Biofilms and contact lenses: problems and solutions
Mark D. P. Willcox A * , Ghayah Bahatheg B , Nicole Carnt A , Parthasarathi Kalaiselvan A , Naresh Kumar B , Rajesh Kuppusamy A B , Binod Rayamajhee A , Manjulatha Sara A , Fiona Stapleton A , Ajay K. Vijay A , Muhammad Yasir A and Tsz Tin Yu BA School of Optometry and Vision Science, University of New South Wales, Sydney, NSW 2052, Australia.
B School of Chemistry, University of New South Wales, Sydney, NSW 2052, Australia.

Mark Willcox (microbiologist) and Naresh Kumar (chemist) are Professors at UNSW Sydney with research interests in microbial colonisation of surfaces and development of new antimicrobials. |

Fiona Stapleton (optometrist) is a Scientia Professor and Nicole Carnt (optometrist) is a Scientia Associate Professor at UNSW Sydney with research interests in the epidemiology of contact lens-associated infection and inflammation. |

Ajay Vijay (optometrist) Parthasarathi Kalaiselvan (optometrist), Rajesh Kuppusamy (chemist) and Muhammad Yasir (microbiologist) are senior research associates at UNSW Sydney with interests in the control of microbial colonisation of surfaces and development of new antimicrobials. |

Ghayah Bahatheg, Binod Rayamajhee, Manjulatha Sara and Tsz Tin Yu are PhD students at UNSW Sydney researching in this area. |
Microbiology Australia 44(2) 96-99 https://doi.org/10.1071/MA23027
Submitted: 9 March 2023 Accepted: 20 April 2023 Published: 9 May 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Contact lenses provide excellent vision correction for many people worldwide. However, they can become colonised by microorganisms and this can result in infections and inflammatory responses at the surface of the eye during wear. If not quickly and appropriately treated, the infections can lead to loss of vision and even loss of the eye. The microorganisms, most commonly bacteria, that colonise the lenses can form biofilms on the lenses. For the past 25 years, we have been studying the epidemiology of contact lens-related infection and inflammation, the causative organisms, risk factors for developing the conditions, and new ways of reducing biofilm formation. This article provides an overview of this research.
Keywords: antimicrobial devices, biofilms, contact lenses, keratitis, risk factors.
Contact lenses are used by over 150 000 000 people worldwide. In Australia, ~13% of the population wear contact lenses (~3.4 million people). Contact lenses are most commonly used to correct myopia (short sightedness) but can be used to correct hyperopia (long sightedness) and other forms of refractive error. The most commonly worn lenses are soft lenses, accounting for 75% of all lenses worn worldwide.1 These soft lenses are worn by 48% of wearers on a daily disposable basis (the lens is discarded at the end of the day and a new lens worn the next day) or by 49% of wearers on a daily wear basis (the lens is cleaned and disinfected when not being worn and re-worn each day for 2 or 4 weeks before being discarded).1 Soft lenses worn on a daily wear basis are disinfected in contact lens cases each night using multipurpose disinfecting solutions by 88% of wearers,1 with the rest usually using hydrogen peroxide. After removing the lens from the eye (with clean recently washed and dried fingers) and before adding it into the contact lens case for disinfection, manufacturers recommend adding some fresh disinfecting solution to the lenses and rubbing the lens between in the palm of one hand with the fingers of the other to help remove any debris. The lenses should then be rinsed with fresh disinfecting solution and added in to the case with fresh disinfecting solution. All manufacturers have a recommended minimum disinfecting time, which is usually between 4 and 6 h.
Contact lenses and ocular surface infection and inflammation
Unfortunately, wearing contact lenses can be associated with ocular infections and inflammation. When lenses are worn on either a daily disposable or daily wear basis, ocular infections occur at ~2 per 10 000 wearers each year.2,3 However, ocular inflammation in the absence of frank infection is much more common, with 2–6 per 100 wearers suffering this each year.2
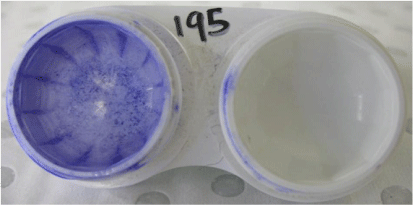
Contact lens-associated ocular infections, commonly called microbial keratitis, are caused by a wide range of different microorganisms, but Pseudomonas aeruginosa is the most common, being isolated from at least 70% of infections worldwide.4 These microorganisms can be found in biofilms on contact lenses of people with microbial keratitis (Fig. 1) as well as in their contact lens cases.5 Contact lens-associated ocular inflammation is often caused by microorganisms, most commonly bacteria such as various Gram-negative bacteria or Staphylococcus aureus, colonising contact lenses during wear.6 These bacteria are also isolated from contact lenses cases7 where they occur in biofilms (Fig. 2).
