Aquaculture: exotic diseases and surveillance
J Brian JonesInvestigation and Diagnostic Centres and Response
Ministry for Primary Industries
PO Box 40742
Upper Hutt, New Zealand
Tel: +64 4 894 5628
Email: brian.jones@mpi.govt.nz
Microbiology Australia 37(3) 124-125 https://doi.org/10.1071/MA16042
Published: 10 August 2016
Aquaculture is a rapidly growing global industry. Half of all seafood is sourced from aquaculture and Australia is part of the trend. A major emerging threat to this industry is disease.
Australian aquaculture production in 2012–13 was valued at >$1 billion with farmed salmonids alone contributing $497 million1. However, although only six species (pearl oysters (Pinctada maxima), Atlantic salmon (Salmo salar), Pacific oysters (Crassostrea gigas), prawns (Penaeus spp.) and southern Bluefin tuna (Thunnus maccoyii)) account for 90% of the production, there are some 40 species under cultivation. A characteristic of Australian aquaculture is that, with a few exceptions, all of the species under cultivation are Australian native animals, the main exceptions being salmonids, introduced from Europe in the 1860s2,3 and Pacific oysters (Crassostrea gigas) introduced4 between 1947 and 1970. Farming native species provides two unique challenges. First, for most of the species under cultivation there is no previous aquaculture experience, and second, as culture intensifies, diseases that are unique to Australia are emerging as a threat to production. Adding to the mix are those disease agents that have either been accidentally introduced and are emerging as a threat to native species or those diseases still offshore that pose a threat to Australian flora and fauna. Because of the intensification of both aquaculture and global trade, diseases are now spreading at a faster rate than regulatory process can respond. This spread has been exacerbated by inadequate biosecurity measures on many farms, though that is changing slowly5,6.
An example of the slow regulatory response is provided by koi herpesvirus. This highly contagious virus affects only common carp (Cyprinus carpio) and carp hybrids. Affected fish die between 24 and 48 hours after the initial onset of gill lesions and mortality may exceed 90%7. Survivors can act as carriers. Common carp are raised as food in many countries and koi carp are a component of the ornamental fish trade. First identified in fish farms in Israel in 1998, the disease spread globally for about 8 years before the World Organisation for Animal Health added koi herpesvirus to the list of internationally notifiable diseases. Australia has remained free of this disease due to the prohibition on importing carp, but research is underway to release the virus in an attempt to control invasive feral carp8.
The detection of potentially exotic diseases in Australian aquaculture farms is facilitated by surveillance, of which there are two types: passive surveillance, which relies on detection of disease signs on farm and a prompt robust system to acquire a diagnosis; and targeted surveillance. Targeted surveillance is intelligence-led and risk based – looking for specific diseases of concern which may establish in specified high risk areas. An Australian example that illustrates both types of sampling is provided by White Spot Syndrome Virus of crustaceans. The disease is exotic to Australia but was detected by passive surveillance (through investigating mortalities) at a hatchery in Darwin. The hatchery was destocked and a nationwide targeted surveillance program was instituted, sampling aquaria and hatcheries where imported frozen prawns (the source of the infection) might have been fed to crustacean brood stock or wild populations. The survey results were negative, allowing Australia to retain its free status9.
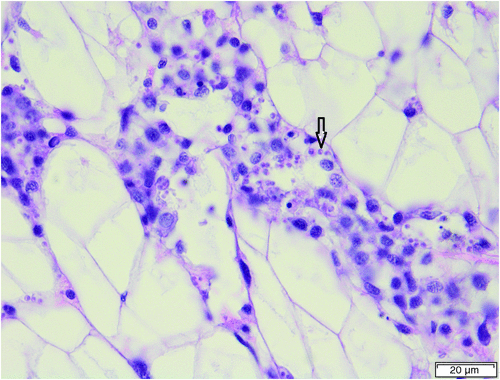
A more complex example is provided by studies of the molluscan parasites informally grouped as ‘microcells’ because of their small size (about 2 microns). The genera Microcytos and Bonamia are relatively easy to detect by histology but species determination is much more problematic. It was by histology and transmission electron microscopy that Bonamia exitiosa with cells of 2–5 µm was found in New Zealand Foveaux Strait oysters (Ostrea chilensis) in 1986. A related parasite, Mikrocytos roughleyi later renamed Bonamia roughleyi was described from Saccostrea glomerata in southeastern Australia in 198810. Unlike B. exitiosa it causes lesions in the host and has smaller cells of 1–3 µm. Subsequently Bonamia sp. a molluscan parasite of Australian flat oysters (Ostreiidae) was reported from Australia in 199111. It has cells of the same size (2–5 µm) as the New Zealand B. exitiosa but there are minor differences in morphology, ultrastructure and histopathology between the New Zealand and Australian microcells12. However, DNA sequencing has shown that, despite the differences, Bonamia sp. and Bonamia exitiosa are both members of a B. exitiosa clade, and that B. roughleyi is a nomen dubium13. Thus, Bonamia exitiosa is no longer regarded as an exotic disease in Australia (Figure 1).
|
Our understanding of pathogens themselves is also changing. Now that the DNA of disease agents can be sequenced it is much easier to not only detect incursions but also the genes that confer virulence. This adds a new layer of complexity on surveillance, since it’s not just the organism that must be detected, but the arrival of more virulent forms of a disease perhaps already well established but tolerated. Herpesvirus-like viruses, associated with mortalities in bivalve hatcheries and detected by histology and transmission electron microscopy in bivalve shellfish, had been recorded in New Zealand, USA, Europe, and in Western Australia in the 1990s14. Subsequently, a micro-variant strain of a herpesvirus (named OSHV-1) emerged in France in 2008 that caused high mortalities only in Crassostrea gigas15. The relationship between the micro-variant strain now detectable by PCR and the herpesvirus-like virus seen in earlier studies by TEM has never been established. The micro-variant form of OSHV-1 spread through European oyster farms, appeared in New Zealand in 2010 and then a few months later in the Georges River, NSW in November 201016. Identification of the herpesvirus by molecular methods, and identification of the characteristic deletions in the genome, confirmed that it was the microvariant strain that was causing the deaths16. A strict imposition of biosecurity controls by the NSW government appeared to limit the geographical spread of infection, but in 2016 the microvariant was also detected in Tasmania following mortalities.
