Maternal Group B Streptococcus colonisation
Lucy Furfaro A C , Barbara Chang B and Matthew Payne AA Division of Obstetrics and Gynaecology, 2nd Floor, Block A, King Edward Memorial Hospital, Subiaco, WA 6008, Australia
B Marshall Center for Infectious Disease Research and Training, School of Biomedical Sciences, The University of Western Australia, WA 6009, Australia
C Tel: +61 8 6488 7969, Email: lucy.furfaro@research.uwa.edu.au
Microbiology Australia 38(3) 134-136 https://doi.org/10.1071/MA17049
Published: 9 August 2017
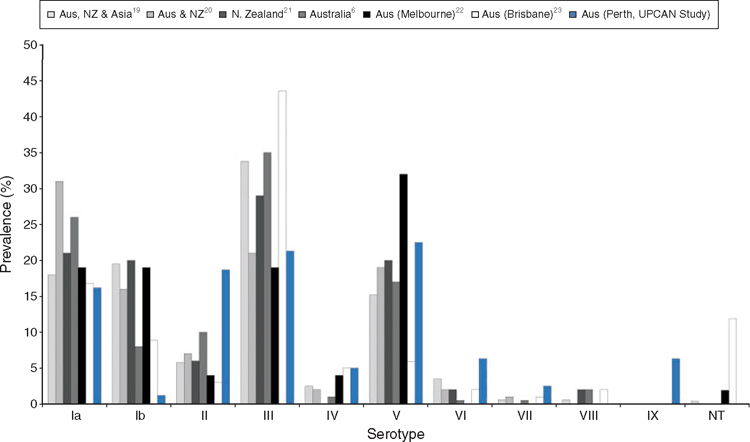
Streptococcus agalactiae, commonly known as Group B Streptococcus (GBS), is an important neonatal pathogen known to cause sepsis, meningitis and pneumonia. Australian pregnant women undergo screening during pregnancy in an effort to eradicate GBS before delivery where transmission to the neonate can occur. Preventative treatment includes intrapartum antibiotic prophylaxis and results in widespread treatment of the 10–40% of pregnant women colonised. GBS are separated into ten different capsular polysaccharide serotypes and previous studies have suggested associations between specific serotypes and disease. At present, however, minimal data exist on serotype distribution within Western Australian-pregnant women, information that may play an important role in future prophylactic treatment regimens. Our preliminary data, obtained from GBS isolated from vaginal swabs from 191 pregnant women, suggests that GBS serotype distributions in Western Australia are different to other parts of Australasia. In particular, compared to the eastern Australian states and New Zealand, in our cohort, serotype Ib prevalence was 7–17 times lower, II was 2–6 times greater and VI was 2–12 times greater. In addition, serotype IX represented 6.3% of all serotypes. Understanding which serotypes are present in our population will provide valuable data for future targeted treatment regimens such as vaccination and bacteriophage therapy.
Group B Streptococcus during pregnancy
Neonates are among the most vulnerable forms of life, they enter this world with minimal immune defences and are faced with a vast array of opportunistic pathogens ready to colonise. One such organism is Streptococcus agalactiae, commonly known as Group B Streptococcus (GBS), which is responsible for morbidity and mortality in the immunocompromised, elderly and in particular, neonatal populations. GBS infection is a leading cause of sepsis and can also lead to meningitis, pneumonia, shock and even death1,2. It is understood that transmission of this organism can occur from a commensally colonised mother to her baby during birth, in utero (vertical) or alternatively through nosocomial transmission once born (horizontal)3. In an effort to prevent infant GBS infection, risk-based and culture-based screening of pregnant women followed by intrapartum antibiotic prophylaxis has been introduced in a number of countries globally4. In Australia, pregnant women are screened for presence of GBS several weeks before expected delivery to determine colonisation status. If a patient is found to carry GBS, antibiotics are administered prior to delivery in an effort to eradicate the organism before the neonate is exposed.