Scanning electron micrograph of a biofilm of the posterior surface of a contact lens from a patient with microbial keratitis. Pseudomonas aeruginosa was grown from the cornea and from the contact lens. Reproduced from Elder et al. (1995)5 with permission.

Biofilm-related risk factors associated with contact lens-associated infection and inflammation
Risk factors for developing contact lens-associated infection include poor compliance with hygiene instructions, disinfecting contact lenses with chlorine or heat, infrequent or no disinfection of lenses,2 using a particular kind of multipurpose contact lens disinfecting solution that contained only polyhexamethylene biguanide as the disinfectant and was marketed to be used without rubbing contact lenses with the solution,8,9 using a disinfecting solution that did not contain a surfactant, and not rubbing and rinsing lenses with a multipurpose disinfecting solution.2
Also, poor lens case hygiene (not air drying lens cases after use) and not replacing lens cases at least every 3 months3,8 are associated with greater risk of developing microbial keratitis during daily wear of contact lenses. In one study, 61% of contact lens wearers reported inadequate cleaning of lens storage cases and 13% inadequate cleaning of lenses.10 A statistical analysis has shown that simply air drying lens cases would reduce the risk of microbial keratitis by 49%, and replacing lens cases at least every 3 months would reduce the risk by 27%.8 If people replaced lens storage cases every 3 months and air dried their lens cases they would reduce their risk of keratitis by 62%.8 It was perhaps not surprising that people did not understand how to appropriately clean and replace their lens cases when we identified that there were inconsistent instructions and limited recommendations about drying positions, rinsing and rubbing of lens cases.11 Our highlighting of this resulted in disinfectant manufacturers changing their instructions to be more consistent and obvious.
Quick fixes for reducing biofilms in lens cases
We examined contact lens cases and how contact lens wearers could best care for them so that they could minimise microbial colonisation and biofilms. All lens cases become contaminated by a range of microorganisms, most commonly bacteria, during use, but the level of contamination and types of microorganisms is associated with use of different multipurpose disinfecting solutions.7 These microorganisms can coaggregate and cohere to form multispecies biofilms.12 We also showed that it was important to use the contact lens case supplied (commonly for free) with the disinfecting solution being used, as mismatching lens cases with disinfecting solutions was a risk factor for lens case contamination.13 In a laboratory study, after growing biofilms of either P. aeruginosa or S. aureus in contact lens storage cases, simply rinsing these in either disinfecting solution or hot water did not affect biofilms of S. aureus and resulted in only partial removal of biofilms of P. aeruginosa.14 Rinsing cases with multipurpose disinfecting solutions, using a clean tissue to wipe cases, and then air-drying cases face down resulted in significant reductions in the biofilms of both bacterial types.14–17 Our clinical trial comparing manufacturers’ guidelines (rinse lens case with disinfectant, air-dry face down) with the new guidelines that incorporated wiping cases with a clean tissue as well as rubbing and rinsing cases and air drying face down, resulted a halving of the median number of bacteria in lens cases (Table 1).18
| Guideline | Median numbers of microorganisms (range) | P-value |
| New: discard old solution in case, fill lens case with new disinfecting solution, rub case with clean fingers for 5 min, discard solution, wipe cases with a clean tissue, air dry face down on clean tissue | 12 (0–10 000) | 0.004 |
| Manufacturers: discard old solution in case, rinse lens case with disinfecting solution, air dry face down on clean tissue | 28 (0–100 000) |
Antimicrobial lens cases to reduce microbial biofilms
Along with recommendations to standardise and change contact lens case cleaning instructions, several manufacturers have developed silver-containing contact lens cases. We have tested these in laboratory and clinical trials. Our laboratory tests showed that silver-containing lens cases could reduce the numbers of live P. aeruginosa and other bacteria that adhered to their surfaces, especially in combination with multipurpose disinfecting solutions.15,19,20 However, this did not translate to the results from our clinical studies. Neither of the clinical trials we have conducted showed reductions in the frequency of contamination of lens cases.21,22 Although one trial found reductions in the numbers of bacteria that colonised silver cases,22 the other study found that this might be related to the types of contact lenses being worn,21 and as each study used different silver-containing cases, this might also have affected the results.
