One Health antimicrobial resistance: stewardship in Australia
Laura Y. Hardefeldt A * and Karin Thursky
A * and Karin Thursky  B
B
A
B

Dr Laura Hardefeldt is a senior lecturer at The University of Melbourne. Her research interests are in antimicrobial use, antimicrobial stewardship and antimicrobial resistance in the veterinary sector and in a One Health context. She is at the forefront of veterinary antimicrobial stewardship research nationally and internationally. |

Prof. Karin Thursky is an infectious disease physician and clinical researcher at the Royal Melbourne Hospital, Peter MacCallum Cancer Centre and The University of Melbourne. She has played a pioneering role in the development of antimicrobial stewardship in the Australian health system and the national antimicrobial resistance strategy and is a renowned leader in antimicrobial stewardship internationally. |
Abstract
Antimicrobial stewardship (AMS) is promoted as a core set of actions that aim to mitigate the emergence of antimicrobial resistance, but to also ensure safety and quality use of antimicrobials. By improving the appropriate use of antimicrobials we can improve patient care, reduce adverse effects and perhaps slow the emergence of antimicrobial resistance. However, changing behaviour of prescribers is challenging, with barriers and facilitators often common across diverse prescribing groups – doctors and veterinarians. Many of the physical challenges facing prescribers are also shared and lessons can be learned about implementation of interventions between groups. In this article we discuss qualitative and quantitative research, and interventions in AMS, in Australia from a One Health perspective.
Keywords: AMR, AMS, antibiotic, antimicrobial resistance, antimicrobial stewardship, behaviour, digital health, implementation, veterinary.
Antimicrobial resistance (AMR) is a global health threat causing high morbidity, mortality, and healthcare costs.1 In Australia, one in five prescriptions in hospitals are inappropriate2 and inappropriate use is prevalent in other sectors like primary care,3,4 dental prescribing5 and the veterinary sector.6–8 The Australian Government’s ‘One Health Master Action Plan for Australia’s National Antimicrobial Resistance Strategy – 2020 and beyond’ emphasises antimicrobial stewardship (AMS) implementation in medical and animal sectors.9 One Health is an integrated, unifying approach that aims to sustainably balance and optimise the health of people, animals and ecosystems (World Health Organization, see https://www.who.int/health-topics/one-health#tab=tab_1). AMR is a perfect example of a crisis for which One Health is a critical approach as antimicrobial use in humans, animals and the environment are contributing to the emergence of AMR. The barriers and facilitators of AMS are often common, and many AMS interventions are useful across sectors.
The barriers and facilitators of AMS are well described in the medical and veterinary sectors in Australia. Lack of awareness and knowledge and lack of access to education and expertise have been found to be key barriers in both medical10,11 and veterinary12 fields, particularly in primary care settings. Scarcity of resources12,13 and pressure from patients, carers and owners12,14,15 are also barriers to AMS affecting doctors and veterinarians. A common facilitator, however, is the desire of prescribers to gain knowledge and contribute to reducing the emergence of AMR.12,16 As with many wicked problems, although prescribers desire improvement, counteracting barriers are present. The common challenge that is faced is that of uncertainty, where the risk of patient deterioration, in conjunction with the prescriber’s own comfort with risk, is perceived as more important than the future risk of AMR.17 The hierarchy of hospitals and veterinary clinics,12,18 fear of loss of autonomy of prescribing12,18 and fear of the moral and legal consequences of patient deterioration15 are also challenging to address and can only be overcome by strong leadership within organisations and reinforcement of evidence-based information on patient safety and quality of care. Although the barriers and facilitators are often common across prescribing settings, the interventions required to implement change are often sector specific and need to be targeted. Addressing behaviour change using methodologies such implementation science and human factors engineering should underpin all improvement initiatives.19
A key outcome from the medical sector is that accreditation has been a successful mechanism to drive resource allocation for AMS programs.13 However, not all states in Australia accredit veterinary hospitals meaning this strategy is not possible without legislative change, and in even in primary care, accreditation is not mandated, although an optional approach has been defined, drawing on the AMS Clinical Care Standard.20 Support of AMS network arrangements involving rural health and veterinary services may also be a useful method, especially since research shows that messages in one sector have influence on the public’s opinion in other sectors.15 Support of nurses and pharmacists to run AMS programs has been successful in Australian rural hospitals,21 bolstered through network arrangements, and is currently being trialled in veterinary hospitals and may overcome some of the resourcing challenges that are faced in rural and primary care – both veterinary and medical.
