Bacteriophage therapy
Kate HodgsonSchool of Pharmacy & Medical Sciences
Division of Health Sciences
University of South Australia
Adelaide, SA 5000, Australia
Tel: +61 8 8302 1374
Email: hodkr002@mymail.unisa.edu.au
Microbiology Australia 34(1) 28-31 https://doi.org/10.1071/MA13009
Published: 20 March 2013
Bacteriophages (phages) are viruses that infect only bacteria. They exhibit one of two types of life cycle; lytic (virulent) or lysogenic (temperate). They are non-toxic to other organisms, infecting, and in the case of lytic phages, multiplying rapidly within the bacterial host, ultimately killing it1. Lysogenic phages can remain in a quiescent state where the genome is integrated into the bacterial chromosome or exist as a plasmid. Some enhance bacterial virulence by encoding genes for toxins or antibiotic resistance2. Lytic phages are preferred for therapy as lysogenic phages may not result in host death and can transfer undesirable genes through transduction1. The history of prophylactic and therapeutic use of phages since their discovery over 90 years ago by d’Herelle (1917) and Twort (1915) are outlined in comprehensive reviews by Sulakvelidze et al, Merril and Hanlon2–4. Inconsistent and unreliable results combined with the discovery of antibiotics led to a decline in research in the West. The emphasis changed to the use of phages as tools for fundamental molecular studies focussing on the nature, replication and regulation of genes5,6. These studies clarified the biology of phages and provided a foundation for investigation into phage therapy and biocontrol.
Williams Smith and Huggins revived interest in phage therapy with their robust and repeatable evaluations of phages on a range of animal species with experimental Escherichia coli infections in the 1980s7–9. They demonstrated that a mixture of phages in a cocktail could significantly reduce morbidity and mortality due to toxigenic E. coli in calves, lambs and piglets. Importantly, protection against enteropathogenic E. coli challenge was afforded by phages present in the pens prior to admission and phage resistant variants were found to be less virulent9.
By the 1990s it was increasingly evident that antibiotic control of pathogens was under threat from the prevalence of antibiotic resistant strains10. This combined with consumer and political pressure to reduce the use of antibiotics, especially in food production, led to a revival in interest in phage therapies as additional or alternative options. The prohibitive cost, time frame and stringent controls on drug development and trials in human medicine have meant that veterinary, agricultural and food safety applications have gained acceptance first. The kinetics of bacteriophage/bacterial host interaction is complex. Successful in vivo application requires analysis of the kinetic properties of each specific phage therapy11. Further to this, optimisation of formulation, delivery routes and long term stability data will be required12. The current pharmaceutical regulatory framework is not compatible with the dynamic nature of phages that do not fit comfortably within current therapeutic categories13.
