Foot-and-mouth disease: a persistent threat
Wilna VoslooAustralian Animal Health Laboratory
Private Bag 24, Geelong East
Vic. 3220, Australia
Tel: +61 3 5227 5015
Fax: +61 3 4227 5555
Email: Wilna.vosloo@csiro.au
Microbiology Australia 34(1) 18-21 https://doi.org/10.1071/MA13006
Published: 20 March 2013
Foot-and-mouth disease (FMD) is a viral infection of cloven-hoofed animals. It is considered one of the most infectious viral diseases known and is feared for its ability to spread rapidly and cause widespread outbreaks in domestic livestock under intensive farming conditions. Remarkably, it does not cause high mortality, but morbidity can reach 100%. The disease has been eradicated from large parts of the world, and countries that are free of FMD take extreme precautions to prevent its reintroduction. For this reason FMD has been called an economic disease due to resultant trade restrictions and subsequent losses in income that have been estimated to reach between $7.1–16 billion for Australia depending on the size and duration of the outbreak1.
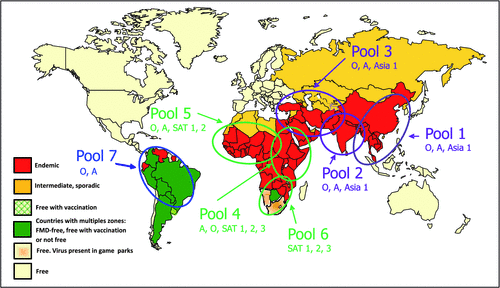
Foot-and-mouth disease virus belongs to the genus Aphthovirus in the family Picornaviridae and exists as seven distinct serotypes (O, A, C, Asia-1 and South African Territories (SAT) 1, 2 and 3) with the latter 3 belonging to a different lineage. There is little to no cross-protection between virus isolates belonging to the different serotypes, complicating control of the disease when using vaccines. It is a single-stranded RNA virus with a small genome (~8.5 kb) that lacks proofreading ability2 and each serotype therefore exists as numerous genetic and antigenic variants that have been classified into topotypes, i.e. geographically linked viruses with limited genetic variation3. The geographic distribution of the serotypes varies and different regions have their particular pools of viruses for which specific vaccine strains are needed. So far, seven pools of viruses have been identified to assist with control plans4 (Fig. 1).
|
The disease has a very wide host range and most cloven-hoofed species are susceptible, although at varying levels5. However, their importance in the maintenance and spread of the infection varies depending on various factors such as the species of animal involved, the virus isolate, the infectious dose and the immune status of the animals. For example, there are FMD virus isolates that are highly infectious to pigs, but not cattle6, while sheep and goats rarely show overt clinical signs7. Impala (Aepyceros melampus) that are found in sub-Saharan Africa are sometimes referred to as indicator species due to their high susceptibility to infection. During infection, that could also be sub-clinical, they can transmit the disease to other susceptible species, but factors such as animal density and contact rates determine that impala do not play an equally important role in the epidemiology of the disease in all regions where the species occur8. The African buffalo (Syncerus caffer), that is limited to sub-Saharan Africa, mostly suffer sub-clinical infection. It is the only species that has been shown to maintain the three SAT serotypes of FMD for long periods of time probably due to co-evolution of host and virus. The virus is present in cells obtained from oro-pharyngeal scrapings more than 28 days after the clinical phase of the disease has ended9. Although it is not clear how buffalo can transmit the disease, there is sufficient evidence that they can act as a source of infection for other domestic and wildlife species10,11.
