Enterovirus infection, β-cell apoptosis and type 1 diabetes
Sandhya Nair A B F , Ammira Akil A C G and Maria E Craig A B C D E HA Virology Research Laboratories, POWH and UNSW Research Laboratories, South Eastern Area Laboratory Services, Prince of Wales Hospital, Sydney
B School of Biotechnology and Biomolecular Science, Faculty of Science, University of New South Wales
C School of Women’s and Children’s Health, University of New South Wales
D Institute of Endocrinology and Diabetes, The Children’s Hospital at Westmead
E Discipline of Paediatrics and Child Health, University of Sydney
F Tel: +61 2 9382 9242, Email: z3267450@student.unsw.edu.au
G Tel: +61 2 9382 9096, Email: ammira.akil@sesiahs.health.nsw.gov.au
H Tel: +61 2 9845 3907, Email: m.craig@unsw.edu.au
Microbiology Australia 34(3) 153-156 https://doi.org/10.1071/MA13051
Published: 4 September 2013
Type 1 diabetes (T1D) results from a complex interplay between genetic and environmental factors, leading to chronic immune mediated destruction of pancreatic β-cells. The inflammatory process is initiated by one or more environmental triggers, such as a viral infection, stimulating release of autoantigens, inflammatory mediators including cytokines and chemokines, and death effectors, with resultant β-cell loss. While multiple enterovirus (EV) serotypes demonstrate β-cell tropism, most studies support a role for the coxsackievirus B (CVB) group in the pathogenesis of T1D. Experimental studies using animal models, insulin-producing cell lines and human islets indicate that the major mechanism of EV-induced β-cell destruction is apoptosis.
Human EV infection has long been implicated in the pathogenesis of T1D. Many epidemiological and cross-sectional studies have demonstrated higher rates of EV infection among individuals with T1D compared with non-diabetic controls1,2. In our meta-analysis of 26 studies using molecular methods for virus detection, there were significant associations between EVs and initiation of autoimmunity (OR 3.7, 95% CI 2.1–6.8) as well as development of T1D (OR 9.8, 95% CI 5.5–17.4)3. The detection of EV particles in pancreatic tissue from people with recent onset and long-standing T1D, with prominent islet tropism4–6, provides direct evidence for the involvement of EVs in T1D. Furthermore, EVs isolated at T1D onset effectively destroyed human islets in culture and the pancreas of experimental animals, supporting a potential causal role for specific EV serotypes in the pathogenesis of T1D4,7,8. Recently, the EV capsid protein VP1 was detected by immunostaining in 44/72 (61%) of pancreatic autopsy specimens from patients with recent onset T1D. The capsid protein was detected in multiple islets and immunostaining was specific to β-cells, with no involvement of other pancreatic cell types5,6. These studies provide some of the most compelling evidence in support of an aetiological role for EVs in at least some cases of T1D.
While pancreatic autopsy specimens provide in vivo data, we and others have shown that a range of EV genotypes, including CVBs and several enteric cytopathic human orphan (ECHO) viruses infect, replicate, impair β-cell function and cause cell death in human islets and insulin producing cells in vitro9–11 (see Figure 1: islets infected with CVB3). In vitro, CVB3 and CVB4 can cause persistent infection in human β-cells, with release of infectious particles up to 1 month after infection, without cell lysis12. Interestingly, EVs isolated from individuals discordant for development of T1D differed in their capacity to infect β-cells in vivo. Four isolates, from a mother and her son diagnosed with T1D on the same day (both infected with CVB5) and from twins (both infected with ECHO 21), one of whom subsequently developed T1D, replicated in human islets and caused slowly progressive β-cell lysis. However, β-cell tropism varied across these isolates, with the least cytolysis manifested by the isolate from the non-diabetic twin13, suggesting divergence of species between individuals. This suggests that recombination events might have resulted in changes in virulence and/or viral persistence, rather than the host immune response determining whether T1D ensues following an EV infection.