Phage based technologies currently used include typing of bacterial strains, as detection agents in ELISA based assays, food preparation/safety and the control of biofilms14–16. Table 1 is an overview of companies researching and producing phage based products.
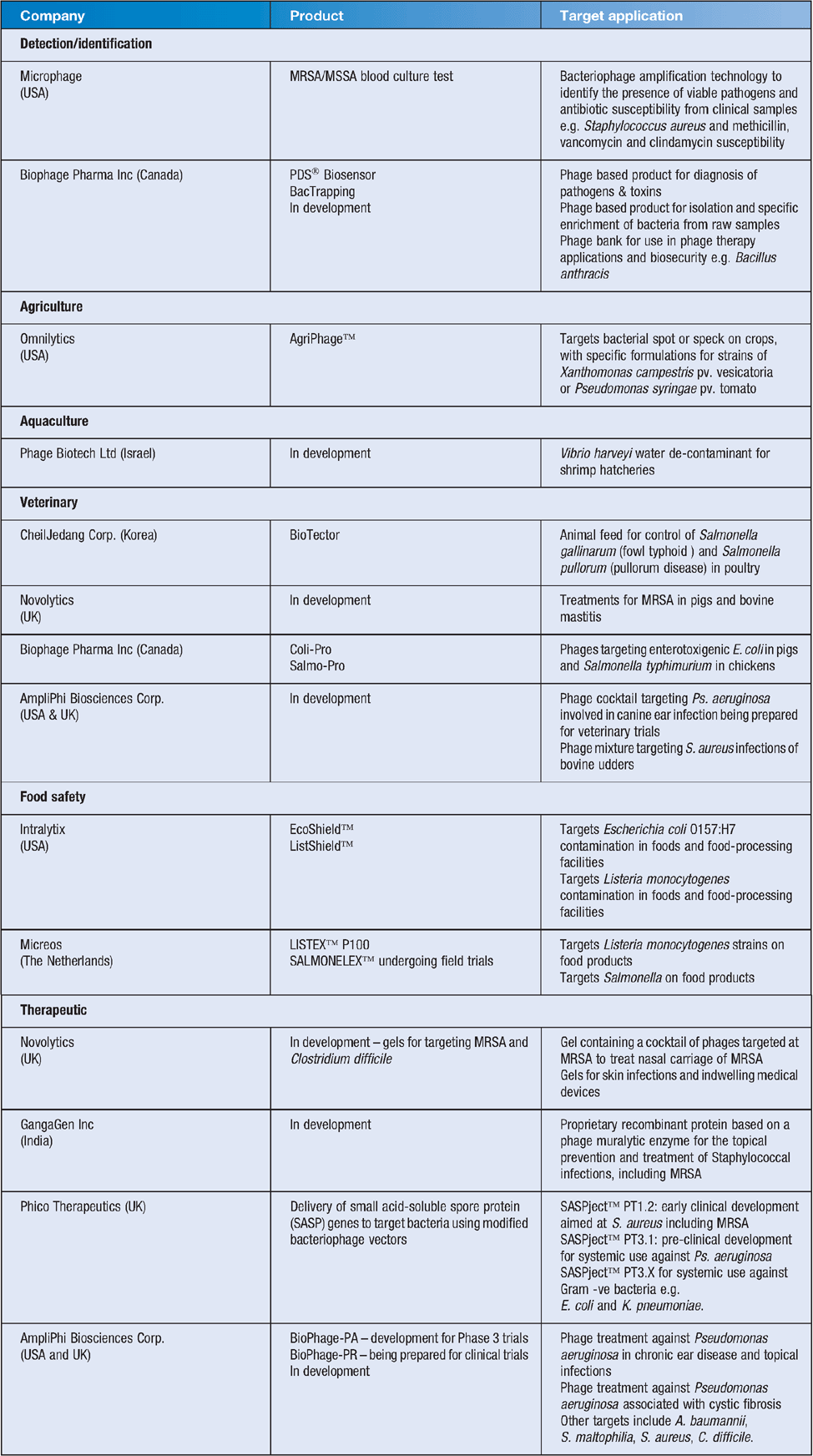
|
Phage applications in food producing animals focus on prevention and treatment of infections or as potential biocontrol agents of zoonotic pathogens and directly on food surfaces. Those most frequently targeted have been Campylobacter,Salmonella, Listeria and E. coli O157:H717–20. Significantly, in August 2006 the U.S Food and Drug Administration (FDA) approved under ‘generally recognised as safe’ (GRAS) regulations a phage cocktail as a spray for ready-to-eat meat to eradicate Listeria monocytogenes21. This was the first of a number of products proven and accepted to be effective and safe for human consumption and has been registered as an additive to organic foods in Europe and was approved for use as a food processing aid by Food Standards Australia & New Zealand (FSANZ) in Aug 201222.
Recent studies of therapeutic use targeting veterinary pathogens include oral administration of cocktails to calves & piglets against enterotoxigenic E. coli, topical treatment of P. aeruginosa otitis in dogs and oral, intratracheal or spray administration to treat colibacillosis in chickens23–25. Aquaculture is an industry where strategies for controlling infectious diseases are limited. Numerous studies of phage biocontrol have been carried out. Pathogens affecting a variety of fish species have been targeted including Vibrio species (vibriosis), Photobacterium (photobacteriosis), Aeromonas salmonicida (furunculosis) and Flavobacterium psychrophilum (bacterial cold water disease)26–28.
Therapeutic and prophylactic phage treatments do have limitations. Studies have noted that phage treatments often result in transient reduction not elimination of the target pathogens29. This is a likely consequence of the dynamic nature of the phage host relationship leading to coexistence of the phage and host and phage-resistant sub-populations. It is recommended that for prevention of food borne pathogens phages should be administered just prior to transport or slaughter to maximise pathogen load reduction and minimise resistance30,31. Prophylactic use of phages in intensive production facilities has risks associated with potential selection of resistant strains. Restricting use to therapeutic application to limit dissemination of phages into the natural environment is recommended32.
In vitro and clinical investigations of combination therapies with antibiotics have been shown to reduce selection of phage resistant variants and facilitate the spread of phages by enhancing lysis of the host bacterial cells33–35. Phage-antibiotic synergy (PAS) has the potential to reduce the amount of antibiotics administered34.