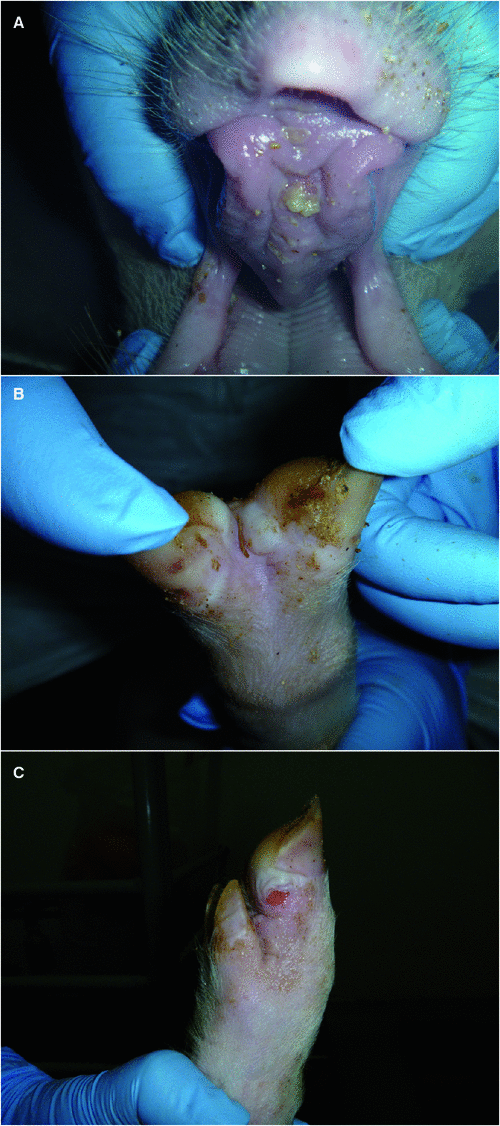
The clinical signs appear in epithelia subject to friction, such as the tongue, the coronary band and the interdigital space of the hoof as well as the teats of lactating animals (Fig. 2). In severe cases, the hoofs can slough. Mortalities occur typically only in young animals as a result of myocarditis, referred to as tiger heart disease. Most animals clear the virus from all excretions within 14 days as the levels of neutralizing antibodies increase, but ruminants can become persistently infected with FMD virus for variable periods of time where virus can be found, sometimes intermittently, in the germinal centres within the dorsal soft palate, pharyngeal tonsil, palatine tonsil, lateral retropharyngeal lymph node and mandibular lymph node12. However, persistently infected domestic animals have not been shown to transmit the infection experimentally while only anecdotal evidence exists of them doing so under natural conditions, and their role in the epidemiology of the disease is still a matter of contention13. Although most animals survive the infection and lesions heal in a relatively short period of time, loss of condition and overall productivity including decreased milk production can result. The greatest impact is seen in intensive farming systems such as feedlots, dairies and piggeries.
|
Pigs are considered to be amplifier hosts as they excrete up to a 1000 times more virus into the environment than infected cattle14. Cattle are more susceptible to airborne infection, most likely due to their large tidal volume. Virus can be present in secretions and excretions up to 4 days before clinical signs are evident (reviewed in Thomson and Bastos15) making products such as milk particularly hazardous as it could contain virus and be distributed widely before any movement control or quarantine measures are in place.
Hand, foot and mouth disease that affects predominantly children is caused mostly by coxsackie A virus and enterovirus 71, both from the Picornaviridae family16. This infection is often confused with FMD. The FMD virus is not a zoonotic agent, therefore products that contain FMD virus are safe for human consumption, although it is not good practice to allow such products into the human or animal food chain. However, these products could be infectious when fed to susceptible animals. Several mitigation steps are available to render products free of infectious virus for trade purposes, such as ultra high heat treatment for milk17 and allowing the pH of meat, especially beef, to decrease below pH6.0 whilst also removing high risk material such as bone and lymph nodes18. These measures are being advocated to allow commodity-based trade from regions where it is difficult to control FMD and to gain better access to export markets19.
FMD is endemic to large parts of South East Asia (SEA) where serotypes O, A and Asia-1 are prevalent. The southern parts of the region such as Indonesia, the Philippines, Singapore and East Malaysia are free of the disease. Under the auspices of the World Organisation for Animal Health (OIE) a regional plan is in place to improve control of FMD progressively and so assist countries to improve productivity and access export markets. Regional control will in turn lower the risk of accidental introduction for Australia. Due to the large number of people moving between the two regions and the risk of illegally imported foodstuffs, SEA is seen as the biggest risk of accidental introduction of FMD into Australia.
