Diagnosis of congenital syphilis and toxoplasmosis
C. R. Robert GeorgeDepartment of Microbiology
SEALS, NSW Health Pathology
The Prince of Wales Hospital
Randwick, NSW 2031, Australia
Tel: +61 2 9382 9054
Fax: +61 2 9382 9098
Email: Robert.George@sesiahs.health.nsw.gov.au
Microbiology Australia 36(4) 184-189 https://doi.org/10.1071/MA15065
Published: 28 October 2015
Syphilis, toxoplasmosis, and cytomegalovirus represent disparate entities. The bacterial spirochaete Treponema pallidum ssp. pallidum causes syphilis, the ‘The Great Imitator’; the organism’s sole natural host is humans and it remains exquisitely sensitive to penicillin. By contrast, the zoonotic parasite Toxoplasma gondii causes toxoplasmosis. Infection is usually self-limited, although serious disease can occur in the immunocompromised. Meanwhile, the human cytomegalovirus (CMV; human herpesvirus 5) is a relatively prevalent enveloped DNA betaherpesvirus with infection specific to humans. Despite nomenclatural, ecological and therapeutic disparities, however, these agents exhibit several concordances, including various, and at times, cryptic syndromes in child and often mother; congenital infections with potentially devastating outcomes; diagnostic dilemmas. This article primarily discusses the latter of these issues in relationship to congenital syphilis and toxoplasmosis in the Australian context.
Syphilis
The number of cases of congenital syphilis has fallen in Australia over the past two decades (1995–2004: 146 cases; 2005–2014: 64 cases)1. However, there has been a recent resurgence of syphilis particularly amongst men who have sex with men2, with the overall incidence of infectious syphilis of less than 2 years duration more than doubling (2004–2006: mean 3.5/100 000; 2012–2014: mean 7.7/100 000)1. Additionally, the burden of disease in indigenous populations is well recognised, and it is possible that fly-in fly-out workers could transmit infections3. In the United States and United Kingdom re-emergence is also underway, and congenital infections have been linked particularly to primary and secondary syphilis in females4–6. Given the potential for further spread in Australia, ongoing vigilance is required.
Vertical transmission is highest in primary and secondary syphilis, continuing throughout pregnancy while the severity of outcomes decreases7. Adverse outcomes can include stillbirth or miscarriage, perinatal death, prematurity, low birthweight, and a series of early congenital manifestations (e.g. snuffles, hepatosplenomegaly, generalised lymphadenopathy, bony lesions) and late congenital manifestations (typically due to chronic granulomatous inflammation)4,8,9.