Thus, it appears that silver may have a small effect on microbial colonisation of lens cases. As we have shown, a more effective way to control colonisation and biofilm formation was to rub, rinse, tissue wipe and air-dry cases.
Development of antimicrobial contact lenses
The same microorganisms can be found in lens cases, on contact lenses and in ulcers on the eye of patients with microbial keratitis.23 This indicates that there is a likely transfer of microorganisms from cases, to lenses and then onto the eye to cause infections. However, not all cases of microbial keratitis occur during daily wear of lenses.2,3,9 Furthermore, we have shown a correlation between contamination of contact lenses by microorganisms and contamination of the domestic water supply.24,25 Thus, we, and others, have been working on developing antimicrobial lenses.
We have tested antimicrobial contact lenses produced using silver (M. D. P. Willcox, unpubl. data), quorum sensing inhibitors26 and cationic peptides27 in laboratory studies and clinical trials. The development of our cationic peptide-coated contact lenses has progressed the furthest and been reviewed previously.27
Since that review, we have tested the cationic peptide-coated contact lenses in a Phase III clinical trial. This trial examined whether wearing our cationic peptide-coated contact lenses could reduce the incidence of bacterially driven ocular surface inflammation during lens wear. The participants of the study wore the antimicrobial lens in one eye and a normal lens in the other for 3 months, replacing lenses every 2 weeks. The study was powered to show a 65% reduction in the incidence of ocular inflammation. The study was successful and showed that the lenses produced a 69% reduction in ocular inflammation.28 We also showed that they did not alter the normal microorganisms that colonise the eyelids or conjunctiva29 and that they were safe and comfortable to wear.30 However, we did show that the lenses slowly lost activity during wear most probably due to proteolysis,31 which may have been the reason they did not show any greater reduction in incidence of inflammation. We have now developed antimicrobial cationic peptide mimics32–34 and tested peptoids,35 which are proteolytically stable and some of which we have recently shown to be able to reduce microbial colonisation, to a greater degree than our cationic peptide, when bound to contact lenses. We are now setting up to test these in pre-clinical tests before embarking on clinical trials.
Conclusions
Biofilms are associated with contact lens-associated ocular infection and inflammation. Our studies have shown that wiping lens cases with a clean tissue or using silver-containing contact lens cases with appropriate disinfectants can reduce the frequency or amount of microorganisms in the cases. We are the first in the world to show that antimicrobial contact lenses can reduce the incidence of bacterially driven contact lens-associated ocular inflammation. Although we need to refine our antimicrobial contact lenses for optimum activity, we remain confident that these, combined with good hygiene practices, are likely to reduce the incidence of potentially blinding infection and inflammation with contact lenses.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors hold patents on peptide mimics US-2019-0256466-A1. The authors declare that they have no other conflicts of interest.
Declaration of funding
The research was supported by grants to M. D. P. Willcox, N. Kumar and F. Stapleton from the Australian Research Council (DP0663368; LP110100475; LP11020063; DP140102195; LP150100752; DP18010084), National Health and Medical Research Council (APP1076206; APP1183597), and Cooperative Research Centres (CRCERT; Vision CRC; IMCRC).
References
[1] Morgan, PB et al. (2023) International contact lens prescribing in 2022: key trends in prescribing highlighted by our 22nd global survey. Contact Lens Spectrum 38, 24–29.[2] Stapleton, F et al. (2007) The epidemiology of contact lens related infiltrates. Optom Vis Sci 84, 257–272.
| The epidemiology of contact lens related infiltrates.Crossref | GoogleScholarGoogle Scholar |
[3] Stapleton, F et al. (2008) The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 115, 1655–1662.
| The incidence of contact lens-related microbial keratitis in Australia.Crossref | GoogleScholarGoogle Scholar |
[4] Willcox, MDP (2017) Contact lens-related keratitis and ocular microbiology. Contact Lens Spectrum 32, 34–40, 42.