The current vaccines consist of concentrated and inactivated virus formulated with adjuvants; the vaccines need to be administered annually or more frequently, depending on the adjuvant, to ensure protective herd immunity in vaccinated livestock (reviewed in Thomson and Bastos15). Countries such as Australia that are free of FMD, keep emergency antigen banks that could be used in the case of an outbreak. These vaccines will be of high potency to induce a rapid immune response and will most likely be administered only once to protect animals against disease and suppress virus circulation to augment the other control measures such as stamping out, quarantine and movement control. Australia’s FMD control plan (AUSVETPLAN)20 is available for scrutiny online and has been used as example by several other countries to develop their own control plans.
It is important to determine whether vaccines will be efficacious against viruses circulating in the field. Currently there are a limited number of vaccine strains available internationally due to the difficulty of adapting FMD virus isolates as effective vaccine strains. Added to that, viruses are continuously mutating and recombining, giving rise to new variants that can escape vaccine immunity (see below). It is therefore important that all countries and regions be vigilant to monitor for potential new variants and ensure vaccines are efficacious. In recent years there have been a number of such incidents, for example new type A variants occurred in Argentina in 2000 and the spread of A-Iran-2005 throughout the Middle East led to the need to develop new vaccine strains21,22.
The gold standard for testing vaccine efficacy remains performing potency tests in animals. Although neutralizing antibodies are important predictors of protection for homologous challenge, the titre of antibodies are not as reliant when predicting protection against heterologous challenge. New methods such as determining the avidity and sub-types of antibodies and cell mediated responses are being investigated to provide more accurate correlates for protection23,24.
Diagnostic tests are available to detect virus antigen, genomic material and antibodies to FMD virus25. However, due to the significant amount of variation between and within serotypes, pan-serotypic diagnostic assays are essential for index case diagnosis and the polymerase chain reaction-based assays that detect conserved regions of the genome are especially valuable in this regard. In addition, these assays are amendable for high-throughput diagnostics, where many samples have to be tested during an outbreak26. To date, it has not been possible to design serological assays that can detect all serotypes and the variants within serotypes and the focus will be on pool specific assays (Fig. 1). The serological assays that are used to distinguish between vaccinated and infected animals are based on the differentiation of antibodies to the structural- (SP) and non-structural proteins (NSP) of the virus; the latter are conserved between the serotypes. NSP tests are therefore useful for detection of infection both in vaccinated and unvaccinated animals. However, the sensitivity and specificity of these tests depend on the immune status of the animals; tests being less sensitive when animals have been vaccinated prior to becoming infected27.
Australia has not had an outbreak of FMD since 1872 and has significant trade advantages due to its freedom, not only from FMD, but also from other infectious diseases. This benefit needs to be protected at all costs, hence a need for both post- and pre-border mitigation of risks. Part of this objective is to ensure the country is prepared for a disease emergency; therefore, the Australian Animal Health Laboratory is executing a project under contract with Animal Health Australia and industry funding via Meat and Livestock Australia to test the efficacy of available vaccine strains in the antigen bank against viruses isolated recently in SEA. All this work has to be performed offshore in facilities that are allowed to work with live FMD virus through international research collaborations. The project also focuses on studying the pathology of the viruses in Australian breeds of cattle, sheep and pigs, validating diagnostic tests and studying the molecular epidemiology of FMD in the SEA region. This project will provide useful information vital for laboratory based surveillance and control actions.
References
[1] http://www.daff.gov.au/animal-plant-health/pests-diseases-weeds/animal/fmd[2] Holland, J. et al. (1982) Rapid evolution of RNA genomes. Science 215, 1577–1585.
| Rapid evolution of RNA genomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XhsFKnt74%3D&md5=ff06bad69ebc7b3d82506b47ace8e6f2CAS |
[3] Knowles, N.J. and Samuel, A.R. (2003) Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 91, 65–80.
| Molecular epidemiology of foot-and-mouth disease virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtF2msg%3D%3D&md5=55ff4268a0a50a8e3a45de3fa5e57932CAS |
[4] Paton, D.J. et al. (2009) Options for control of foot-and-mouth disease: knowledge, capability and policy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2657–2667.
| Options for control of foot-and-mouth disease: knowledge, capability and policy.Crossref | GoogleScholarGoogle Scholar |
[5] Condy, J.B. et al. (1969) Foot-and-mouth disease in wildlife in Rhodesia and other African territories. A serological survey. J. Comp. Pathol. 79, 27–31.
