. The researchers calculated the predicted environmental concentration (PECriver) in the Neva River to be circa 5 ng/L. At the same time, a significantly increased diclofenac concentration in the wastewater effluent was found compared to that in the influent. This phenomenon is apparently explained by the release of conjugated diclofenac metabolites during secondary wastewater treatment. The ethinylestradiol concentration was 0.4 ng/L.
Sampling performed at water intakes and reservoirs (Moscow region) revealed 105 pharmaceuticals and their residues21. Diclofenac (0.025‒0.35 ng/L), caffeine (26 ng/L) and tetracycline (0.662 ng/L) were most frequently detected. Considering the potential risks of pharmaceutical pollutants for living organisms, the authors used the PASS program (Prediction of Activity Spectra for Substances) tailored to simulate the drug toxicity. According to the structural formula of an organic compound the PASS program estimates its probable biological activity21. They predicted the possible toxic effects (embryotoxicity, carcinogenicity, mutagenicity, etc.) on living organisms and developed an ecotoxicological map of some Moscow aquatic ecosystems.
Because of the limited knowledge on the topic of this review, the priority research in Russia is still focused on environmental detection and identification of pharmaceuticals, their effective analyses in wastewater, and clinical trials of low drug concentrations against humans and other living organisms, including environmentally relevant microorganisms. The latter are capable of pharmaceutical pollutant detoxification in natural ecosystems.
Biodegradation of pharma pollutants
The role of microorganisms role in the environmental degradation of xenobiotics is pivotal. Of those involved in water and soil ecosystem self-cleaning processes, Rhodococcus actinobacteria exhibit nonspecific enzymatic actions. They are first to attack compounds novel for microbial cells. In recent years, rhodococci are often considered as promising biodegraders and biotransformers of various xenobiotics. Rhodococcus’ ecological versatility and exceptional polyfunctionality, high catalytic activity in extreme environments, and biodegradation of organic compounds from many known classes clearly indicate the suitability of rhodococci for degradation of pharmaceutical pollutants22,23.
Biocatalysis activity studies revealed Rhodococcus’ ability to decompose chemically diverse pharmaceuticals. Gauthier et al.24 used R. rhodochrous ATCC 13808 to biodegrade heterocyclic nitrogen-containing pharmaceuticals, such as sulfamethisole (43.4 mg/L), sulfamethoxazole (32 mg/L), and carbamazepine (9.5 mg/L). These compounds were biodegraded only in the presence of glucose. In co-metabolic conditions, biodegradations of sulfamethisole, sulfamethoxazole and carbamazepine were 14, 20, and 15%, respectively. Each drug biodegradation process did not exceed 36 days and metabolites formed were unstable.
Yoshimoto et al.25 studied rhodococcal interactions with steroid compounds (specifically17β-estradiol). R. zopfii Y 50158 degraded this pharmaceutical in the presence of glucose within 24 hours.
R. erythropolis, R. equi and R. rhodochrous were examined as potential 17α-ethinylestradiol (1.4 mg/L) biodegraders, with R. erythropolis being the most active. 17α-ethinylestradiol depletion as the sole carbon and energy source was 10% after 75 hours, and 47% in the presence of glucose within 13 hours26. According Larcher and Yargeau27, R. rhodochrous ATCC 13808 completely utilised 17α-ethinylestradiol (5 mg/L) after 48 hours. Complete testosterone (1 mg/ml) decomposition was achieved with resting cells of R. equi ATCC 1488728 within 2 days28. Biological testosterone degradation proceeded in two stages: formation of 9α,17β-dihydroxyandrost-4-en-3-one and androst-4-ene-3,17-dione further converted into 9α-hydroxyandrost-4-ene-3,17-dione and 9α-hydroxy-1,4-adrostadien-3,17-dione, respectively. The final products contained 3-hydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione (3-HSA) further degraded to CO2 and H2O28. There are also studies published on Rhodococcus biodegradation of ester-based drugs. Possible clofibric acid biodegradation to clofibrate using R. rhodochrous was shown29.