Phages are released from the host cell through the action of two phage genome encoded proteins - holins disrupt the cell membrane and endolysins digest the cell wall36. Endolysins are also able to lyse gram positive bacteria when applied exogenously. Advantages over antibiotic or phage therapy include reduced likelihood of resistance, endolysin specificity, the potential for genetic modification and the fact that they can be sourced from lytic or lysogenic phages37–39.
The host specificity of phages based on interaction with bacterial surface receptors has been exploited in a number of novel technologies for targeted gene, drug and vaccine delivery40,41. Phage tail-spike proteins (Tsp) mediate specificity through interaction with host receptors. Many have endoglycosidase activity leading to the hydrolysis of the receptor that has been shown to interfere with bacterial cell motility, significantly reducing colonisation and invasiveness42. Advantages are a reduction in resistance of target cells and no release of potentially toxic bacterial cell components during lysis.
A novel approach is the reversal of antibiotic resistance of pathogens using lysogenic phages to introduce genes conferring sensitivity to resistant strains43.
Phages and phage-based products are not the panacea to control bacterial infections or food borne diseases however when carefully evaluated and in combination with other strategies they have tremendous potential.
References
[1] Kutter, E. and Sulakvelidze, A. (2005) eds. Bacteriophages Biology and Applications. 2005, CRC Press: Florida. 510.[2] Merril, C. et al. (2003) The prospect for bacteriophage therapy in Western medicine. Nat. Rev. Drug Discov. 2, 489–497.
| The prospect for bacteriophage therapy in Western medicine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFOhu7g%3D&md5=66331210303b505c97a35fc122c4e447CAS |
[3] Sulakvelidze, A. et al. (2001) Bacteriophage therapy. Antimicrob. Agents Chemother. 45, 649–659.
| Bacteriophage therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsFWgt78%3D&md5=8aae121c73bfad37bb80a4da2c9503e3CAS |
[4] Hanlon, G.W. (2007) Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int. J. Antimicrob. Agents 30, 118–128.
| Bacteriophages: an appraisal of their role in the treatment of bacterial infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntFart74%3D&md5=0650434e7c88859a1e33a87f888b16d8CAS |
[5] Ellis, E.L. and Delbruck, M. (1939) The growth of bacteriophage. J. Gen. Physiol. 22, 365–384.
| The growth of bacteriophage.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3crjsl2itQ%3D%3D&md5=b8d06a9b2b8028904e342b253475e4aeCAS |
[6] Lwoff, A. (1953) Lysogeny. Bacteriol. Rev. 17, 269–337.
| 1:CAS:528:DyaG2cXhvVygsw%3D%3D&md5=c3da6532bcb01e865e2dcbdb42a987bdCAS |
[7] Williams Smith, H. and Huggins, M.B. (1983) Effectiveness of phages in treating experimental Escherichia coli diarhoea in calves, piglets and lambs. J. Gen. Microbiol. 129, 2659–2675.
[8] Williams Smith, H. et al. (1987) Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J. Gen. Microbiol. 133, 1127–1135.
[9] Williams Smith, H. et al. (1987) The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J. Gen. Microbiol. 133, 1111–1126.
[10] French, G.L. (2010) The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents 36, S3–S7.
| The continuing crisis in antibiotic resistance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFant7%2FK&md5=a7b3bd8a1ca10dde69f30245aa27e031CAS |
[11] Cairns, B.J. et al. (2009) Quantitative models of in vitro bacteriophage-host dynamics and their application to phage therapy. PLoS Pathog. 5, e1000253.
[12] Ryan, E.M. et al. (2011) Recent advances in bacteriophage therapy: How delivery routes, formulation, concentration and timing influence the success of phage therapy. J. Pharm. Pharmacol. 63, 1253–1264.
| Recent advances in bacteriophage therapy: How delivery routes, formulation, concentration and timing influence the success of phage therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Citr3N&md5=66aac2a4f6df94dd3f5a52099dd2d450CAS |
[13] Pirnay, J.P. et al. (2012) Introducing yesterday’s phage therapy in today’s medicine. Future Virol. 7, 379–390.
| Introducing yesterday’s phage therapy in today’s medicine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkvV2nu74%3D&md5=5c3aa20c5edbb6fa1da3042864312f69CAS |
[14] Hagens, S. and Loessner, M. (2007) Application of bacteriophages for detection and control of foodborne pathogens. Appl. Microbiol. Biotechnol. 76, 513–519.