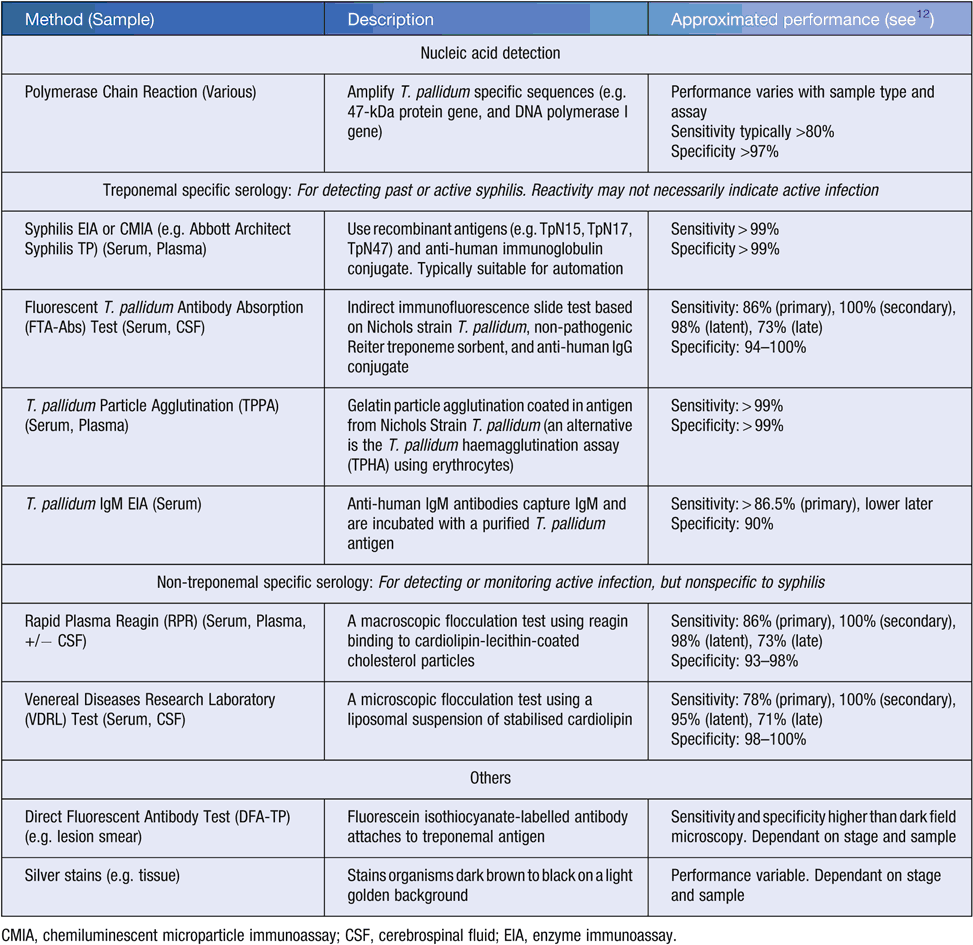
In Australia, universal screening for syphilis is recommended in pregnancy, with a treponemal-specific serological assay performed at the first antenatal visit, and repeat screening in high-risk populations should be considered at 28 weeks10,11. Modalities available for diagnosing congenital syphilis in Australia include both treponemal-specific and non-treponemal specific serology, nucleic acid detection, direct fluorescent antibody testing, and histochemical staining (e.g. Warthin-Starry silver stain) (Table 1); methods of historical interest include dark field microscopy and in vivo culture using rabbit testes. Test selection and interpretation is complicated by the variety of tests available, with performance characteristics varying with disease progression. The Public Health Laboratory Network (PHLN) laboratory case definitions provide definitive and suggestive criteria for congenital, acquired active, and acquired active re-infective syphilis12; these criteria define testing strategies and complement the ‘Syphilis (congenital) case definition’ published by the Communicable Diseases Network Australia13.
|
It is beyond the scope of this article to detail the interpretation and implications of the gamut of serological profiles potentially observed in maternal and congenital syphilis. Instead, the reader is referred to diagnostic algorithms and case definitions published elsewhere (e.g.12–14). When investigating potential congenital syphilis, areas of consideration include:
-
Undertaking a rigorous history including exposure history, treatment history and its adequacy, and assessment of factors that pre-dispose to false positive and negative results such as pregnancy and auto-immune disease.
-
Undertaking a rigorous examination of mother and infant.
-
Reviewing maternal and newborn serology. For example, assessing maternal or newborn syphilis titres for significant increases, or for newborn titres that are 4-fold greater than maternal titres.
-
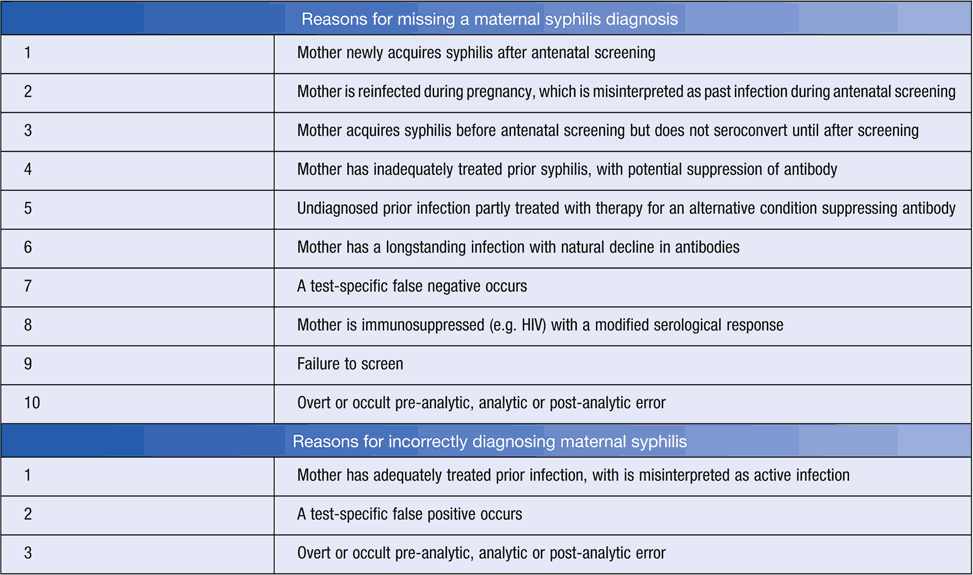
Awareness of the performance of non-treponemal serological tests (see Table 1), and reasons for misleading results (Table 2). For example, have false positives (e.g. biological) and false negatives (e.g. potentially methodological such as prozone15, or biological such as natural titre decline irrespective of therapy) been considered? Were separate serum specimens tested in parallel given inter-observer and inter-laboratory variation in endpoint determination12?