The authors of this review employed resources of the Regional Specialized Collection of Alkanotrophic Microorganisms (acronym IEGM, WDCM 768, http://www.iegmcol.ru)30 to investigate biodegradation of pharmaceutical pollutants typically found in ecosystems and widely used in Russia, such as non-steroidal anti-inflammatory drugs (phenylacetic acid derivatives diclofenac and ibuprofen), spasmolytic (an isoquinoline derivative drotaverine or No-Spa)31,32, analgesic (a phenol group-containing n-acetaminophen or paracetamol)33. To elucidate biodegradation mechanisms, the genomes of environmentally significant R. erythropolis IEGM 267 and R. ruber IEGM 231, which actively biodegrade a variety of complex organic compounds, were sequenced34. Comparative bioinformatic analysis applied to sequencing data allowed analysis of functional genes, which control the pharma pollutant biodegradation. Kinetics and decomposition patterns of pharma pollutants were studied depending on physiological state of biodegraders (growing, washed, immobilised, and dormant bacterial cells) and their culture conditions (mineral composition, aeration rate and acidity, temperature, initial ecotoxicant concentrations, and selection of effective co-substrates). Regulation mechanisms (induction, inhibition) of Rhodococcus catalytic activity towards pharmaceutical pollutants were elucidated. Less hazardous metabolites were identified. The main biotransformation pathways of the parent ecotoxicants were determined. Features of rhodococcal interaction with pharmaceutical pollutants were studied32. The pharmaceutical biodegradation process was most significantly enhanced with immobilised rhodococci31. The wooden waste available in the Perm region was used as carrier material. To enhance the adsorbent surface affinity to bacterial cells, the carrier was treated with selected hydrophobisers35. Stable polyfunctional biocatalytic systems based on immobilised Rhodococcus cells were developed. They increased the rate of the biodegradation processes of pharmaceutical pollutants and their metabolites. Additionally, they are characterised by high functional stability over 3‒8-month storage and are reusable.
The research results indicate that Rhodococcus actinobacteria may act as important bio-oxidants of chemically diverse pharma pollutants typically detected in the environment. The experimental data on mechanisms and tentative detoxification and bioconversion pathways of pharma pollutants together with biochemically and catalytically characterised biodegrader strains may offer new environmentally safe methods for hazardous pharmaceutical waste management. Another practical application is to use actinobacteria-based biodegraders as a suitable model to study the novel pathways of drug metabolism that allows prediction of metabolites expected from degradation of closely related pharmaceutical pollutants.
Conclusion
There is a rapid growth in pharmaceuticals and novel pharmaceutical agents on the market. This, combined with their exponentially increasing annual consumption, only partial removal of pharmaceuticals and their metabolites in wastewater treatment, and a lack of highly effective disposal methods for hazardous pharmaceutical wastes contribute to the dramatically growing pharmaceutical pollution of the biosphere and an imbalance in natural ecosystems. Society only recently realised the real scope of the imminent danger and the urgent need to work out ways to deal with the pharmaceutical pollution challenge. Environmental disamenities stimulate novel technical solutions on how to mitigate the environmental burden and to assess environmental risks due to possible pharmaceutical pollutant exposure. They force us to seek detoxification and removal methods of these anthropogenic toxicants from aquatic and terrestrial ecosystems to reduce and even exclude the problem completely in future.
In Russia, such studies are still at the stage of intensive accumulation of factual data on both the expansion and analysis of pharmaceutical pollution of the environment (principally water bodies) and drug conversion by microbes. Attempts are being made to propose a set of measures reducing environmental risks associated with drug pollution. However, it will take years of research to evolve fundamentally new, experimentally based solutions that require large investment, and more deliberate strategies to prevent drug release into the environment.