| Application of bacteriophages for detection and control of foodborne pathogens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpt1aht78%3D&md5=4b83291031afa8d487064f616d6cbecfCAS |
[15] Carson, L. et al. (2010) The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol. Med. Microbiol. 59, 447–455.
| 1:CAS:528:DC%2BC3cXhtVeju7zN&md5=a0be5056dd7f38e8b96535f712fc1bc1CAS |
[16] Chibeu, A. et al. (2012) Bacteriophages with the ability to degrade uropathogenic Escherichia coli biofilms. Viruses 4, 471–487.
| Bacteriophages with the ability to degrade uropathogenic Escherichia coli biofilms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmslOqsbo%3D&md5=1fe374e12146f2c136514848df675589CAS |
[17] Abuladze, T. et al. (2008) Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef with Escherichia coli O157:H7. Appl. Environ. Microbiol. 74, 6230–6238.
| Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef with Escherichia coli O157:H7.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1ygu7jE&md5=913ec31bb13a5972fda7a87706acb41aCAS |
[18] Bardina, C. et al. (2012) Significance of the bacteriophage treatment schedule in reducing salmonella colonization of poultry. Appl. Environ. Microbiol. 78, 6600–6607.
| Significance of the bacteriophage treatment schedule in reducing salmonella colonization of poultry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlalurrP&md5=0280bb4bf2929e0e9ae4af5cf1eb0cb2CAS |
[19] Bigot, B. et al. (2011) Control of Listeria monocytogenes growth in a ready-to-eat poultry product using a bacteriophage. Food Microbiol. 28, 1448–1452.
| Control of Listeria monocytogenes growth in a ready-to-eat poultry product using a bacteriophage.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MfksVGltA%3D%3D&md5=d9aa72dff92fe4177f031757d7b0d5e5CAS |
[20] Carvalho, C.M. et al. (2010) The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol. 10, 232.
| The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens.Crossref | GoogleScholarGoogle Scholar |
[21] FDA (2006) Food and Drugs Title 21, Volume 3, Subchapter B - Food for Human Consumption, in Chapter I.
[22] FSANZ, F.S.A.N.Z., (2012) Approval Report – Application A1045 in Bacteriophage Preparation P100 as Processing Aid.
[23] Jamalludeen, N. et al. (2009) Evaluation of bacteriophages for prevention and treatment of diarrhea due to experimental enterotoxigenic Escherichia coli O149 infection of pigs. Vet. Microbiol. 136, 135–141.
| Evaluation of bacteriophages for prevention and treatment of diarrhea due to experimental enterotoxigenic Escherichia coli O149 infection of pigs.Crossref | GoogleScholarGoogle Scholar |
[24] Hawkins, C. et al. (2010) Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: A before/after clinical trial. Vet. Microbiol. 146, 309–313.
| Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: A before/after clinical trial.Crossref | GoogleScholarGoogle Scholar |
[25] Oliveira, A. et al. (2010) In vivo efficiency evaluation of a phage cocktail in controlling severe colibacillosis in confined conditions and experimental poultry houses. Vet. Microbiol. 146, 303–308.
| In vivo efficiency evaluation of a phage cocktail in controlling severe colibacillosis in confined conditions and experimental poultry houses.Crossref | GoogleScholarGoogle Scholar |
[26] Stenholm, A.R. et al. (2008) Isolation and characterization of bacteriophages infecting the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 74, 4070–4078.
| Isolation and characterization of bacteriophages infecting the fish pathogen Flavobacterium psychrophilum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXot1Omsbk%3D&md5=bb6aaebce51714e6ae677c0cb4ad5370CAS |
[27] Defoirdt, T. et al. (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 14, 251–258.
| Alternatives to antibiotics for the control of bacterial disease in aquaculture.Crossref | GoogleScholarGoogle Scholar |
[28] Oliveira, J. et al. (2012) Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquacult. Int. 20, 879–910.
| Bacteriophage therapy as a bacterial control strategy in aquaculture.Crossref | GoogleScholarGoogle Scholar |
[29] Sheng, H. et al. (2006) Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl. Environ. Microbiol. 72, 5359–5366.
| Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosVKlsL4%3D&md5=929b05d3b9cecdc353c83fea4c3c540dCAS |
[30] Wall, S.K. et al. (2010) Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Appl. Environ. Microbiol. 76, 48–53.
| Phage therapy to reduce preprocessing Salmonella infections in market-weight swine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntFOnug%3D%3D&md5=429b14190c50135f558aa84463e10df8CAS |
[31] Doyle, M.P. and Erickson, M.C. (2012) Opportunities for mitigating pathogen contamination during on-farm food production. Int. J. Food Microbiol. 152, 54–74.