-
Awareness of potential weaknesses in treponemal-specific serological tests (see Table 1) and reasons for misleading results (Table 2). For example, has a false negative in a very recent exposure (i.e. before seroconversion) been considered? Such consideration is particularly warranted when history identifies possible exposure to syphilis (e.g. an infected partner), or examination reveals a chancre-like lesion.
-
Awareness of sample selection. As an example, umbilical cord blood samples may be less preferable than infant serum given maternal blood contamination could incorrectly suggest congenital infection16.
-
Awareness of sample management. As an example, recognition that seroreversion can occur with inappropriate storage conditions or freeze–thaw cycling (e.g. after repeated parallel testing)17.
-
Awareness of the utility of nucleic acid testing (NAT) testing. For example, confirmation of diagnosis may be achieved using paraffin-embedded tissue samples12.
-
Adequate follow-up. For example, testing of an infant might be considered if maternal diagnosis of syphilis occurs within one year of delivery18.
|
Despite such considerations and well characterised testing pathways and definitions, and universally recommended testing, numerous factors could contribute to missed or incorrect diagnoses (Table 2). Suspicious or discordant cases may be evaluated through reviewing patient history, retesting (potentially with an alternate assay) and comparing results. Expert advice should be sought when interpreting discordant results in relationship to interpretive criteria. Additionally, all diagnoses of maternal or congenital syphilis require notification in Australia.
Toxoplasmosis
Like congenital syphilis, congenital toxoplasmosis is believed to be uncommon in Australia. Being non-notifiable, diagnostic records are not collated, and epidemiological analysis is challenging. Between 2001 and 2009, incidence rates of T. gondii based on seropositivity analysis were estimated at 0.017 per 1000 live births in New South Wales19. International estimates vary considerably20, dependant on disease burden and methodology. While possibly fewer than 20 symptomatic congenital cases are diagnosed in Australia annually, the disease burden remains poorly understood particularly amongst indigenous and remote communities.
Disease occurs after maternal exposure to T. gondii via, for example, consumption of undercooked meat, or ingestion of oocyst contaminated soil and water21,22. The frequency of vertical transmission after exposure increases as pregnancy proceeds, while disease severity typically declines23. Although most cases are subclinical, manifestations can be broad-ranging with sequelae unrecognised for decades23. Outcomes may include spontaneous abortion, still birth or prematurity, or manifestations such as hydrocephalus, chorioretinitis, intracranial calcifications, jaundice, hepatomegaly, thrombocytopenia, cerebral spinal fluid abnormalities, and motor abnormalities23,24.
The role of screening for toxoplasmosis has long been debated (e.g.25). The low incidence of toxoplasmosis in Australia translates to a low pre-test probability of infection. Combined with issues of imperfect testing and the risks associated with potentially invasive confirmatory tests, routine antenatal screening for T. gondii infection is not recommended. Instead, pregnant women are presumed non-immune and advised to routinely avoid risk activities, although evidence supporting prenatal education as a method of preventing congenital toxoplasmosis remains sparse26. Similar approaches are recommended in other low prevalence countries (e.g. Canada27). Conversely, higher prevalence countries such as Denmark, France, Italy and Germany promote screening for congenital toxoplasmosis28. In Australia, selective testing is only recommended in women at ‘substantially increased risk of infection’10, although evidence based criteria are not available. Women whose history and examination indicates a substantially increased risk of infection, including those who are symptomatic, should be tested.