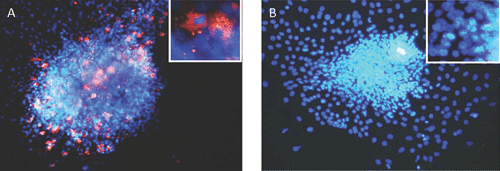
|
It is generally accepted that activation and infiltration of autoimmune cells within the islets is initiated largely by cytokines and chemokines produced by macrophages and other immune cells14–16. Recently, several studies have demonstrated that pancreatic islets are actively involved in signalling immune cells to invade the site of EV infection. CVB4 infection upregulates expression of cytokines such as interleukins (IL) eg; IL-1β, IL-6 and IL-8 and the chemokines; C-C motif ligand-5 (CCL-5) and C-C motif ligand-2 (CCL-2) by human islets17. Similarly, CVB5 induces β-cell expression of IL-15, CCL-5 and interferon (IFN)-γ induced protein 10 (IP-10), as we and others have shown9,18.
The constant activation of pro-inflammatory cytokines leads to the expression of high levels acute inflammatory mediators, such as IL-1β, CCL-2, tumour necrosis factor-α (TNF-α) and IL-819,20, with progression to T1D through activation of apoptotic signalling factors. In addition, acute or chronic viral infection activates the innate immune response21 via interaction of ssRNA with pattern recognition receptors such as toll-like receptor (TLR) 7 and TLR8, activation of signalling pathways and production of antiviral cytokines such as IFN-α22.
There are two major apoptotic pathways: intrinsic and extrinsic. The intrinsic pathway is regulated by B-cell lymphoma 2 (Bcl-2), which is associated with the outer mitochondrial membrane, while the extrinsic pathway is induced by death receptors such as Fas and TNF receptor-1 (TNF-R1). Either pathway involves activation of MAPK kinase, NF-κB and JAK/STAT pathways triggering, downstream cysteine proteases (caspases) – the final step of apoptosis23.
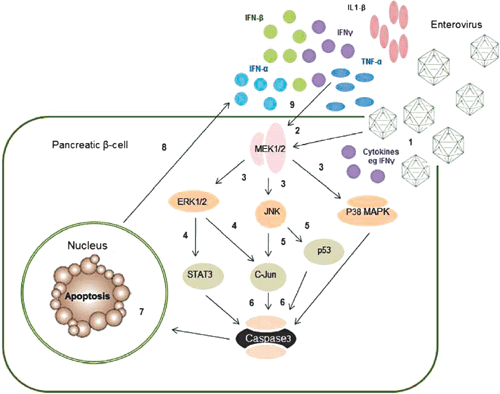
|
Most studies examining mechanisms of EV mediated cell death have utilised cell types other than human β-cells. There is some evidence that the MAPK kinase pathway is involved (Figure 2); for example CVB3 infection in HeLa cells24,25 and Jurkat T cells26 phosphorylated p38 MAPK, JNK and ERK1/2. Similarly, c-Jun and p44 MAPK were phosphorylated following EV71 infection in rat brain astrocytes27.
There is also more limited evidence that EVs induce β-cell death via apoptosis. Following CVB5 and 4 infection of islets derived from human pancreatic progenitor cells, we observed increases in ERK1/2, JNK and p38 (data not shown), confirming activation of the MAPK pathway (Figure 2). Similarly, EV infection in pancreatic islets activated the intrinsic pathway via an increase in Bim and a decrease in induced myeloid leukemia cell differentiation protein (Mcl-1), an anti-apoptotic factor,6,28 as well as the NF-κB promoter29. Collectively, these data indicate that apoptosis is the major mechanism of cell death following EV infection of β-cells. However, the involvement of other signalling pathways has not been investigated.
Although EVs are ubiquitous, their contribution to the burden of T1D remains poorly understood. Furthermore, the putative role of other viruses such as rotavirus, rubella and mumps, as initiators and/or accelerators of T1D, is even less studied30. While it is essential to identify specific serotypes and molecular characteristics of EVs that infect and destroy β-cells, a better understanding of the mechanisms of β-cell death may provide insights into development of novel strategies for prevention and treatment of T1D. In particular, EV induced β-cell death may be prevented through intervening in the production and/or action of immune mediators and apoptotic pathways. Development of vaccines targeting ‘diabetogenic’ EVs is another promising approach that might pave the way to reducing the burden of this chronic life-long disease.