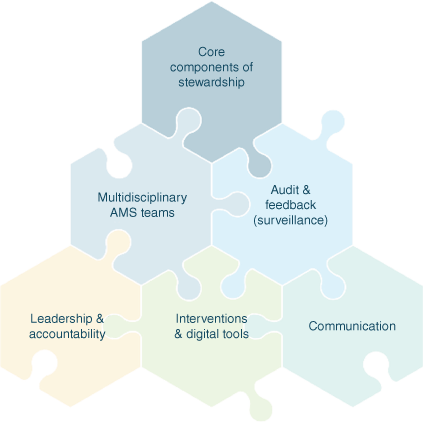
Effective AMS requires a coherent set of actions that promote using antimicrobials responsibly, and includes governance, surveillance of both quantity and quality of antimicrobials, including restrictions where appropriate, audit and feedback and reporting (Fig. 1).22 Australia has a long history of innovative stewardship interventions in hospitals with leading innovations such as the National Antimicrobial Prescribing Survey (NAPS, Melbourne Health, see https://www.naps.org.au), computerised decision support systems,23 allergy delabelling programs24 and development of appropriateness as the key process metric in AMS interventions.25 Australia has also been on the forefront of AMS implementation in veterinary environments.26 Veterinary antimicrobial guideline development by the Australian Veterinary Association and NCAS, and models of AMS that are acceptable, feasible and sustainable,27 have been foundational changes.
Antimicrobial stewardship in Australian hospitals is well established although implementation challenges remain in the rural and regional setting.28 The major driver for the rapid maturation of structural and process measures was the introduction of a dedicated AMS accreditation standard in 2011 (updated in 2017 and 2021).29 The standard explicitly supports the need to provide access to national antimicrobial prescribing guidelines, to use antimicrobial restriction and approval processes, and to monitor consumption and appropriateness of antimicrobial use. More recently, the adoption of electronic medical records has facilitated AMS programs by enabling efficient antimicrobial usage auditing and linkages to computerised clinical decision-support systems.
Although AMS in hospitals in Australia is well developed, the primary care setting remains a major challenge, as the infrastructure and resources to support implementation of AMS programs are limited. Awareness of AMS is high (69%) among Australian GPs and many recognise the value of AMS programs (62%) but uptake of strategies is relatively poor and GPs did not feel that improving their prescribing would affect AMR overall (53%),16 a finding consistent with the attitudes of veterinarians.12 Access to the Therapeutic Guidelines was also relatively low in this survey (51%), a finding that should represent low-hanging fruit for action but is challenging to address due to the structure of general practice in Australia. Strategies like delayed prescribing are popular (72% always or often using when appropriate).16
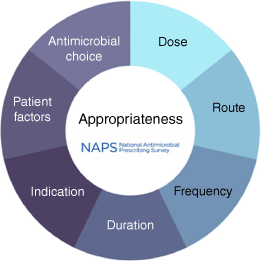
Australian researchers have made a major contribution to the AMS landscape. One example is the NAPS, which supports AMS programs in hospitals and residential aged care homes and provides data for the Antimicrobial Use and Resistance in Australia (AURA) program (Fig. 2). The web-based platform has undergone continuous improvement since 2013, and now comprises five modules: the Hospital NAPS, Surgical NAPS, Aged Care NAPS, the Antifungal NAPS and the Quality Improvement NAPS. Despite the voluntary nature of the survey activities, participation has continued to increase across both public and private institutions and is being used in 12 countries (including low- and middle-income countries).25 Unique internationally, the platform has demonstrated the feasibility and acceptability of measuring the appropriateness of antimicrobials being used, rather than a limited assessment of guideline compliance.
