Diagnosis of human taeniasis
Abdul Jabbar , Charles Gauci and Marshall W LightowlersFaculty of Veterinary and Agricultural Sciences
The University of Melbourne
Werribee, Vic. 3030, Australia
Email: jabbara@unimelb.edu.au
Microbiology Australia 37(1) 43-45 https://doi.org/10.1071/MA16011
Published: 11 February 2016
Taenia solium, T. saginata and T. asiatica are taeniid tapeworms that cause taeniasis in humans and cysticercosis in intermediate host animals. T. solium can also cause cysticercosis in humans. A number of diagnostic methods have been developed to diagnose Taenia species that infect humans. This article is aimed at providing an overview of currently available diagnostic methods for human taeniasis.
Human taeniasis is an important zoonotic health problem and is caused by adult stages of taeniid cestodes, including Taenia solium, T. saginata, and T. asiatica. These parasites have indirect life cycles where humans act as definitive hosts whereas pigs (for T. solium and T. asiatica) and cattle (T. saginata) serve as intermediate hosts. Humans become infected by ingesting parasite cysts present in raw or undercooked infected meat/liver; when the cyst reaches the human intestine, it develops into an adult tapeworm, releasing segments and/or eggs in the stools or motile segments (e.g. T. saginata) are expelled actively. Intermediate hosts such as cattle and pigs become infected when they ingest taeniid eggs via contaminated feed or water and the larval stage (cysticercus) forms in muscular and sometimes other tissues1. The adult tapeworm stage of Taenia spp. is relatively innocuous and does not cause pathogenic effects in humans; however, the intermediate stage of T. solium can also develop in human brains causing neurocysticercosis, a major cause of neurological disease in many developing countries as well as other organs causing intramuscular, ocular, subcutaneous and spinal cysticercoses2,3. T. saginata is endemic in Australia, whereas T. solium and T. asiatica are exotic. However, people coming and/or returning to Australia from endemic countries can be infected with T. solium, leading to the possibility that infected individuals may pass segments of the parasite in their stools, which can serve as a source of infection for human cysticercosis. Therefore, differentiation of Taenia species becomes significant for surveillance and control of human taeniasis.
A number of diagnostic methods have been used to differentiate the common human cestodes, T. saginata and T. solium; however, each method has its advantages and disadvantages, and careful attention should be paid to determining which particular test is best to use for differentiation of the two species4. The following sections provide a quick rundown on various diagnostic methods available to differentiate Taenia spp. that infect humans.
Microscopic diagnosis
Traditionally, diagnosis of taeniasis has been based on the detection of eggs by microscopic examination but this method lacks sensitivity and specificity as T. solium and T. saginata eggs are morphologically identical, making species identification impossible5. However, morphological examination of gravid proglottids can allow the differentiation of T. solium and T. saginata provided the internal structures (i.e. uterine branches) are intact5. Sometimes, even the morphological examination of proglottids does not allow the differentiation of T. solium and T. saginata, thus requiring alternate methods for the differentiation of human Taenia spp.
Immunodiagnosis
The first method of coproantigen (parasite antigens in human stool) detection using enzyme-linked immunosorbent assay (ELISA) was developed by Allan et al.6. Although the test displayed a higher sensitivity and specificity than microscopic diagnosis for the detection of Taenia spp.7, it did not allow differentiation of T. saginata and T. solium. Recently, Guezala et al.8 developed another coproantigen ELISA and successfully differentiated T. solium from T. saginata. These tests were developed in individual labs using in-house reagents and have not been independently validated in different laboratories nor have they been widely used in diagnostic laboratories.
The first serological assay to detect specific antibodies against T. solium infection in humans was developed by Wilkins et al.9 Subsequently, a number of studies reported various immunoassays for the diagnosis of human taeniasis, primarily caused by T. solium10,11 and these studies used either native excretory-secretory products collected from adult tapeworms9 or cloned and expressed excretory-secretory products of adult T. solium10,11. Like coproantigen ELISAs, the detection of Taenia species-specific antibodies are also more specific and sensitive than microscopic techniques. However, these tests have been found to have some degree of cross-reactivity in sera from patients with cystic echinococcosis, ascariasis, and schistosomiasis11. Furthermore, currently available immunodiagnostic tests may give false positive results as specific circulating antibodies in taeniasis patients could possibly remain detectable for some time either after treatment and recent past infections4.