| Foot-and-mouth disease in wildlife in Rhodesia and other African territories. A serological survey.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1M7ktFagsg%3D%3D&md5=a1d2f933c32d47f16d0ecee04167f2efCAS |
[6] Huang, C.C. et al. (2000) Characteristics of foot and mouth disease virus in Taiwan. J. Vet. Med. Sci. 62, 677–679.
| Characteristics of foot and mouth disease virus in Taiwan.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FktF2muw%3D%3D&md5=3f64491b67a54148ca49986fe360424aCAS |
[7] Kitching, R.P. and Hughes, G.J. (2002) Clinical variation in foot and mouth disease: sheep and goats. Rev. Sci. Tech. 21, 505–512.
| 1:STN:280:DC%2BD3s%2Fhs12jtQ%3D%3D&md5=c7ace95c5bf455be70d8518c62cd1ccbCAS |
[8] Vosloo, W. et al. (2009) Longitudinal study to investigate the role of impala (Aepyceros melampus) in foot-and-mouth disease maintenance in the Kruger National Park, South Africa. Transbound. Emerg. Dis 56, 18–30.
| Longitudinal study to investigate the role of impala (Aepyceros melampus) in foot-and-mouth disease maintenance in the Kruger National Park, South Africa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M7islSksA%3D%3D&md5=61b0104b53d26e1cf849dbb16e5e1355CAS |
[9] Condy, J.B. et al. (1985) The duration of the foot-and-mouth disease virus carrier state in African buffalo (i) in the individual animal and (ii) in a free-living herd. Comp. Immunol. Microbiol. Infect. Dis. 8, 259–265.
| The duration of the foot-and-mouth disease virus carrier state in African buffalo (i) in the individual animal and (ii) in a free-living herd.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL287jsVCksQ%3D%3D&md5=1312dddb51aca493806b1710c7bbd7fdCAS |
[10] Bastos, A.D. et al. (2000) Natural transmission of foot-and-mouth disease virus between African buffalo (Syncerus caffer) and impala (Aepyceros melampus) in the Kruger National Park, South Africa. Epidemiol. Infect. 124, 591–598.
| Natural transmission of foot-and-mouth disease virus between African buffalo (Syncerus caffer) and impala (Aepyceros melampus) in the Kruger National Park, South Africa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvjs1egtQ%3D%3D&md5=ccb7537e8f6e1cb2193d1c4440e096c1CAS |
[11] Vosloo, W. et al. (2002) The possible role that buffalo played in the recent outbreaks of foot-and-mouth disease in South Africa. Ann. N. Y. Acad. Sci. 969, 187–190.
| The possible role that buffalo played in the recent outbreaks of foot-and-mouth disease in South Africa.Crossref | GoogleScholarGoogle Scholar |
[12] Juleff, N. et al. (2008) Foot-and-mouth disease virus persists in the light zone of germinal centres. PLoS ONE 3, e3434.
| Foot-and-mouth disease virus persists in the light zone of germinal centres.Crossref | GoogleScholarGoogle Scholar |
[13] Sutmoller, P. and Casas, O.R. (2002) Unapparent foot and mouth disease infection (sub-clinical infections and carriers): implications for control. Rev. Sci. Tech. 21, 519–529.
| 1:STN:280:DC%2BD3s%2Fhs12juw%3D%3D&md5=b950f256ffccb6e24e18a2a936a3e019CAS |
[14] Donaldson, A.I. and Alexandersen, S. (2002) Predicting the spread of foot and mouth disease by airborne virus. Rev. Sci. Tech. 21, 569–575.
| 1:STN:280:DC%2BD3s%2Fhs12isQ%3D%3D&md5=1c48403adbd7e3c9afbdcebc763f2956CAS |
[15] Thomson, G.R. and Bastos, A.D.S. (2004) Foot and mouth disease. In: Infectious Diseases of Livestock (Coetzer, J.A.W. and Tustin, R.C., eds), pp. 1324–1366, Oxford University Press.