Acknowledgements
The study was supported by the Russian Foundation for Basic Research and the Ministry of Education and Science of Perm Krai (grant no. 17-44-590567), and the Integrated Program of Fundamental Research of the Ural Branch of the Russian Academy of Sciences (18-4-8-21).
References
[1] Bell, K.Y. et al . (2011) Emerging Pollutants. Water Environ. Res. 83, 1906–1984.| Emerging Pollutants.Crossref | GoogleScholarGoogle Scholar |
[2] Geissen, V. et al . (2015) Emerging pollutants in the environment: a challenge for water resource management. Int. Soil Water Conserv. Res. 3, 57–65.
| Emerging pollutants in the environment: a challenge for water resource management.Crossref | GoogleScholarGoogle Scholar |
[3] Network of reference laboratories, research centres and related organisations for monitoring of emerging environmental substances (2016) http://www.norman-network.net
[4] aus der Beek, T. et al . (2016) Pharmaceuticals in the environment – global occurrences and perspectives. Environ. Toxicol. Chem. 35, 823–835.
| Pharmaceuticals in the environment – global occurrences and perspectives.Crossref | GoogleScholarGoogle Scholar |
[5] Kolpin, D.W. et al . (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ. Sci. Technol. 36, 1202–1211.
| Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance.Crossref | GoogleScholarGoogle Scholar |
[6] Nikiforov, V. et al.. (2014) BASE project 2012–2014: pilot activity to identify sources and flow patterns of pharmaceuticals in St. Petersburg to the Baltic Sea. Helcom. , 1–53.
[7] Narvaez, Jh. and Jimenez, C.C. (2012) Pharmaceutical products in the environment: sources, effects and risks. Vitae, Revista de la facultad de química farmaceutica 19, 93–108.
[8] Archer, E. et al . (2017) The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminant (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 174, 437–446.
| The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminant (EDCs), metabolites and illicit drugs in a WWTW and environmental waters.Crossref | GoogleScholarGoogle Scholar |
[9] EWG (2008) Bottled water quality investigation: test results: chemicals in bottled water. http://www.ewg.org/research/bottled-water-quality-investigation/test-results-chemicals-bottled-water (accessed 3 July 2017).
[10] Stumm-Zollinger, E. and Fair, G.M. (1965) Biodegradation of steroid hormones. J. Water Pollut. Control Fed. 37, 1506–1510.
[11] Hignite, C. and Azarmoff, D.L. (1977) Drugs and drug metabolites as environmental contaminants−chlorophenoxysobutyrate and salicylic-acid in sewage water effluent. Life Sci. 20, 337–341.
| Drugs and drug metabolites as environmental contaminants−chlorophenoxysobutyrate and salicylic-acid in sewage water effluent.Crossref | GoogleScholarGoogle Scholar |
[12] Richardson, M.L. and Bowron, J. (1985) The fate of pharmaceutical chemicals in the aquatic environment. J. Pharm. Pharmacol. 37, 1–12.
| The fate of pharmaceutical chemicals in the aquatic environment.Crossref | GoogleScholarGoogle Scholar |
[13] Ternes, T.A. (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 32, 3245–3260.
| Occurrence of drugs in German sewage treatment plants and rivers.Crossref | GoogleScholarGoogle Scholar |
[14] Oaks, J.L. et al . (2004) Diclofenac residues as the cause of vulture population declines in Pakistan. Nature 427, 630–633.
| Diclofenac residues as the cause of vulture population declines in Pakistan.Crossref | GoogleScholarGoogle Scholar |
[15] Richards, N.L. et al . (2011) Qualitative detection of the NSAIDs diclofenac and ibuprofen in the hair of Eurasian otters (Lutra lutra) occupying UK waterways with GC–MS. Eur. J. Wildl. Res. 57, 1107–1114.