When required, diagnosis frequently relies on the interpretation of serological profiles. A typical approach might include assessment of IgG and IgM reactivity (e.g.14,29), whereby:
-
IgM non-reactivity with IgG non-reactivity usually indicates no past or recent infection;
-
IgM non-reactivity with IgG reactivity usually indicates past infection; and
-
IgM reactivity with IgG reactivity or non-reactivity could indicate recent infection, prolonged IgM persistence or a falsely positive IgM.
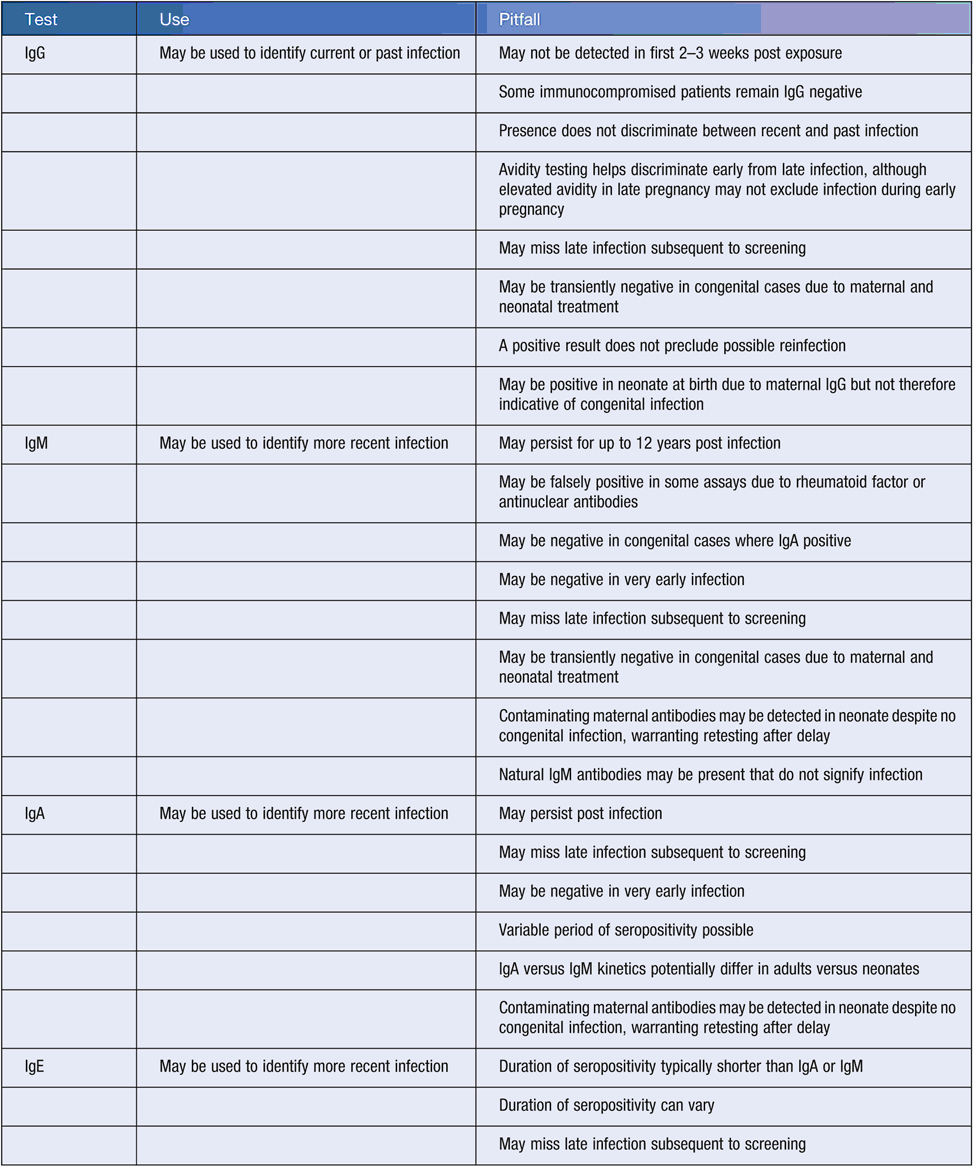
Numerous pitfalls are associated with the interpretation of Toxoplasma serology (Table 3). Additionally, no unified Australian case definition for human toxoplasmosis exists, although definitions are available elsewhere32,33. Key considerations when diagnosing maternal toxoplasmosis include:
|
-
Differentiating uninfected cases from those with IgM reactivity (e.g. when IgG is non-reactive and IgM reactive). Consider testing stored prenatal samples (if available), repeat testing after two weeks to identify seroconversion indicative of recent infection, and retesting the same specimen with an alternative IgM assay. If the IgG remains non-reactive, revisit possible reasons for the reactive IgM (Table 3).