Factors considered in evaluating appropriateness in the National Antimicrobial Prescribing Survey.
Although NAPS has had wide uptake in hospitals (~600 sites) and aged care homes (>1000 sites), the labour-intensive nature of the audit means that its usefulness in community practice is reduced although a successful pilot was undertaken in the three general practices.30 Digital health records represent an important tool for antimicrobial use surveillance and the use of data science methodologies may overcome the challenges of NAPS implementation in general practice and veterinary clinics. Research databases have been leveraged in primary care31 and veterinary care32 and collaboration with computer scientists has allowed in-depth evaluation of veterinary clinical notes at a population scale.8 The National Centre for Antimicrobial Stewardship is leading a data science initiative33 that is examining the use of natural language processing, and machine learning, to assist antimicrobial prescribing surveillance and audit and feedback in veterinary clinics, primary care and in hospitals. Key to this is the establishment of data models and standards that support scaling of these digital health initiatives.
Veterinary clinic implementation of AMS has dramatically improved since 2018. An implementation trial in 135 general veterinary practices,26 AMR as one of the key priorities of the Australian Veterinary Association, and the development of the National Centre for Antimicrobial Stewardship as a One Health Centre for Research Excellence have all contributed to the elevation of AMS as a priority within the veterinary profession. Antimicrobial stewardship guidelines are now commonplace in veterinary clinics and interventions such as traffic light programs have been effective for emphasising the importance of antimicrobials and reducing the use of drugs such as cefovecin (a third-generation cephalosporin) and enrofloxacin. The Australian Veterinary Association has coordinated the development of antimicrobial use guidelines for sheep, dairy and beef feedlot cattle, pigs, and chickens, and with guidelines for horses underway. The AMR Vet Collective hosts an online learning course on antimicrobial use, resistance and stewardship, which was funded by the Commonwealth and developed in conjunction with the Australian Veterinary Schools.
The Australian red meat, dairy, pork, and poultry industries (meat and eggs) have collaborated to form the Animal Industry Antimicrobial Stewardship Research, Development and Extension Strategy (AIAS). These groups have also prepared a joint report – Antimicrobial Stewardship in Australian Livestock Industries.34 Most livestock industries also have stewardship guidelines. There is a heavy focus on reducing the need for antimicrobial use through preventative measures, such as preparation of animals prior to feedlot entry, vaccination and biosecurity, and replacing antimicrobials with products such as probiotics, yeasts, plant extracts and organic acids. As an example, AMS was selected as one of six key priorities in the Australian Beef Sustainability Framework and within 1 year of the release of the guidelines, 39% of feedlots had voluntarily implemented an AMS plan. This increased to 62% within 2 years and, in January 2022, became mandatory.35
Conclusion
There are many common challenges across both human and animal sectors in the efforts to optimise antimicrobial use. A shared understanding of appropriateness in antimicrobial prescribing across the community, hospital and policy settings will be important. Similarly, there great opportunities to work together to align methodologies for surveillance of antimicrobial use and implementation of AMS programs. However, it is essential that the context and resources are taken into account when designing implementation of AMS programs. A One Health approach to AMS does not require the same interventions in every sector – this is necessary as the interventions must be tailored to achieve the best outcome, but working together is useful for shared learnings, shared challenges and recognition of where efforts can be cross-sectoral. Government and granting bodies should recognise and promote these activities.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Declaration of funding
L. Y. Hardefeldt was funded by an Australian Research Council Discovery Early Career Research Grant (grant number DE200100030).