| Qualitative detection of the NSAIDs diclofenac and ibuprofen in the hair of Eurasian otters (Lutra lutra) occupying UK waterways with GC–MS.Crossref | GoogleScholarGoogle Scholar |
[16] Gross-Sorokin, M.Y. et al . (2006) Assessment of feminization of male fish in English rivers by the Environment Agency of England and Wales. Environ. Health Perspect. 114, 147–151.
| Assessment of feminization of male fish in English rivers by the Environment Agency of England and Wales.Crossref | GoogleScholarGoogle Scholar |
[17] Kidd, K.A. et al . (2007) Collapse of a fish population after exposure to synthetic estrogen. Proc. Natl. Acad. Sci. USA 104, 8897–8901.
| Collapse of a fish population after exposure to synthetic estrogen.Crossref | GoogleScholarGoogle Scholar |
[18] An, J. et al . (2009) Ecotoxicological effects of paracetamol on seed germination and seedling development of wheat (Triticum aestivum L.). J. Hazard. Mater. 169, 751–757.
| Ecotoxicological effects of paracetamol on seed germination and seedling development of wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar |
[19] Schmidt, W. and Redshaw, C.H. (2015) Evaluation of biological endpoints in crop plants after exposure to non-steroidal anti-inflammatory drugs (NSAIDs): implications for phytotoxicological assessment of novel contaminants. Ecotoxicol. Environ. Saf. 112, 212–222.
| Evaluation of biological endpoints in crop plants after exposure to non-steroidal anti-inflammatory drugs (NSAIDs): implications for phytotoxicological assessment of novel contaminants.Crossref | GoogleScholarGoogle Scholar |
[20] Russkikh, Ya.V. et al . (2014) Medicines in water objects of the Northwest of Russia. Region. Ecolog. 1-2, 77–83.
[21] Barenboim, G.M. et al . (2014) Characteristics of the surface water pollution with drugs residues. Water Sector Russia: Probl. Technol. Manag. 3, 131–141.
[22] Larkin, M.J. et al . (2006) Biodegradation by members of the genus Rhodococcus: biochemistry, physiology, and genetic adaptation. Adv. Appl. Microbiol. 59, 1–29.
| Biodegradation by members of the genus Rhodococcus: biochemistry, physiology, and genetic adaptation.Crossref | GoogleScholarGoogle Scholar |
[23] Martínková, L. et al . (2009) Biodegradation potential of the genus Rhodococcus. Environ. Int. 35, 162–177.
| Biodegradation potential of the genus Rhodococcus.Crossref | GoogleScholarGoogle Scholar |
[24] Gauthier, H. et al . (2010) Biodegradation of pharmaceuticals by Rhodococcus rhodochrous and Aspergillus niger growing by co-metabolism. Sci. Total Environ. 408, 1701–1706.
| Biodegradation of pharmaceuticals by Rhodococcus rhodochrous and Aspergillus niger growing by co-metabolism.Crossref | GoogleScholarGoogle Scholar |
[25] Yoshimoto, T. et al . (2004) Degradation of estrogens by Rhodococcus zopfii and Rhodococcus equi isolates from activated sludge in wastewater treatment plants. Appl. Environ. Microbiol. 70, 5283–5289.
| Degradation of estrogens by Rhodococcus zopfii and Rhodococcus equi isolates from activated sludge in wastewater treatment plants.Crossref | GoogleScholarGoogle Scholar |
[26] O’Grady, D. et al . (2009) Removal of aqueous 17-α-ethinilestradiol by Rhodococcus species. Environ. Eng. Sci. 26, 1393–1400.
| Removal of aqueous 17-α-ethinilestradiol by Rhodococcus species.Crossref | GoogleScholarGoogle Scholar |
[27] Larcher, S. and Yargeau, V. (2013) Biodegradation of 17-α-ethinilestradiol by heterotrophic bacteria. Environ. Pollut. 173, 17–22.