-
Differentiating recent infections from past infections (e.g. when both IgG and IgM are reactive). Approaches include IgG avidity testing34, and retesting the same specimen using a different IgM assay. Antenatal bloods may be retrospectively tested for IgM and IgG reactivity, and avidity. Confirmatory testing in low IgG avidity cases suggestive of recent infection may be via amniocentesis and NAT. Amniocentesis is not completely without risk35, and is not normally recommended in HIV positive patients30. However, it is associated with lower rates of pregnancy loss and has better diagnostic performance versus demonstration of fetal immunoglobulin by cordocentesis (e.g.36). In high IgG avidity and/or IgM non-reactive cases suggestive of probable past infection, further investigation depends on gestation and exposure history.
When assessing for suspected congenital toxoplasmosis in a newborn, clinicians should perform a full clinical examination including ophthalmological assessment. Additional considerations include:
-
Placental histopathology (e.g. with Wright-Giemsa and immunoperoxidase stains) and NAT29. Historical methods include culture (e.g. cell line or mouse inoculation)30.
-
Serology of peripheral blood. Infant IgM and/or IgA reactivity likely suggests congenital infection, although exceptions exist (Table 3). Other indicators include persistent IgG at 6 and 12 months30,37. Single avidity scores from newborns may be difficult to interpret without further testing38,39. Umbilical cord blood may be contaminated with maternal blood30.
-
Analysis of other sample types including cerebrospinal fluid and urine37,38. Methods include NAT and serology (where appropriate). Diagnostic findings include IgM reactivity or target detection on NAT from cerebrospinal fluid37.
-
Imaging for hydrocephalus and calcifications37.
As for congenital syphilis, despite such considerations, diagnosis of congenital toxoplasmosis can still remain challenging and expert advice should be sought when considering testing, or interpreting test results. Ideally, investigation in women with substantially increased risk of infection should be considered before pregnancy10, and education regarding preventative measures provided.
Acknowledgement
Thanks to Ross Whybin for his comments and review.
References
[1] Australian Government Department of Health (2015) National Notifiable Diseases Surveillance System 2015, 10 August 2015. http://www9.health.gov.au/cda/source/rpt_2_sel_a.cfm[2] Read, P. et al. (2015) Increasing trends of syphilis among men who have sex with men in high income countries. Sex. Health 12, 155–163.
| Increasing trends of syphilis among men who have sex with men in high income countries.Crossref | GoogleScholarGoogle Scholar |
[3] Kwan, K.S. et al. (2012) Syphilis epidemiology and public health interventions in Western Australia from 1991 to 2009. Sex. Health 9, 272–279.
| Syphilis epidemiology and public health interventions in Western Australia from 1991 to 2009.Crossref | GoogleScholarGoogle Scholar | 22697145PubMed |
[4] Chakraborty, R. and Luck, S. (2007) Managing congenital syphilis again? The more things change. Curr. Opin. Infect. Dis. 20, 247–252.
| Managing congenital syphilis again? The more things change.Crossref | GoogleScholarGoogle Scholar | 17471033PubMed |
[5] Simms, I. and Ward, H. (2006) Congenital syphilis in the United Kingdom. Sex. Transm. Infect. 82, 1.
| Congenital syphilis in the United Kingdom.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28%2Fnslaksw%3D%3D&md5=1859080ea48bd55d3ce0afcaff16a4daCAS | 16461590PubMed |
[6] Centers for Disease Control and Prevention (2014) 2013 Sexually Transmitted Diseases Surveillance. Atlanta: US Department of Health and Human Services.
[7] Wicher, V. and Wicher, K. (2001) Pathogenesis of maternal-fetal syphilis revisited. Clin. Infect. Dis. 33, 354–363.
| Pathogenesis of maternal-fetal syphilis revisited.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzosVCktQ%3D%3D&md5=0e1d6ae22294be14914efaac56476621CAS | 11438902PubMed |
[8] World Health Organization (2007) The Global elimination of congenital syphilis: rationale and strategy for action. World Health Organization.