Serotypes
Global carriage rates among pregnant women are estimated at 10–40% which results in widespread antibiotic use in this population4–6. Due to contraindications of a number of drug classes during pregnancy the antibiotics of choice include penicillin or if the woman is sensitised, cephazolin or clindamycin4. Penicillin resistance has rarely been described, however, clindamycin resistance is rising and has been reported recently in Australia7. Our current culture detection gives a presence/absence result and does not define characteristics of colonisation such as serotype. GBS are encapsulated and have a capsular polysaccharide (cps) locus that determines one of 10 serotypes (Ia, Ib, II–IX)8–10. Global distributions of these serotypes have shown variation in each region: for example, most countries have cps types Ia, Ib, II, III and V as the most common, although Japan has found prevalence of cps VIII, which globally is considered rare11–13. The capsule is considered an important virulence factor and some serotypes are associated with invasive disease more so than others14. For example, cps III has been observed in association with neonatal bloodstream infection, while cps V more so in cases of adult disease15. Understanding serotype distribution and its role in disease may improve the way we treat women during pregnancy.
GBS in Western Australia
Our research aims to determine which serotypes are prevalent amongst Western Australian pregnant women and explore alternative targeted treatment options. Our study is currently recruiting 1000 pregnant women at King Edward Memorial Hospital, Perth, Western Australia and collecting vaginal and rectal specimens at 14–22 and 34–38 weeks’ gestation. The specimens are cultured and PCR tested for GBS presence and common serotypes Ia, Ib and III using our novel multiplex qPCR assay16. Other remaining serotypes are confirmed through methods described by Imperi et al.17. Initial retrospective studies of vaginal specimens from the UPCAN study18 found interesting results compared to those previously reported in Australasia (Figure 1). The main differences in serotypes compared to other studies are seen for cps Ib, II, VI and IX in our WA cohort. We have identified a lower incidence of common serotype cps Ib and higher incidence of cps II, VI and IX. It must be noted, however, that a number of these previous studies had not tested for cps IX due to it only being proposed as a new cps type in 200910. Comparison of cps IX to the Australia-wide study by Ko et al.6 is appropriate, as testing for this new serotype was included, but no cases were detected.
|
Clinical impact and future directions
Monitoring of GBS strains within the pregnant community generates clinically useful information about this pathogen and can equip us for future targeted prevention and treatment strategies. For example, vaccination development targeting the capsule has now progressed with a number of candidate vaccines targeting multiple cps types such as Ia, Ib and III24. Knowledge of prevalent serotypes could impact our vaccination strategy as we discover differences in serotype distribution amongst different geographical populations. Another alternative targeted therapy that we are researching is bacteriophage therapy. The major principle behind this is that the specificity and lytic activity of these bacterial viruses could provide a targeted GBS treatment solution that would concurrently help to prevent emerging antibiotic resistance and microbiome dysbiosis, in addition to avoiding the unknown impacts of antibiotic exposure on the newborn. We are currently isolating and testing novel bacteriophages for lytic activity against clinical GBS strains to assess future potential.
This research is all about defining our target in an effort to improve clinical detection and refining treatment strategies, to ensure we protect our vulnerable neonates.
Acknowledgements
Funding for this research was received by MSP from the Women’s and Infants’ Research Foundation (WIRF) and the Channel 7 Telethon Trust. LLF is supported by the Australian Government Research Training Program Scholarship and the Professor Gordon King Postgraduate Scholarship provided by WIRF. MSP is supported by a NHMRC Project Grant [1077931]. The authors thank Professor Lyn Gilbert and Dr Fanrong Kong for providing the GBS reference isolates.