In response to the growing threats posed by emerging diseases, attention is moving from a reliance on country border protection to a much greater emphasis on farm biosecurity. This is accompanied by a greatly increased awareness of the need for both passive and active surveillance, not only at the state level, but also at the level of the individual farm.
References
[1] Department of Agriculture Forests and Fisheries (2016) Aquaculture industry in Australia. http://www.agriculture.gov.au/fisheries/aquaculture/aquaculture-industry-in-australia[2] Clements, J. (1988) Salmon at the antipodes: a history and review of trout, salmon and char and introduced coarse fish in Australasia.
[3] Ovenden, J.R. et al. (1993) Mitochondrial DNA nucleotide sequence variation in Atlantic salmon (Salmo salar), brown trout (S. trutta), rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis) from Tasmania, Australia. Aquaculture 114, 217–227.
| Mitochondrial DNA nucleotide sequence variation in Atlantic salmon (Salmo salar), brown trout (S. trutta), rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis) from Tasmania, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXlslOrurg%3D&md5=f49ef374851ef898cda5910695a9f5cdCAS |
[4] Nell, J.A. (2001) The history of oyster farming in Australia. Mar. Fish. Rev. 63, 14–25.
[5] Perera, R.P. et al. (2008) Maintaining biosecurity in aquaculture systems: a constraint or a challenge. In Diseases in Asian Aquaculture VI (Bondad-Reantaso, M.G. et al., eds), pp. 3–20, Fish Health Section, Asian Fisheries Society, Manila, Philippines.
[6] Farm Biosecurity (2013) Nothing fishy about biosecurity for aquaculture business. http://www.farmbiosecurity.com.au/nothing-fishy-about-biosecurity-for-aquaculture-business/
[7] Pokorova, D. et al. (2005) Current knowledge on koi herpesvirus (KHV): a review. Vet. Med. Czech. 50, 139–147.
[8] McColl, K. (2016) Cyprinid herpesvirus-3 (CyHV-3) as a biological control agent for carp in Australia. Invasive Animals CRC Research Programs 2012–2017. http://www.invasiveanimals.com/research/phase2/inland-waters/cyhv-3-as-a-biological-control-for-carp/
[9] East, I.J. et al. (2004) Survey for the presence of White Spot Syndrome virus in Australian crustaceans. Aust. Vet. J. 82, 236–240.
| Survey for the presence of White Spot Syndrome virus in Australian crustaceans.Crossref | GoogleScholarGoogle Scholar |
[10] Farley, C.A. et al. (1988) A long-term study of ‘microcell’ disease in oysters with a description of new genus Mikrocytos (g.n.), and two new species, Mikrocytos mackini (sp.n.) and Mikrocytos roughleyi (sp. n.). Fish. Bull. (Wash. D. C.) 86, 581–593.
[11] Hine, P.M. and Jones, J.B. (1994) Bonamia and other aquatic parasites of importance to New Zealand. N.Z. J. Zool. 21, 49–56.
| Bonamia and other aquatic parasites of importance to New Zealand.Crossref | GoogleScholarGoogle Scholar |
[12] Corbeil, S. et al. (2009) Bonamiasis in Australian Ostrea angasi. ANZSDP, SCAHLS.
[13] Carnegie, R.B. et al. (2014) The haplosporidian Bonamia exitiosa is present in Australia, but the identity of the parasite described as Bonamia (formerly Mikrocytos) roughleyi is uncertain. J. Invertebr. Pathol. 115, 33–40.
| The haplosporidian Bonamia exitiosa is present in Australia, but the identity of the parasite described as Bonamia (formerly Mikrocytos) roughleyi is uncertain.Crossref | GoogleScholarGoogle Scholar | 24211185PubMed |
[14] Hine, P.M. and Thorne, T. (1997) Replication of herpes-like viruses in haemocytes of adult flat oysters Ostrea angasi: an ultrastructural study. Dis. Aquat. Organ. 29, 189–196.
| Replication of herpes-like viruses in haemocytes of adult flat oysters Ostrea angasi: an ultrastructural study.Crossref | GoogleScholarGoogle Scholar |
[15] Segarra, A. et al. (2010) Detection and description of a particular Ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008. Virus Res. 153, 92–99.
| Detection and description of a particular Ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFamsrrM&md5=d043b0babb72a2df482aac1021376e1aCAS | 20638433PubMed |
[16] Jenkins, C. et al. (2013) Identification and characterisation of an ostreid herpesvirus-1 microvariant (OsHV-1 µ-var) in Crassostrea gigas (Pacific oysters) in Australia. Dis. Aquat. Organ 105, 109–126.
| 1:CAS:528:DC%2BC3sXhsFynsbvL&md5=70296bbb94ee5b1c073ed317d9e7f76cCAS | 23872855PubMed |
Biography
Brian Jones was formerly Principal Fish Pathologist for Fisheries Western Australia and is currently Adjunct Professor at Murdoch University, Western Australia, and also Principal Advisor Aquatic Animal Health at the Ministry for Primary Industries in New Zealand.