[9] De Santis, M. et al. (2012) Syphilis infection during pregnancy: fetal risks and clinical management. Infect. Dis. Obstet. Gynecol. 2012, 430585.
| Syphilis infection during pregnancy: fetal risks and clinical management.Crossref | GoogleScholarGoogle Scholar | 22829747PubMed |
[10] The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (2015) College Statement C-Obs 3 (b): Routine Antenatal Assessment in the Absence of Pregnancy Complications.
[11] Australian Health Ministers’ Advisory Council (2012) Clinical Practice Guidelines: Antenatal Care – Module 1. Canberra. http://www.health.gov.au/antenatal
[12] Public Health Laboratory Network (2012) Syphilis Laboratory Case Definition (LCD). (Australian Government Department of Health, ed.) Australian Government Department of Health.
[13] Communicable Diseases Network Australia (2015) Syphilis (congenital) case definition. (Australian Government Department of Health, ed.) Australian Government Department of Health.
[14] Palasanthiran, P. et al. eds (2014) Management of Perinatal Infections. Australasian Society for Infectious Diseases, Sydney.
[15] Liu, L.L. et al. (2014) Incidence and risk factors for the prozone phenomenon in serologic testing for syphilis in a large cohort. Clin. Infect. Dis. 59, 384–389.
| Incidence and risk factors for the prozone phenomenon in serologic testing for syphilis in a large cohort.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtlSrurnP&md5=81848cc10bbd0b8882210511acb8da4fCAS | 24803377PubMed |
[16] Centers for Disease Control (1988) Guidelines for the prevention and control of congenital syphilis. MMWR Morb. Mortal. Wkly. Rep. 37, 1–13.
| 2842647PubMed |
[17] van Dyck, E. et al. (2001) Effect of adverse storage conditions of antigen reagent on performance of the rapid plasma reagin test. Int. J. STD AIDS 12, 299–301.
| Effect of adverse storage conditions of antigen reagent on performance of the rapid plasma reagin test.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M3nslSmtQ%3D%3D&md5=b823581cf1f811e015e0f975dfd275c8CAS | 11368802PubMed |
[18] Arnold, S.R. and Ford-Jones, E.L. (2000) Congenital syphilis: a guide to diagnosis and management. Paediatr. Child Health 5, 463–469.
| 1:STN:280:DC%2BC3c7ivFSquw%3D%3D&md5=3ab19f33e10f0f39ac4cd373ecf03ea9CAS | 20177559PubMed |
[19] Jayamaha, J.C. et al. (2012) Congenital toxoplasmosis over 10 years in a low-incidence population. Med. J. Aust. 196, 443–444.
| Congenital toxoplasmosis over 10 years in a low-incidence population.Crossref | GoogleScholarGoogle Scholar | 22509871PubMed |
[20] FSANZ (2013) Toxoplasma gondii. In Agents of Foodborne Illness, 2nd edn (Food Standards Australia New Zealand, ed.) pp. 103–114. Canberra.
[21] Hill, D. and Dubey, J.P. (2002) Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 8, 634–640.
| Toxoplasma gondii: transmission, diagnosis and prevention.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38njt1emtA%3D%3D&md5=5b2f80e719080ad414bd176c2158cd7cCAS | 12390281PubMed |
[22] Gilbert, G.L. (2002) 1: Infections in pregnant women. Med. J. Aust. 176, 229–236.
| 11999241PubMed |
[23] McAuley, J.B. (2014) Congenital Toxoplasmosis. J. Pediatric Infect. Dis. Soc. 3, S30–S35.
| Congenital Toxoplasmosis.Crossref | GoogleScholarGoogle Scholar | 25232475PubMed |
[24] McAuley, J. et al. (1994) Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin. Infect. Dis. 18, 38–72.
| Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2czjtFGjtQ%3D%3D&md5=1030a4233757c50f2b64a491e0832c27CAS | 8054436PubMed |
[25] Foulon, W. (1992) Congenital toxoplasmosis: is screening desirable? Scand. J. Infect. Dis. Suppl. 84, 11–17.
| 1:STN:280:DyaK3s7nsVajuw%3D%3D&md5=01a0a1440a317f9ba38f127a9845ec58CAS | 1290068PubMed |
[26] Di Mario, S. et al. (2013) Prenatal education for congenital toxoplasmosis. Cochrane Database Syst. Rev. , CD006171.