References
[1] Heath, P.T. and Schuchat, A. (2007) Perinatal group B streptococcal disease. Best Pract. Res. Clin. Obstet. Gynaecol. 21, 411–424.| Perinatal group B streptococcal disease.Crossref | GoogleScholarGoogle Scholar |
[2] Schuchat, A. (1998) Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin. Microbiol. Rev. 11, 497–513.
| 1:STN:280:DyaK1czjtlKksg%3D%3D&md5=bb7159bb61ed58cc272517378058a688CAS |
[3] Band, J.D. et al. (1981) Transmission of group B streptococci. Traced by use of multiple epidemiologic markers. Am. J. Dis. Children 135, 355–358.
| Transmission of group B streptococci. Traced by use of multiple epidemiologic markers.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M7lslWnsQ%3D%3D&md5=3bebc4846fadcc1264cc67be108300a1CAS |
[4] Verani, J.R. et al. (2010) Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR 59, 1–36.
[5] Stoll, B.J. and Schuchat, A. (1998) Maternal carriage of group B streptococci in developing countries. Pediatr. Infect. Dis. J. 17, 499–503.
| Maternal carriage of group B streptococci in developing countries.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1czhvVaktg%3D%3D&md5=9861cbc633f4d0bf790c433bccaecba9CAS |
[6] Ko, D.W. et al. (2015) Group B streptococcal disease and genotypes in Australian infants. J. Paediatr. Child Health 51, 808–814.
| Group B streptococcal disease and genotypes in Australian infants.Crossref | GoogleScholarGoogle Scholar |
[7] Chang, L.W. et al. (2015) Increasing clindamycin resistance among Australian group B streptococcus isolates. Intern. Med. J. 45, 465–466.
| Increasing clindamycin resistance among Australian group B streptococcus isolates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXlsFakt7c%3D&md5=f416967091173bb92863b0ab010ff2ceCAS |
[8] Haug, R.H. et al. (1981) Serotyping and bacteriophage typing of human and bovine group-B streptococci. J. Med. Microbiol. 14, 479–482.
| Serotyping and bacteriophage typing of human and bovine group-B streptococci.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL38%2Fnsl2ktg%3D%3D&md5=d0248810186a98d33bff62c3d56a50caCAS |
[9] Harrison, L.H. et al. (1998) Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. Maryland Emerging Infections Program. J. Infect. Dis. 177, 998–1002.
| Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. Maryland Emerging Infections Program.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7psVGqtQ%3D%3D&md5=dc7f953fb17eb47206fb9948f6f71a16CAS |
[10] Slotved, H.C. et al. (2007) Serotype IX, a proposed new Streptococcus agalactiae serotype. J. Clin. Microbiol. 45, 2929–2936.
| Serotype IX, a proposed new Streptococcus agalactiae serotype.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFemtLrF&md5=5a295d0f2229f7c833cba21c1d93cbc9CAS |
[11] Matsubara, K. et al. (2002) Seroepidemiologic studies of serotype VIII group B Streptococcus in Japan. J. Infect. Dis. 186, 855–858.
| Seroepidemiologic studies of serotype VIII group B Streptococcus in Japan.Crossref | GoogleScholarGoogle Scholar |
[12] Terakubo, S. et al. (2003) [Serotypes and antibody levels of group B streptococci in pregnant women]. Kansenshogaku zasshi. The Journal of the Japanese Association for Infectious Diseases 77, 121–126.
[13] Lachenauer, C.S. et al. (1999) Serotypes VI and VIII predominate among group B streptococci isolated from pregnant Japanese women. J. Infect. Dis. 179, 1030–1033.
| Serotypes VI and VIII predominate among group B streptococci isolated from pregnant Japanese women.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M7mvFWquw%3D%3D&md5=6f057998401a91a4b45e27d649c8b466CAS |
[14] Rajagopal, L. (2009) Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiol. 4, 201–221.
| Understanding the regulation of Group B Streptococcal virulence factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXislWrtrc%3D&md5=10f87afcab88a223f3607e2605952ad6CAS |
[15] Darbar, A.A. and Gilbert, G.L. (2007) Phenotypical and genotypical characteristics of invasive group B Streptococcus isolates in Western Sydney 2000–2005. Pathology 39, 589–593.
| Phenotypical and genotypical characteristics of invasive group B Streptococcus isolates in Western Sydney 2000–2005.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlWktrnJ&md5=bbc82f947e0c74f4e70d60f279be3c08CAS |
[16] Furfaro L.L. et al 2017 A novel one-step real-time multiplex PCR assay to detect Streptococcus agalactiae presence and serotypes Ia, Ib and III. Diagn. Microbiol. Infect. Dis.