| Opportunities for mitigating pathogen contamination during on-farm food production.Crossref | GoogleScholarGoogle Scholar |
[32] Sillankorva, S. et al. (2010) Salmonella Enteritidis bacteriophage candidates for phage therapy of poultry. J. Appl. Microbiol. 108, 1175–1186.
| Salmonella Enteritidis bacteriophage candidates for phage therapy of poultry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlt1Oqsb0%3D&md5=a83360ee5092d468075115079f472f49CAS |
[33] Verma, V. et al. (2009) Restricting ciprofloxacin-induced resistant variant formation in biofilm of Klebsiella pneumoniae B5055 by complementary bacteriophage treatment. J. Antimicrob. Chemother. 64, 1212–1218.
| Restricting ciprofloxacin-induced resistant variant formation in biofilm of Klebsiella pneumoniae B5055 by complementary bacteriophage treatment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVWqtLvE&md5=b5fa33b180fbd30da72170c03591f869CAS |
[34] Comeau, A.M. et al. (2007) Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS ONE 2, e799.
| Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth.Crossref | GoogleScholarGoogle Scholar |
[35] Kutateladze, M. and Adamia, R. (2010) Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 28, 591–595.
| Bacteriophages as potential new therapeutics to replace or supplement antibiotics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVags7nK&md5=25d55ce7fa7b68291deb45fbc28bcb92CAS |
[36] Hermoso, J.A. et al. (2007) Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr. Opin. Microbiol. 10, 461–472.
| Taking aim on bacterial pathogens: from phage therapy to enzybiotics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1yltbzM&md5=e09645d9ca974bfc1862293c23bd1b8bCAS |
[37] O’Flaherty, S. et al. (2009) Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol. Rev. 33, 801–819.
| Bacteriophage and their lysins for elimination of infectious bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnslGhu70%3D&md5=1c9561a280301a5353699bf5511a1cd8CAS |
[38] Fischetti, V.A. (2010) Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 300, 357–362.
| Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVantrvM&md5=fec9c441003a84adf41abee71e636d59CAS |
[39] Borysowski, J. et al. (2011) Potential of bacteriophages and their lysins in the treatment of MRSA: Current status and future perspectives. BioDrugs 25, 347–355.
| Potential of bacteriophages and their lysins in the treatment of MRSA: Current status and future perspectives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1aiurs%3D&md5=824406ac27f5186f6c8903b6bb269251CAS |
[40] Vaks, L. and Benhar, I. I. (2011) Antibacterial application of engineered bacteriophage nanomedicines: antibody-targeted, chloramphenicol prodrug loaded bacteriophages for inhibiting the growth of Staphylococcus aureus bacteria, in Methods in molecular biology S.J. Hurst, Editor. Springer Science+Business Media: Clifton, N.J. p. 187–206.
[41] Clark, J.R. et al. (2011) Comparison of a bacteriophage-delivered DNA vaccine and a commercially available recombinant protein vaccine against hepatitis B. FEMS Immunol. Med. Microbiol. 61, 197–204.
| Comparison of a bacteriophage-delivered DNA vaccine and a commercially available recombinant protein vaccine against hepatitis B.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjtFahtLw%3D&md5=3e1c9b32948b65b4244b354e74134b93CAS |
[42] Waseh, S. et al. (2010) Orally administered P22 phage tailspike protein reduces Salmonella colonization in chickens: Prospects of a novel therapy against bacterial infections. PLoS ONE 5, e13904.
| Orally administered P22 phage tailspike protein reduces Salmonella colonization in chickens: Prospects of a novel therapy against bacterial infections.Crossref | GoogleScholarGoogle Scholar |
[43] Edgar, R. et al. (2012) Reversing bacterial resistance to antibiotics by phage-mediated delivery of dominant sensitive genes. Appl. Environ. Microbiol. 78, 744–751.
| Reversing bacterial resistance to antibiotics by phage-mediated delivery of dominant sensitive genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVOiur4%3D&md5=c5b21c2835489c503b9fe77995435908CAS |
Biography
Dr Kate Hodgson has many years experience working in microbiology in industry and research laboratories in Adelaide, Canberra and London. She has also taught Microbiology in the School of Pharmacy and Medical Sciences at the University of South Australia. Her PhD research involved the isolation and characterisation of bacteriophages specific for enterotoxigenic Escherichia coli associated with post-weaning diarrhoea in piglets.