| 23450566PubMed |
[27] Paquet, C. and Yudin, M.H. (2013) Toxoplasmosis in pregnancy: prevention, screening, and treatment. J. Obstet. Gynaecol. Can. 35, 78–81.
| 23343802PubMed |
[28] Bénard, A. et al. (2008) Survey of European programmes for the epidemiological surveillance of congenital toxoplasmosis. Euro Surveill. 13, 18834.
| 18445459PubMed |
[29] Montoya, J.G. and Remington, J.S. (2008) Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 47, 554–566.
| Management of Toxoplasma gondii infection during pregnancy.Crossref | GoogleScholarGoogle Scholar | 18624630PubMed |
[30] Montoya, J.G. (2002) Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 185, S73–S82.
| Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis.Crossref | GoogleScholarGoogle Scholar | 11865443PubMed |
[31] Sensini, A. (2006) Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin. Microbiol. Infect. 12, 504–512.
| Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283nt1Srtw%3D%3D&md5=a53d41f36aad95a262923ecfc1a9e503CAS | 16700697PubMed |
[32] Lebech, M. et al. (1996) Classification system and case definitions of Toxoplasma gondii infection in immunocompetent pregnant women and their congenitally infected offspring. European Research Network on Congenital Toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 15, 799–805.
| Classification system and case definitions of Toxoplasma gondii infection in immunocompetent pregnant women and their congenitally infected offspring. European Research Network on Congenital Toxoplasmosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s7gslygsA%3D%3D&md5=1e3917f0bb10648cf69d42d5bdaeef0fCAS | 8950557PubMed |
[33] Stronati, M. et al. (1998) Application and evaluation of a classification system and case definitions of Toxoplasma gondii infection. Eur. J. Clin. Microbiol. Infect. Dis. 17, 67–68.
| Application and evaluation of a classification system and case definitions of Toxoplasma gondii infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7ntFWntg%3D%3D&md5=2ad5f7c65cb70dcaffa400c0629d4de9CAS | 9512192PubMed |
[34] Liesenfeld, O. et al. (2001) Effect of testing for IgG avidity in the diagnosis of Toxoplasma gondii infection in pregnant women: experience in a US reference laboratory. J. Infect. Dis. 183, 1248–1253.
| Effect of testing for IgG avidity in the diagnosis of Toxoplasma gondii infection in pregnant women: experience in a US reference laboratory.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M3mtVaktw%3D%3D&md5=1e2972c532aab8a0bbed59137876eb94CAS | 11262207PubMed |
[35] Akolekar, R. et al. (2015) Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 45, 16–26.
| Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cbktVKgsQ%3D%3D&md5=97ec4dd0e61a6b7b9fe5348c6da65b71CAS | 25042845PubMed |
[36] Antsaklis, A. et al. (2002) Prenatal diagnosis of congenital toxoplasmosis. Prenat. Diagn. 22, 1107–1111.
| Prenatal diagnosis of congenital toxoplasmosis.Crossref | GoogleScholarGoogle Scholar | 12454967PubMed |
[37] Moncada, P.A. and Montoya, J.G. (2012) Toxoplasmosis in the fetus and newborn: an update on prevalence, diagnosis and treatment. Expert Rev. Anti Infect. Ther. 10, 815–828.
| Toxoplasmosis in the fetus and newborn: an update on prevalence, diagnosis and treatment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1yqtbfP&md5=d065153129c8f3d256f11b4217c7d7fbCAS | 22943404PubMed |
[38] Fuentes, I. et al. (1996) Urine sample used for congenital toxoplasmosis diagnosis by PCR. J. Clin. Microbiol. 34, 2368–2371.
| 1:STN:280:DyaK2s%2FjsVyhtw%3D%3D&md5=c68289a71083d46e6c1aed9cdb03beb6CAS | 8880481PubMed |
[39] Holliman, R.E. et al. (1994) The diagnosis of toxoplasmosis using IgG avidity. Epidemiol. Infect. 112, 399–408.
| The diagnosis of toxoplasmosis using IgG avidity.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c3gtVejtg%3D%3D&md5=c0b69831fe55cea5946b832ff67bcb02CAS | 8150014PubMed |
Biography
Dr Robert George is the Microbiology Registrar based at South Eastern Area Laboratory Services (SEALS), Randwick Campus. Previously, he completed a Doctor of Philosophy at the University of Queensland where he worked on spatial modelling and the prediction of outbreak systems.