[16] Nasri, D. et al. (2007) Basic rationale, current methods and future directions for molecular typing of human enterovirus. Expert Rev. Mol. Diagn. 7, 419–434.
| Basic rationale, current methods and future directions for molecular typing of human enterovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnsV2jurk%3D&md5=a786245d38c720c173db2f530070b542CAS |
[17] Tomasula, P.M. et al. (2007) Thermal inactivation of foot-and-mouth disease virus in milk using high-temperature, short-time pasteurization. J. Dairy Sci. 90, 3202–3211.
| Thermal inactivation of foot-and-mouth disease virus in milk using high-temperature, short-time pasteurization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntlCksrw%3D&md5=e14bdff833d7895a11c6bff0521cf66cCAS |
[18] Paton, D.J. et al. (2010) Qualitative assessment of the commodity risk for spread of foot-and-mouth disease associated with international trade in deboned beef. Transbound. Emerg. Dis. 57, 115–134.
| Qualitative assessment of the commodity risk for spread of foot-and-mouth disease associated with international trade in deboned beef.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3crkt1Glug%3D%3D&md5=daf40fc96cc5f9facf7c15d9e3a744efCAS |
[19] Thomson, G.R. et al. (2004) International trade in livestock and livestock products: the need for a commodity-based approach. Vet. Rec. 155, 429–433.
| 1:STN:280:DC%2BD2crjsl2ksg%3D%3D&md5=b770db56f6c0c669b94aa73eac6863dbCAS |
[20] http://www.animalhealthaustralia.com.au/wp-content/uploads/2011/04/FMD3_2-12-FINAL6May10.pdf
[21] Mattion, N. et al. (2004) Reintroduction of foot-and-mouth disease in Argentina: characterisation of the isolates and development of tools for the control and eradication of the disease. Vaccine 22, 4149–4162.
| Reintroduction of foot-and-mouth disease in Argentina: characterisation of the isolates and development of tools for the control and eradication of the disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXotlWqtb0%3D&md5=95517f2e54cbaea97b772529891de56bCAS |
[22] Knowles, N.J. et al. (2009) Recent spread of a new strain (A-Iran-05) of foot-and-mouth disease virus type A in the Middle East. Transbound. Emerg. Dis 56, 157–169.
| Recent spread of a new strain (A-Iran-05) of foot-and-mouth disease virus type A in the Middle East.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MzjslWqsA%3D%3D&md5=3aab8f18d0b0c61913299eeb76832552CAS |
[23] Lavoria, M.A. et al. (2012) Avidity and subtyping of specific antibodies applied to the indirect assessment of heterologous protection against Foot-and-Mouth Disease Virus in cattle. Vaccine 30, 6845–6850.
| Avidity and subtyping of specific antibodies applied to the indirect assessment of heterologous protection against Foot-and-Mouth Disease Virus in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVWnurbI&md5=b202e89ab7064e660016332e23d1d836CAS |
[24] Eble, P.L. et al. (2006) Comparison of immune responses after intra-typic heterologous and homologous vaccination against foot-and-mouth disease virus infection in pigs. Vaccine 24, 1274–1281.
| Comparison of immune responses after intra-typic heterologous and homologous vaccination against foot-and-mouth disease virus infection in pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xhs1Smsbc%3D&md5=1e257ef4bafd2e257cae5e181805913dCAS |
[25] OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2012 http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.05_FMD.pdf
[26] Reid, S.M. et al. (2001) Diagnosis of foot-and-mouth disease by real-time fluorogenic PCR assay. Vet. Rec. 149, 621–623.
| Diagnosis of foot-and-mouth disease by real-time fluorogenic PCR assay.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FjtlCruw%3D%3D&md5=d85d997b214f666ee631e216b9845044CAS |
[27] Brocchi, E. et al. (2006) Comparative evaluation of six ELISAs for the detection of antibodies to the non-structural proteins of foot-and-mouth disease virus. Vaccine 24, 6966–6979.
| Comparative evaluation of six ELISAs for the detection of antibodies to the non-structural proteins of foot-and-mouth disease virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Shu7jN&md5=498ca202786abee40d49dd62676845e6CAS |
Biography
Wilna Vosloo has been working on FMD for over 20 years with vast experience in the disease, first gained in Africa and more recently in South East Asia. She is currently a senior research scientist at the Australian Animal Health Laboratory where she leads a major research project on FMD.