Molecular diagnosis
A number of molecular methods using PCR-based technologies have been developed to either determine the presence of Taenia species-specific DNA in human stools or differentiate Taenia spp. (T. solium, T. saginata or T. asiatica) based on the analysis of DNA extracted directly from tapeworm12. PCR-based methods have higher sensitivity in the detection of taeniasis cases (i.e. the detection of parasite DNA in human stools) than microscopy alone. In addition, a combined use of PCR and microscopy have been found to improve diagnostic sensitivity where Yamasaki et al.13 showed that some proven egg-positive cases were negative by PCR. Specificity of PCR is high with control faecal samples, including samples from patients with other parasitic infections, being almost always negative in PCR.
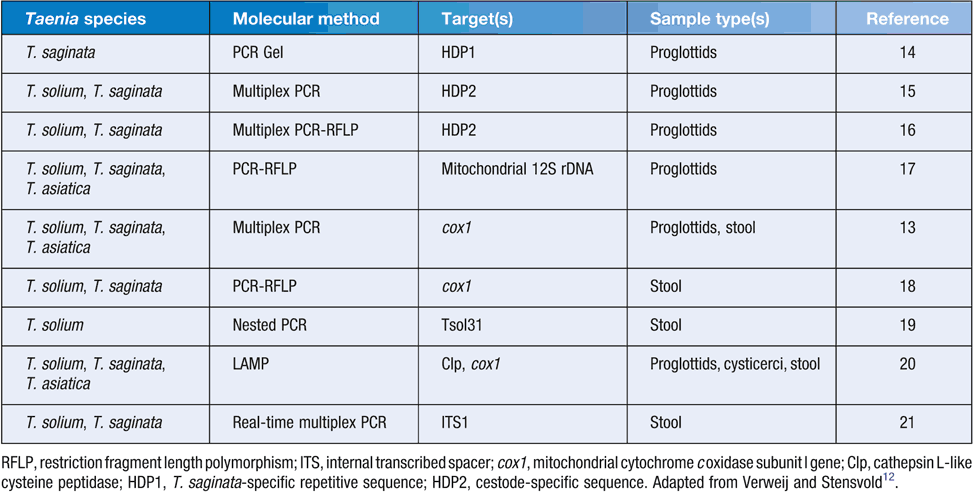
To date, predominantly conventional and multiplex PCR, and PCR-restriction fragment length polymorphism (PCR-RFLP) have utilized various markers, including internal transcribed spacer, mitochondrial cytochrome c oxidase subunit I gene as well as 12S rDNA, cathepsin L-like cysteine peptidase, T. saginata-specific repetitive sequence (HDP1) and cestode-specific sequence (HDP2) to discriminate between human Taenia spp13–21 (Table 1). Mayta et al.19 developed a nested PCR utilizing two rounds of PCR amplification of the Tso31 gene, which is more sensitive than conventional PCR but pose technical difficulties. A field DNA-based test known as loop mediated isothermal amplification (LAMP) was developed by Nkouawa et al.20, which amplifies the cox1 gene. To date, only one real-time PCR to discriminate T. solium and T. saginata has been developed which targets the internal transcribed spacer 1 of the nuclear ribosomal RNA21. The majority of DNA-based methods have utilised DNA isolated from proglottids for Taenia species identification while relatively fewer studies isolated DNA from stool samples (Table 1). In addition, almost all studies have been tested only on small numbers of usually known positive and negative samples.
|
Conclusions and future perspectives
Human taeniasis is a worldwide parasitic disease and detection and discrimination of T. solium, T. saginata and T. asiatica remains a public health concern. Microscopic, immunological and molecular methods have been used to detect and differentiate Taenia spp., and a combination of two or more methods appears to provide higher sensitivity13,21. Almost all of the immunological and molecular methods developed so far have not been independently tested and validated on controlled negative and positive samples as well as field samples. Among existing molecular methods, the nested PCR19 provides the highest sensitivity and specificity of those methods that have been developed and validated using unselected faecal samples from parasitological proven taeniasis carriers. Although the method is technically challenging and could be expected to be relatively expensive to use, the reagents required are available worldwide and laboratories capable of undertaking PCR competently can run this PCR4.
To the best of our knowledge, no standardised PCRs are available in Australia to discriminate human Taenia spp. Future studies should focus on independent validation of the nested PCR19 and the LAMP technique20 as these two DNA-based tests offer higher sensitivity and a user-friendly option without sophisticated equipment, respectively. In addition, future studies should be aimed at extracting DNA from sodium acetate-acetic acid-formalin (SAF) fixed proglottids using different methods as the current DNA extraction protocols do not provide reliable and consistent DNA yield for PCRs (Jabbar, unpublished data). To date only one study has reported the extraction of DNA from long-term stored Taenia specimens in formalin22 and this has not been independently verified.