References
1 O’Neill J (2016) Tackling drug-resistant infections globally: final report and recommendations. The Wellcome Trust and Her Majesty’s Government, London, UK. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
2 Australian Commission on Safety and Quality in Health Care (2021) AURA 2021: fourth Australian report on antimicrobial use and resistance in human health. ACSQHC, Sydney, NSW, Australia. https://www.safetyandquality.gov.au/sites/default/files/2021-09/aura_2021_-_report_-_final_accessible_pdf_-_for_web_publication.pdf
3 Australian Commission on Safety and Quality in Health Care (2019) AURA 2019: third Australian report on antimicrobial use and resistance in human health. ACSQHC, Sydney, NSW, Australia. https://www.safetyandquality.gov.au/sites/default/files/2019-06/AURA-2019-Report.pdf
4 Manski-Nankervis JA et al. (2018) A need for action: results from the Australian General Practice National Antimicrobial Prescribing Survey (GP NAPS). Society for Academic Primary Care. https://sapc.ac.uk/conference/2018/abstract/need-action-results-australian-general-practice-national-antimicrobial
5 Teoh L et al. (2023) Exploring the appropriateness of antibiotic prescribing for dental presentations in Australian general practice – a pilot study. Br J Clin Pharmacol 89, 1554-1559.
| Crossref | Google Scholar | PubMed |
6 Hardefeldt LY et al. (2017) Antimicrobial prescribing in dogs and cats in Australia: results of the Australasian Infectious Disease Advisory Panel survey. J Vet Intern Med 31, 1100-1107.
| Crossref | Google Scholar | PubMed |
7 Hardefeldt LY et al. (2017) Cross-sectional study of antimicrobials used for surgical prophylaxis by bovine veterinary practitioners in Australia. Vet Rec 181, 426.
| Crossref | Google Scholar | PubMed |
8 Hur B et al. (2022) Evaluating the dose, indication and agreement with guidelines of antimicrobial use in companion animal practice with natural language processing. JAC Antimicrob Resist 4, dlab194.
| Crossref | Google Scholar | PubMed |
9 Department of Health and Aged Care and Department of Agriculture, Fisheries and Forestry (2021) One Health Master Action Plan for Australia’s National Antimicrobial Resistance Strategy – 2020 and Beyond. Commonwealth of Australia. https://www.amr.gov.au/resources/one-health-master-action-plan-australias-national-antimicrobial-resistance-strategy-2020-and-beyond
10 James R et al. (2015) A mixed methods study of the barriers and enablers in implementing antimicrobial stewardship programmes in Australian regional and rural hospitals. J Antimicrob Chemother 70, 2665-2670.
| Crossref | Google Scholar | PubMed |
11 Rzewuska M et al. (2020) Barriers and facilitators to implementation of antibiotic stewardship programmes in hospitals in developed countries: insights from transnational studies. Front Sociol 5, 41.
| Crossref | Google Scholar | PubMed |
12 Hardefeldt LY et al. (2018) Barriers to and enablers of implementing antimicrobial stewardship programs in veterinary practices. J Vet Intern Med 32, 1092-1099.
| Crossref | Google Scholar | PubMed |
13 Bishop JL et al. (2019) Qualitative study of the factors impacting antimicrobial stewardship programme delivery in regional and remote hospitals. J Hosp Infect 101, 440-446.
| Crossref | Google Scholar | PubMed |
14 Biezen R et al. (2019) Dissonant views – GPs’ and parents’ perspectives on antibiotic prescribing for young children with respiratory tract infections. BMC Fam Pract 20, 46.
| Crossref | Google Scholar | PubMed |
15 Scarborough RO et al. (2023) ‘Brave enough’: a qualitative study of veterinary decisions to withhold or delay antimicrobial treatment in pets. Antibiotics 12, 540.
| Crossref | Google Scholar | PubMed |
16 Saha SK et al. (2020) A nationwide survey of Australian general practitioners on antimicrobial stewardship: awareness, uptake, collaboration with pharmacists and improvement strategies. Antibiotics 9, 310.
| Crossref | Google Scholar | PubMed |
17 Davis M et al. (2024) Risk individualisation and moral injury in the treatment of infection as impediments to the tackling of antimicrobial resistance. Health Risk Soc 1-18.
| Crossref | Google Scholar |
18 Ierano C et al. (2019) Influences on surgical antimicrobial prophylaxis decision making by surgical craft groups, anaesthetists, pharmacists and nurses in public and private hospitals. PLoS ONE 14, e0225011.
| Crossref | Google Scholar | PubMed |
19 Thursky KA et al. (2021) Antimicrobial stewardship in Australia: the role of qualitative research in programme development. JAC Antimicrob Resist 3, dlab166.