References
[1] Craig, M.E. et al. (2003) Reduced frequency of HLA DRB1*03-DQB1*02 in children with type 1 diabetes associated with enterovirus RNA. J. Infect. Dis. 187, 1562–1570.| Reduced frequency of HLA DRB1*03-DQB1*02 in children with type 1 diabetes associated with enterovirus RNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXksFeisb4%3D&md5=1bd25979e3c005c67b56ee9d6d6768c9CAS | 12721936PubMed |
[2] Oikarinen, M. et al. (2012) Type 1 diabetes is associated with enterovirus infection in gut mucosa. Diabetes 61, 687–691.
| Type 1 diabetes is associated with enterovirus infection in gut mucosa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XovFegur0%3D&md5=841a55e22be1de6fcbeed9b3c98efe0aCAS | 22315304PubMed |
[3] Yeung, W.C. et al. (2011) Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 342, d35.
| Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies.Crossref | GoogleScholarGoogle Scholar | 21292721PubMed |
[4] Dotta, F. et al. (2007) Coxsackie B4 virus infection of β cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. USA 104, 5115–5120.
| Coxsackie B4 virus infection of β cells and natural killer cell insulitis in recent-onset type 1 diabetic patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjvFChu7o%3D&md5=34dea8e9e23fbdddb1b7b3f48eed71f5CAS | 17360338PubMed |
[5] Richardson, S.J. et al. (2009) The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52, 1143–1151.
| The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlt1Gqu70%3D&md5=3b3eb13cd0a283726fe3957a61f67c40CAS | 19266182PubMed |
[6] Richardson, S.J. et al. (2013) Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia 56, 185–193.
| Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVKgsrfO&md5=f642139b51fd12d20b90e8f536df21d3CAS | 23064357PubMed |
[7] Champsaur, H.F. et al. (1982) Virologic, immunologic, and genetic factors in insulin-dependent diabetes mellitus. J. Pediatr. 100, 15–20.
| Virologic, immunologic, and genetic factors in insulin-dependent diabetes mellitus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL387itF2qsg%3D%3D&md5=e5c03c88f3bdd55b7896a495cec09fc5CAS | 7035634PubMed |
[8] Yoon, J.W. et al. (1979) Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 300, 1173–1179.
| Isolation of a virus from the pancreas of a child with diabetic ketoacidosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1M7ls1Ogsg%3D%3D&md5=d1bd22c2bc2fe50b52320498b75afea7CAS | 219345PubMed |
[9] Nair, S. et al. (2010) Enterovirus infection induces cytokine and chemokine expression in insulin-producing cells. J. Med. Virol. 82, 1950–1957.
| Enterovirus infection induces cytokine and chemokine expression in insulin-producing cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht12kt7jJ&md5=141173d26509b5211908c889cab6cb87CAS | 20872723PubMed |
[10] Ylipaasto, P. et al. (2004) Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47, 225–239.
| Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c7gtValsg%3D%3D&md5=d6fb1c528f31136eb54ed66a7ecc42cbCAS | 14727023PubMed |
[11] Roivainen, M. et al. (2002) Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia 45, 693–702.
| Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlsVWktLw%3D&md5=5b9831b384c3397aa437d3103d0dd155CAS | 12107750PubMed |
[12] Chehadeh, W. et al. (2000) Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in β cells. J. Virol. 74, 10153–10164.
| Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in β cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnsFOltbo%3D&md5=0f2db6b4500fb8be1eeb6e7033a2dc3eCAS | 11024144PubMed |
[13] Elshebani, A. et al. (2007) Effects on isolated human pancreatic islet cells after infection with strains of enterovirus isolated at clinical presentation of type 1 diabetes. Virus Res. 124, 193–203.