References
[1] Roberts, L.S. et al. (2013) Foundations of Parasitology, 9th edn. McGraw-Hill Education, USA.[2] Mahanty, S. et al. (2010) Cysticercosis and neurocysticercosis as pathogens affecting the nervous system. Prog. Neurobiol. 91, 172–184.
| Cysticercosis and neurocysticercosis as pathogens affecting the nervous system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltF2qsbY%3D&md5=3fe75aa0c690c08f17b9c6250e372d13CAS | 20035822PubMed |
[3] García, H.H. et al. (2003) Taenia solium cysticercosis. Lancet 362, 547–556.
| Taenia solium cysticercosis.Crossref | GoogleScholarGoogle Scholar | 12932389PubMed |
[4] Lightowlers, M.W. et al. (2015) Monitoring the outcomes of interventions against Taenia solium: option and suggestion. Parasite Immunol. , .
| Monitoring the outcomes of interventions against Taenia solium: option and suggestion.Crossref | GoogleScholarGoogle Scholar | 26538513PubMed |
[5] Chapman, A. et al. (1995) Isolation and characterization of species-specific DNA probes from Taenia solium and Taenia saginata and their use in an egg detection assay. J. Clin. Microbiol. 99, 1283–1288.
[6] Allan, J.C. et al. (1990) Immunodiagnosis of taeniasis by coproantigen detection. Parasitology 101, 473–477.
| Immunodiagnosis of taeniasis by coproantigen detection.Crossref | GoogleScholarGoogle Scholar | 2092303PubMed |
[7] Allan, J.C. et al. (1996) Field trial of the coproantigen-based diagnosis of Taenia solium taeniasis by enzymelinked immunosorbent assay. Am. J. Trop. Med. Hyg. 54, 352–356.
| 1:STN:280:DyaK283gvVSguw%3D%3D&md5=f7db0d91f04b9281e27263cb8d799fe2CAS | 8615446PubMed |
[8] Guezala, M.C. et al. (2009) Development of a species-specific coproantigen ELISA for human Taenia solium taeniasis. Am. J. Trop. Med. Hyg. 81, 433–437.
| 1:CAS:528:DC%2BD1MXhtFequrrK&md5=8f2b9b0b80a24638be5ede401aee781bCAS | 19706909PubMed |
[9] Wilkins, P.P. et al. (1999) Development of a serologic assay to detect Taenia solium taeniasis. Am. J. Trop. Med. Hyg. 60, 199–204.
| 1:CAS:528:DyaK1MXhvFamsro%3D&md5=18b4dd0cadb56755a5ab7821b59f779bCAS | 10072136PubMed |
[10] Levine, M.Z. et al. (2004) Characterization, cloning, and expression of two diagnostic antigens for Taenia solium tapeworm infection. J. Parasitol. 90, 631–638.
| Characterization, cloning, and expression of two diagnostic antigens for Taenia solium tapeworm infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlvFOls7k%3D&md5=5d03d66a5b1f3762fb9b433a04f843bfCAS | 15270112PubMed |
[11] Levine, M.Z. et al. (2007) Development of an enzyme-linked immunoelectrotransfer blot (EITB) assay using two baculovirus expressed recombinant antigens for diagnosis of Taenia solium taeniasis. J. Parasitol. 93, 409–417.
| Development of an enzyme-linked immunoelectrotransfer blot (EITB) assay using two baculovirus expressed recombinant antigens for diagnosis of Taenia solium taeniasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmt1emtr0%3D&md5=3c79a1a6372eb5b23c767774e7357bf5CAS | 17539427PubMed |
[12] Verweij, J.J. and Stensvold, C.R. (2014) Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clin. Microbiol. Rev. 27, 371–418.
| Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections.Crossref | GoogleScholarGoogle Scholar | 24696439PubMed |
[13] Yamasaki, H. et al. (2003) Cysticercosis/taeniasis: recent advances in serological and molecular diagnoses. Southeast Asian J. Trop. Med. Public Health 34, 98–102.
| 1:CAS:528:DC%2BD2cXotl2rsw%3D%3D&md5=6b381deca0e5940d28516c74c4680ad8CAS | 19230578PubMed |
[14] Gonzalez, L.M. (2000) Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. J. Clin. Microbiol. 38, 737–744.