[5] Elder, MJ et al. (1995) Biofilm-related infections in ophthalmology. Eye 9, 102–109.
| Biofilm-related infections in ophthalmology.Crossref | GoogleScholarGoogle Scholar |
[6] Willcox, M et al. (2011) External ocular surface and lens microbiota in contact lens wearers with corneal infiltrates during extended wear of hydrogel lenses. Eye Contact Lens 37, 90–95.
| External ocular surface and lens microbiota in contact lens wearers with corneal infiltrates during extended wear of hydrogel lenses.Crossref | GoogleScholarGoogle Scholar |
[7] Willcox, MDP et al. (2010) Contact lens case contamination during daily wear of silicone hydrogels. Optom Vis Sci 87, 456–464.
| Contact lens case contamination during daily wear of silicone hydrogels.Crossref | GoogleScholarGoogle Scholar |
[8] Stapleton, F et al. (2012) Risk factors for moderate and severe microbial keratitis in daily wear contact lens users. Ophthalmology 119, 1516–1521.
| Risk factors for moderate and severe microbial keratitis in daily wear contact lens users.Crossref | GoogleScholarGoogle Scholar |
[9] Lim, CHL et al. (2016) Risk factors for contact lens-related microbial keratitis in Singapore. Eye 30, 447–455.
| Risk factors for contact lens-related microbial keratitis in Singapore.Crossref | GoogleScholarGoogle Scholar |
[10] Wu, Y et al. (2010) Contact lens user profile, attitudes and level of compliance to lens care. Cont Lens Anterior Eye 33, 183–188.
| Contact lens user profile, attitudes and level of compliance to lens care.Crossref | GoogleScholarGoogle Scholar |
[11] Wu, Y et al. (2010) Contact lens and lens storage case cleaning instructions: whose advice should we follow? Eye Contact Lens 36, 68–72.
| Contact lens and lens storage case cleaning instructions: whose advice should we follow?Crossref | GoogleScholarGoogle Scholar |
[12] Datta, A et al. (2018) Bacterial coaggregation and cohesion among isolates from contact lens cases. Invest Ophthalmol Vis Sci 59, 2729–2735.
| Bacterial coaggregation and cohesion among isolates from contact lens cases.Crossref | GoogleScholarGoogle Scholar |
[13] Wu, YT et al. (2015) The effect of contact lens hygiene behavior on lens case contamination. Optom Vis Sci 92, 167–174.
| The effect of contact lens hygiene behavior on lens case contamination.Crossref | GoogleScholarGoogle Scholar |
[14] Vijay, AK et al. (2015) Contact lens storage case hygiene practice and storage case contamination. Eye Contact Lens 41, 91–97.
| Contact lens storage case hygiene practice and storage case contamination.Crossref | GoogleScholarGoogle Scholar |
[15] Vijay, AK et al. (2020) Bacterial biofilm in silver-impregnated contact lens cases. Cont Lens Anterior Eye 43, 408–412.
| Bacterial biofilm in silver-impregnated contact lens cases.Crossref | GoogleScholarGoogle Scholar |
[16] Wu, YT et al. (2011) The effectiveness of various cleaning regimens and current guidelines in contact lens case biofilm removal. Invest Ophthalmol Vis Sci 52, 5287–5292.
| The effectiveness of various cleaning regimens and current guidelines in contact lens case biofilm removal.Crossref | GoogleScholarGoogle Scholar |
[17] Nakagawa, M et al. (2021) Effect of hygiene procedures on lens case contamination with povidone-iodine or multipurpose disinfecting solutions. Optom Vis Sci 98, 563–569.
| Effect of hygiene procedures on lens case contamination with povidone-iodine or multipurpose disinfecting solutions.Crossref | GoogleScholarGoogle Scholar |
[18] Wu, YT et al. (2011) Impact of lens case hygiene guidelines on contact lens case contamination. Optom Vis Sci 88, E1180–E1187.
| Impact of lens case hygiene guidelines on contact lens case contamination.Crossref | GoogleScholarGoogle Scholar |
[19] Dantam, J et al. (2011) Biocidal efficacy of silver-impregnated contact lens storage cases in vitro. Invest Ophthalmol Vis Sci 52, 51–57.
| Biocidal efficacy of silver-impregnated contact lens storage cases in vitro.Crossref | GoogleScholarGoogle Scholar |
[20] Datta, A et al. (2019) in vitro antimicrobial efficacy of silver lens cases used with a multipurpose disinfecting solution. Transl Vis Sci Technol 8, 52.
| in vitro antimicrobial efficacy of silver lens cases used with a multipurpose disinfecting solution.Crossref | GoogleScholarGoogle Scholar |
[21] Datta, A et al. (2021) In vivo efficacy of silver-impregnated barrel contact lens storage cases. Cont Lens Anterior Eye 44, 101357.