| Crossref | Google Scholar | PubMed |
20 Australian Commission on Safety and Quality in Health Care (2020) Antimicrobial Stewardship Clinical Care Standard. ACSQHC, Sydney, NSW, Australia. https://www.safetyandquality.gov.au/our-work/clinical-care-standards/antimicrobial-stewardship-clinical-care-standard
21 Bishop JL et al. (2021) Impact of time to first antimicrobial dose on length of stay and 30-day hospital readmission in patients with lower limb cellulitis. J Glob Antimicrob Resist 25, 367-369.
| Crossref | Google Scholar | PubMed |
22 Dyar OJ et al. (2017) What is antimicrobial stewardship? Clin Microbiol Infect 23, 793-798.
| Crossref | Google Scholar | PubMed |
23 Buising KL et al. (2008) Electronic antibiotic stewardship – reduced consumption of broad-spectrum antibiotics using a computerized antimicrobial approval system in a hospital setting. J Antimicrob Chemother 62, 608-616.
| Crossref | Google Scholar | PubMed |
24 Chua KYL et al. (2021) The Penicillin Allergy Delabeling Program: a multicenter whole-of-hospital health services intervention and comparative effectiveness study. Clin Infect Dis 73, 487-496.
| Crossref | Google Scholar | PubMed |
25 James R et al. (2022) The feasibility and generalizability of assessing the appropriateness of antimicrobial prescribing in hospitals: a review of the Australian National Antimicrobial Prescribing Survey. JAC Antimicrob Resist 4, dlac012.
| Crossref | Google Scholar | PubMed |
26 Hardefeldt LY et al. (2022) Antimicrobial stewardship in companion animal practice: an implementation trial in 135 general practice veterinary clinics. JAC Antimicrob Resist 4, dlac015.
| Crossref | Google Scholar |
28 Bishop JL et al. (2020) Sustainability of antimicrobial stewardship programs in Australian rural hospitals: a qualitative study. Aust Health Rev 44, 415-420.
| Crossref | Google Scholar | PubMed |
30 Biezen R et al. (2021) Evaluating the implementation of a pilot quality improvement program to support appropriate antimicrobial prescribing in general practice. Antibiotics 10, 867.
| Crossref | Google Scholar | PubMed |
31 Hawes L et al. (2018) Use of electronic medical records to describe general practitioner antibiotic prescribing patterns. Aust J Gen Pract 47, 796-800.
| Crossref | Google Scholar | PubMed |
32 Hur BA et al. (2020) Describing the antimicrobial usage patterns of companion animal veterinary practices; free text analysis of more than 4.4 million consultation records. PLoS ONE 15, e0230049.
| Crossref | Google Scholar | PubMed |
33 The National Centre for Antimicrobail Stewardship (2022) MRFFRD000113: appropriate antimicrobial use: scaling surveillance using digital health. NCAS. https://www.ncas-australia.org/digital-health-research-data-infrastructure.
34 Coombe P (2023) Antimicrobial Stewardship in Australian Livestock Industries. 3rd edn. Animal Industries Antimicrobial Stewardship RD&E Strategy. https://aiasrdestrategy.com.au/wp-content/uploads/2023/12/AMS-Report-2023-v3.pdf
35 Australian Beef Sustainability Framework (2022) Annual update 2022. ABSF. https://www.sustainableaustralianbeef.com.au/globalassets/beef-sustainability/documents/absf_update_2022_web.pdf
 Dr Laura Hardefeldt is a senior lecturer at The University of Melbourne. Her research interests are in antimicrobial use, antimicrobial stewardship and antimicrobial resistance in the veterinary sector and in a One Health context. She is at the forefront of veterinary antimicrobial stewardship research nationally and internationally. |
 Prof. Karin Thursky is an infectious disease physician and clinical researcher at the Royal Melbourne Hospital, Peter MacCallum Cancer Centre and The University of Melbourne. She has played a pioneering role in the development of antimicrobial stewardship in the Australian health system and the national antimicrobial resistance strategy and is a renowned leader in antimicrobial stewardship internationally. |