| 1:CAS:528:DC%2BD3cXht1ejsbg%3D&md5=68f418c0d2f7dddc49332b7477640589CAS | 10655377PubMed |
[15] González, L.M. et al. (2002)a Differential diagnosis of Taenia saginata and Taenia solium infections: from DNA probes to polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 96, S243–S250.
| Differential diagnosis of Taenia saginata and Taenia solium infections: from DNA probes to polymerase chain reaction.Crossref | GoogleScholarGoogle Scholar | 12055846PubMed |
[16] González, L.M. et al. (2002)b PCR tools for the differential diagnosis of Taenia saginata and Taenia solium taeniasis/cysticercosis from different geographical locations. Diagn. Microbiol. Infect. Dis. 42, 243–249.
| PCR tools for the differential diagnosis of Taenia saginata and Taenia solium taeniasis/cysticercosis from different geographical locations.Crossref | GoogleScholarGoogle Scholar | 12007441PubMed |
[17] Rodriguez-Hidalgo, R. et al. (2002) Comparison of conventional techniques to differentiate between Taenia solium and Taenia saginata and an improved polymerase chain reaction-restriction fragment length polymorphism assay using a mitochondrial 12S rDNA fragment. J. Parasitol. 88, 1007–1011.
| Comparison of conventional techniques to differentiate between Taenia solium and Taenia saginata and an improved polymerase chain reaction-restriction fragment length polymorphism assay using a mitochondrial 12S rDNA fragment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XptVOjt7c%3D&md5=8f647b601a3f0ed87f7a15dfd176a141CAS | 12435145PubMed |
[18] Nunes, C.M. et al. (2005) Taenia saginata: differential diagnosis of human taeniasis by polymerase chain reaction-restriction fragment length polymorphism assay. Exp. Parasitol. 110, 412–415.
| Taenia saginata: differential diagnosis of human taeniasis by polymerase chain reaction-restriction fragment length polymorphism assay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlt1Gqur8%3D&md5=87246379128cc5cf6dba5675f0d66447CAS | 15882866PubMed |
[19] Mayta, H. et al. (2008) Nested PCR for specific diagnosis of Taenia solium taeniasis. J. Clin. Microbiol. 46, 286–289.
| Nested PCR for specific diagnosis of Taenia solium taeniasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhs1aquro%3D&md5=0cbfe95aa95181d61b8f502a25ee7f30CAS | 17989190PubMed |
[20] Nkouawa, A. et al. (2009) Loop-mediated isothermal amplification method for differentiation and rapid detection of Taenia species. J. Clin. Microbiol. 47, 168–174.
| Loop-mediated isothermal amplification method for differentiation and rapid detection of Taenia species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsFaru7g%3D&md5=274329592ebe2a754240bcbe089c9926CAS | 19005142PubMed |
[21] Praet, N. et al. (2013) Bayesian modelling to estimate the test characteristics of coprology, coproantigen ELISA and a novel real-time PCR for the diagnosis of taeniasis. Trop. Med. Int. Health 18, 608–614.
| Bayesian modelling to estimate the test characteristics of coprology, coproantigen ELISA and a novel real-time PCR for the diagnosis of taeniasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlvVegtr0%3D&md5=52b368350e6c4fc7c8e4e4c4fda3207cCAS | 23464616PubMed |
[22] Jeon, H.-K. et al. (2011) Molecular identification of Taenia specimens after long-term preservation in formalin. Parasitol. Int. 60, 203–205.
| Molecular identification of Taenia specimens after long-term preservation in formalin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFSqs70%3D&md5=bee95f92e4e90ff665990b7f99457bb0CAS | 21163367PubMed |
Biographies
Dr Abdul Jabbar is a Senior Lecturer in Veterinary Parasitology at The University of Melbourne. His main research interests cover epidemiology and diagnosis of parasites of socioeconomic importance using next-generation molecular tools.
Dr Charles Gauci is a Senior Research Fellow at The University of Melbourne. He has worked with Prof Marshall Lightowlers throughout his career and his research interests focus on recombinant vaccines for prevention of transmission of the parasite causing neurocysticercosis and the related parasite that causes hydatid disease.
Professor Marshall W Lightowlers has been a full-time research scientist supported by medical research funding for more-or-less all of his working life. He currently holds appointments as Laureate Professor at the University of Melbourne’s Faculty of Veterinary and?Agricultural Sciences, and Principal Research Fellow with the NHMRC.