| Biodegradation of 17-α-ethinilestradiol by heterotrophic bacteria.Crossref | GoogleScholarGoogle Scholar |
[28] Kim, Y.-U. et al . (2007) Steroid 9α-hydroxylation during testosterone degradation by resting Rhodococcus equi cells. Arch. Pharm. 340, 209–214.
| Steroid 9α-hydroxylation during testosterone degradation by resting Rhodococcus equi cells.Crossref | GoogleScholarGoogle Scholar |
[29] Evangelista, S. et al . (2008) The recalcitrance of clofibric acid to microbial degradation. Water Pollut. 9, 273–278.
| The recalcitrance of clofibric acid to microbial degradation.Crossref | GoogleScholarGoogle Scholar |
[30] Catalogue of Strains of Regional Specialized Collection of Alkanotrophic Microorganisms. http://www.iegmcol.ru/strains/index.html (accessed 3 July 2017).
[31] Ivshina, I.B. et al . (2012) Biodegradation of drotaverine hydrochloride by free and immobilized cells of Rhodococcus rhodochrous IEGM 608. World J. Microbiol. Biotechnol. 28, 2997–3006.
| Biodegradation of drotaverine hydrochloride by free and immobilized cells of Rhodococcus rhodochrous IEGM 608.Crossref | GoogleScholarGoogle Scholar |
[32] Ivshina, I.B. et al . (2015) Drotaverine hydrochloride degradation using cyst-like dormant cells of Rhodococcus ruber. Curr. Microbiol. 70, 307–314.
| Drotaverine hydrochloride degradation using cyst-like dormant cells of Rhodococcus ruber.Crossref | GoogleScholarGoogle Scholar |
[33] Ivshina, I.B. et al . (2006) Catalysis of the biodegradation of unusable medicines by alkanotrophic rhodococci. Appl. Biochem. Microbiol. 42, 392–395.
| Catalysis of the biodegradation of unusable medicines by alkanotrophic rhodococci.Crossref | GoogleScholarGoogle Scholar |
[34] Ivshina, I.B. et al . (2014) Draft genome sequence of propane and butane oxidizing actinobacterium Rhodococcus ruber IEGM 231. Genome Announc. 2, e01297–14.
| Draft genome sequence of propane and butane oxidizing actinobacterium Rhodococcus ruber IEGM 231.Crossref | GoogleScholarGoogle Scholar |
[35] Podorozhko, E.A. et al . (2008) Hydrophobised sawdust as a carrier for immobilisation of the hydrocarbon-oxidizing bacterium Rhodococcus ruber. Bioresour. Technol. 99, 2001–2008.
| Hydrophobised sawdust as a carrier for immobilisation of the hydrocarbon-oxidizing bacterium Rhodococcus ruber.Crossref | GoogleScholarGoogle Scholar |
Biographies
Professor Irina Ivshina carries out research in environmental microbiology and industrial biotechnology. She leads the Laboratory of Alkanotrophic Microorganisms at the Institute of Ecology and Genetics of Microorganisms, Ural Branch of the Russian Academy of Sciences, and performs teaching activities at the Microbiology and Immunology Department, Perm State University. She is an academician of the Russian Academy of Sciences, awarded Laureate of the Russian Federation Government for the development and implementation of a complex of remediation biotechnologies for hydrocarbon-polluted biogeocenoses, and Vice-President of the Russian Interregional Microbiological Society.
Elena Tyumina, MS (Biotechnology), graduated with honors from Perm State University, and is now a post-graduate student of the Microbiology and Immunology Department, Perm State University. Her research interests are general microbiology, ecology, microbial diversity. She is awarded a Laureate of the V.I. Vernadsky Non-governmental Ecological Foundation in the field of science.
Professor Elena Vikhareva, Dr Sci. (Pharmacology), is Head of the Department of Analytical Chemistry, Perm State Pharmaceutical Academy. Her research interests are ecology, biotechnology and analysis of pharmaceuticals.