| In vivo efficacy of silver-impregnated barrel contact lens storage cases.Crossref | GoogleScholarGoogle Scholar |
[22] Dantam, J et al. (2012) In vivo assessment of antimicrobial efficacy of silver-impregnated contact lens storage cases. Invest Ophthalmol Vis Sci 53, 1641–1648.
| In vivo assessment of antimicrobial efficacy of silver-impregnated contact lens storage cases.Crossref | GoogleScholarGoogle Scholar |
[23] Konda, N et al. (2014) Microbial analyses of contact lens-associated microbial keratitis. Optom Vis Sci 91, 47–53.
| Microbial analyses of contact lens-associated microbial keratitis.Crossref | GoogleScholarGoogle Scholar |
[24] Willcox, MDP et al. (1997) Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci 74, 1030–1038.
| Potential sources of bacteria that are isolated from contact lenses during wear.Crossref | GoogleScholarGoogle Scholar |
[25] Rayamajhee, B et al. (2020) Investigating domestic shower settings as a risk factor for Acanthamoeba keratitis. Water 12, 3493.
| Investigating domestic shower settings as a risk factor for Acanthamoeba keratitis.Crossref | GoogleScholarGoogle Scholar |
[26] Zhu, H et al. (2008) Fimbrolide-coated antimicrobial lenses: their in vitro and in vivo effects. Optom Vis Sci 85, 292–300.
| Fimbrolide-coated antimicrobial lenses: their in vitro and in vivo effects.Crossref | GoogleScholarGoogle Scholar |
[27] Willcox, MDP et al. (2020) The development of an antimicrobial contact lens – from the laboratory to the clinic. Curr Protein Pept Sci 21, 357–368.
| The development of an antimicrobial contact lens – from the laboratory to the clinic.Crossref | GoogleScholarGoogle Scholar |
[28] Kalaiselvan, P et al. (2021) Effect of antimicrobial contact lenses on corneal infiltrative events: a randomized clinical trial. Transl Vis Sci Technol 10, 32.
| Effect of antimicrobial contact lenses on corneal infiltrative events: a randomized clinical trial.Crossref | GoogleScholarGoogle Scholar |
[29] Kalaiselvan, P et al. (2022) Ocular microbiota and lens contamination following Mel4 peptide-coated antimicrobial contact lens (MACL) extended wear. Cont Lens Anterior Eye 45, 101431.
| Ocular microbiota and lens contamination following Mel4 peptide-coated antimicrobial contact lens (MACL) extended wear.Crossref | GoogleScholarGoogle Scholar |
[30] Kalaiselvan, P et al. (2022) Biocompatibility and comfort during extended wear of Mel4 peptide-coated antimicrobial contact lenses. Antibiotics 11, 58.
| Biocompatibility and comfort during extended wear of Mel4 peptide-coated antimicrobial contact lenses.Crossref | GoogleScholarGoogle Scholar |
[31] Kalaiselvan, P et al. (2023) Effect of deposition and protease digestion on the ex vivo activity of antimicrobial peptide-coated contact lenses. Nanomaterials 13, 349.
| Effect of deposition and protease digestion on the ex vivo activity of antimicrobial peptide-coated contact lenses.Crossref | GoogleScholarGoogle Scholar |
[32] Bahatheg, G et al. (2022) Short tryptamine-based peptoids as potential therapeutics for microbial keratitis: structure–function correlation studies. Antibiotics 11, 1074.
| Short tryptamine-based peptoids as potential therapeutics for microbial keratitis: structure–function correlation studies.Crossref | GoogleScholarGoogle Scholar |
[33] Kuppusamy, R et al. (2018) Design and synthesis of short amphiphilic cationic peptidomimetics based on biphenyl backbone as antibacterial agents. Eur J Med Chem 143, 1702–1722.
| Design and synthesis of short amphiphilic cationic peptidomimetics based on biphenyl backbone as antibacterial agents.Crossref | GoogleScholarGoogle Scholar |
[34] Yu, TT et al. (2021) Polyphenylglyoxamide-based amphiphilic small molecular peptidomimetics as antibacterial agents with anti-biofilm activity. Int J Mol Sci 22, 7344.
| Polyphenylglyoxamide-based amphiphilic small molecular peptidomimetics as antibacterial agents with anti-biofilm activity.Crossref | GoogleScholarGoogle Scholar |
[35] Czyzewski, AM et al. (2016) In vivo, in vitro, and in silico characterization of peptoids as antimicrobial agents. PLoS One 11, e0135961.
| In vivo, in vitro, and in silico characterization of peptoids as antimicrobial agents.Crossref | GoogleScholarGoogle Scholar |