[17] Imperi, M. et al. (2010) A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J. Microbiol. Methods 80, 212–214.
| A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVGls7g%3D&md5=f43af2eee7832ed8124fa16e62248977CAS |
[18] Payne, M.S. et al. (2016) Ureaplasma parvum genotype, combined vaginal colonisation with Candida albicans, and spontaneous preterm birth in an Australian cohort of pregnant women. BMC Pregnancy Childbirth 16, 312.
| Ureaplasma parvum genotype, combined vaginal colonisation with Candida albicans, and spontaneous preterm birth in an Australian cohort of pregnant women.Crossref | GoogleScholarGoogle Scholar |
[19] Zeng, X. et al. (2006) Simultaneous detection of nine antibiotic resistance-related genes in Streptococcus agalactiae using multiplex PCR and reverse line blot hybridization assay. Antimicrob. Agents Chemother. 50, 204–209.
| Simultaneous detection of nine antibiotic resistance-related genes in Streptococcus agalactiae using multiplex PCR and reverse line blot hybridization assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XivVGktA%3D%3D&md5=879145b217a1c0926df1d13a99e4210eCAS |
[20] Zhao, Z. et al. (2008) Distribution of genotypes and antibiotic resistance genes among invasive Streptococcus agalactiae (group B streptococcus) isolates from Australasian patients belonging to different age groups. Clin. Microbiol. Infect. 14, 260–267.
| Distribution of genotypes and antibiotic resistance genes among invasive Streptococcus agalactiae (group B streptococcus) isolates from Australasian patients belonging to different age groups.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c%2FpslSjsQ%3D%3D&md5=f947a645aeed69c8b6822bfab5a81fdfCAS |
[21] Grimwood, K. et al. (2002) Late antenatal carriage of group B Streptococcus by New Zealand women. Aust. N. Z. J. Obstet. Gynaecol. 42, 182–186.
| Late antenatal carriage of group B Streptococcus by New Zealand women.Crossref | GoogleScholarGoogle Scholar |
[22] Ellis, S. et al. (1996) Restriction endonuclease analysis of group B streptococcal isolates from two distinct geographical regions. J. Hosp. Infect. 33, 279–287.
| Restriction endonuclease analysis of group B streptococcal isolates from two distinct geographical regions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FhvVehtg%3D%3D&md5=28b66fb58dcdfc780806877988b1e17eCAS |
[23] Taylor, K. (2006) A study of group B streptococcus in Brisbane: the epidemiology, detection by PCR assay and serovar prevalence. Masters Thesis.
[24] Heath, P.T. (2016) Status of vaccine research and development of vaccines for GBS. Vaccine 34, 2876–2879.
| Status of vaccine research and development of vaccines for GBS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XksFGqu7s%3D&md5=f8c4d1bf08aa92e411b637d4ff0d7844CAS |
Biographies
Lucy Furfaro is a final year PhD candidate researching GBS dynamics in Western Australian pregnant women and a potential alternative treatment using bacteriophage therapy. She has developed a novel multiplex PCR assay to detect GBS and clinically relevant serotypes with the potential for diagnostic use.
Barbara Chang is a Professor within the School of Biomedical Sciences at the University of Western Australia, known for her expertise in molecular bacteriology and bacteriophage research.
Matthew Payne is a Research Fellow within the School of Medicine at the University of Western Australia, and a highly experienced molecular microbiologist with expertise in perinatal microbiology.