| Effects on isolated human pancreatic islet cells after infection with strains of enterovirus isolated at clinical presentation of type 1 diabetes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhs1Cjurk%3D&md5=81c64307dab215403ea813638d24ebc7CAS | 17169456PubMed |
[14] He, J. and Haskins, K. (2008) Pathogenicity of T helper 2 T-cell clones from T-cell receptor transgenic non-obese diabetic mice is determined by tumour necrosis factor-alpha. Immunology 123, 108–117.
| Pathogenicity of T helper 2 T-cell clones from T-cell receptor transgenic non-obese diabetic mice is determined by tumour necrosis factor-alpha.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnsFWjtw%3D%3D&md5=1eb623b365600b7a160ade8fd93a0c64CAS | 17983440PubMed |
[15] Mallone, R. and van Endert, P. (2008) T cells in the pathogenesis of type 1 diabetes. Curr. Diab. Rep. 8, 101–106.
| T cells in the pathogenesis of type 1 diabetes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXms1WgtrY%3D&md5=54e41f74aab522f24393ae0c60be2129CAS | 18445351PubMed |
[16] Morran, M.P. et al. (2008) Innate and adaptive autoimmunity in type 1 diabetes. Pediatr. Diabetes 9, 152–161.
| Innate and adaptive autoimmunity in type 1 diabetes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsV2hs77J&md5=40e1e4c3a79c25bd809177d55d1d213dCAS | 18221432PubMed |
[17] Olsson, A. et al. (2005) Inflammatory gene expression in Coxsackievirus B-4-infected human islets of Langerhans. Biochem. Biophys. Res. Commun. 330, 571–576.
| Inflammatory gene expression in Coxsackievirus B-4-infected human islets of Langerhans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXis1Gku7c%3D&md5=180c02c9e76028f4d2718a3d7e60d50eCAS | 15796921PubMed |
[18] Ylipaasto, P. et al. (2005) Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 48, 1510–1522.
| Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXnslyrtL4%3D&md5=83310efaa184be769fbf1d173c3424a7CAS | 15991020PubMed |
[19] Kolb, H. and Mandrup-Poulsen, T. (2005) An immune origin of type 2 diabetes? Diabetologia 48, 1038–1050.
| An immune origin of type 2 diabetes?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlt1KmtL4%3D&md5=660687c1385b1488056d0173ba1afec2CAS | 15864529PubMed |
[20] Donath, M.Y. et al. (2003) Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes. J. Mol. Med. 81, 455–470.
| Inflammatory mediators and islet β-cell failure: a link between type 1 and type 2 diabetes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmsV2jtLw%3D&md5=51c718396dcb0ab67febf1c595e38dc9CAS | 12879149PubMed |
[21] Xagorari, A. and Chlichlia, K. (2008) Toll-like receptors and viruses: induction of innate antiviral immune responses. Open Microbiol. J. 2, 49–59.
| Toll-like receptors and viruses: induction of innate antiviral immune responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1Whu74%3D&md5=4309c20f16d4c237c4823e4a88d183eaCAS | 19088911PubMed |
[22] Colli, M.L. et al. (2010) MDA5 and PTPN2, two candidate genes for type 1 diabetes, modify pancreatic beta-cell responses to the viral by-product double-stranded RNA. Hum. Mol. Genet. 19, 135–146.
| MDA5 and PTPN2, two candidate genes for type 1 diabetes, modify pancreatic beta-cell responses to the viral by-product double-stranded RNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGhu7vM&md5=1bf1e60f988909cdb0c7157807f3b689CAS | 19825843PubMed |
[23] McKenzie, M.D. et al. (2008) Proapoptotic BH3-only protein Bid is essential for death receptor-induced apoptosis of pancreatic beta-cells. Diabetes 57, 1284–1292.
| Proapoptotic BH3-only protein Bid is essential for death receptor-induced apoptosis of pancreatic beta-cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvVKjtLg%3D&md5=95203537629dc1f52c9420f4eaf8bb9cCAS | 18252892PubMed |
[24] Luo, H. et al. (2002) Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 76, 3365–3373.
| Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xit1Kjsro%3D&md5=9c8032138362dda49c9fe4f0f4c14f56CAS | 11884562PubMed |
[25] Cunningham, K.A. et al. (2003) Caspase-3 activation and ERK phosphorylation during CVB3 infection of cells: influence of the coxsackievirus and adenovirus receptor and engineered variants. Virus Res. 92, 179–186.
| Caspase-3 activation and ERK phosphorylation during CVB3 infection of cells: influence of the coxsackievirus and adenovirus receptor and engineered variants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXis12rtL4%3D&md5=95a30c4a5de1a8f5aa565e0640eaa62cCAS | 12686427PubMed |
[26] Opavsky, M.A. et al. (2002) Enhanced ERK-1/2 activation in mice susceptible to coxsackievirus-induced myocarditis. J. Clin. Invest. 109, 1561–1569.
| Enhanced ERK-1/2 activation in mice susceptible to coxsackievirus-induced myocarditis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xks12ltLc%3D&md5=ae1daf38e3fcf56e7e5cecaf5d5a6ffeCAS | 12070303PubMed |
[27] Tung, W.H. et al. (2010) EV71 induces COX-2 expression via c-Src/PDGFR/PI3K/Akt/p42/p44 MAPK/AP-1 and NF-κB in rat brain astrocytes. J. Cell. Physiol. 224, 376–386.
| EV71 induces COX-2 expression via c-Src/PDGFR/PI3K/Akt/p42/p44 MAPK/AP-1 and NF-κB in rat brain astrocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmslWitb4%3D&md5=b72439e0e5553309f82e986b816cef47CAS | 20333648PubMed |
[28] Colli, M.L. et al. (2011) Exposure to the viral by-product dsRNA or Coxsackievirus B5 triggers pancreatic beta cell apoptosis via a Bim / Mcl-1 imbalance. PLoS Pathog. 7, e1002267.
| Exposure to the viral by-product dsRNA or Coxsackievirus B5 triggers pancreatic beta cell apoptosis via a Bim / Mcl-1 imbalance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtleis7zJ&md5=3b4c1f275cf96bc6f1abfc60917cb74bCAS | 21977009PubMed |
[29] García, M. et al. (2009) Regulation and function of the cytosolic viral RNA sensor RIG-I in pancreatic beta cells. Biochim. Biophys. Acta 1793, 1768–1775.
| Regulation and function of the cytosolic viral RNA sensor RIG-I in pancreatic beta cells.Crossref | GoogleScholarGoogle Scholar | 19747951PubMed |
[30] Craig, M.E. et al. (2013) Viruses and type 1 diabetes: a new look at an old story. Pediatr. Diabetes 14, 149–158.
| 1:CAS:528:DC%2BC3sXhtVWms7rM&md5=457b8d4072d28bc3023137f859b48187CAS | 23517503PubMed |
Biographies
Sandhya Nair completed a year of honours with the Virology Research Laboratory at the Prince of Wales Hospital and continued as a PhD student, furthering her research into the mechanism behind type 1 diabetes and enterovirus. Sandhya’s current research is examining viral induction of signalling pathways in beta cells infected with different enterovirus subtypes, to further understand the pathogenesis of virus-induced diabetes.
Dr Ammira Akil is a Postdoctoral research fellow in the School of Women’s and Children’s Health, Faculty of Medicine, University of New South Wales. Her work in the Virology Research Laboratory is focused on the molecular mechanisms for enterovirus-induced pancreatic beta-cell destruction. Currently she is investigating the viral pathogenesis of type 1 diabetes, particularly enteroviruses, aiming to expand the basic understanding of type 1 diabetes progression to allow the development of better therapeutic and prevention strategies by using a range of systems including human and non-human models.
Associate Professor Maria E Craig is a NHMRC Practitioner Fellow and a Staff Specialist in Paediatric Endocrinology at The Children’s Hospital at Westmead. After training in paediatric endocrinology, she was awarded a NHMRC Postgraduate Medical Research Scholarship for her PhD studies at the Virology Research Laboratory, investigating the association between enterovirus infection and the onset of type 1 diabetes. She has since been undertaking further larger-scale cohort studies of at-risk children to investigate the link between viruses and diabetes, including several NHMRC-funded project grants.


