Candida biofilm formation and recurrent vulvovaginal candidiasis
Yao Sun A B , Xenia Kostoulias A C and Yue Qu A C *A Department of Microbiology, Faculty of Medicine, Nursing and Health Sciences, Biomedicine Discovery Institute, Monash University, Clayton, Vic. 3800, Australia.
B Department of Laboratory Medicine, The First Affiliated Hospital of Wenzhou Medical University, Zhejiang, 325000, PR China.
C Department of Infectious Diseases, The Alfred Hospital and Central Clinical School, Monash University, Melbourne, Vic. 3004, Australia.

Dr Yao Sun is a visiting PhD student at Monash Biomedicine Discovery Institute. Her research interests include biofilm-related hospital-acquired infections, antimicrobial resistance of Gram-negative bacteria, and collateral sensitivity of bacterial pathogens in the clinical settings. |

Dr Xenia Kostoulias is a senior research assistant at Monash Biomedicine Discovery Institute and Central Clinical School. Xenia is interested in virulence and mechanisms of antimicrobial resistance in hospital-acquired microorganisms, with a special interest in the contribution of different microbial growth modes to this process. Xenia uses a range of methods including in vivo infection models as well as static and dynamic biofilm models to conduct her investigations. |

Dr Yue Qu is a senior research fellow at the Department of Infectious Diseases, the Alfred Hospital and Monash University. His expertise is in translational medical research and has contributed significantly to the field of medical device-related biofilm infections, from disease pathogenesis to prevention and treatment. His research has a broad coverage of bacterial and fungal pathogens, different infectious diseases, including bloodstream infections, recurrent vaginal candidiasis, ventricular assist device driveline infections, and other medical device-related infections. |
Microbiology Australia 44(2) 92-95 https://doi.org/10.1071/MA23026
Submitted: 24 March 2023 Accepted: 21 April 2023 Published: 8 May 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Recurrent vulvovaginal candidiasis (RVVC), a recalcitrant Candida infection of the lower female reproductive tract, is a difficult-to-treat medical condition affecting the morbidity of many otherwise healthy women. Cumulative experimental evidence supports the involvement of biofilm formation of Candida in the pathogenesis of RVVC and its treatment failure. In this review, we will discuss important insights into the roles of Candida biofilms in RVVC.
Keywords: antifungal resistance, biofilm formation, Candida species, pathogenesis, persistence, recurrence, vulvovaginal candidiasis.
Introduction
Like many other opportunistic pathogens, Candida species inhabit the mucosal surfaces of the human gastrointestinal tract, genitourinary tract and skin as harmless commensals that become pathogenic in hosts with elevated sensitivity to fungal colonisation.1 Candida has gained considerable importance as an infective agent in women’s health, causing vulvovaginal candidiasis (VVC) in females of reproductive age.2 According to the US Centers for Disease Control and Prevention, VVC can be broadly categorised as uncomplicated VVC that is sporadic and mild-to-moderate infections caused by Candida albicans in immunocompetent women and complicated VVC that includes recurrent VVC (RVVC), severe VVC, VVC caused by non-albicans Candida, and VVC in immunocompromised patients (see https://www.cdc.gov/std/treatment-guidelines/candidiasis.htm). The terms sporadic VVC and RVVC are widely used in clinical diagnosis of VVC cases; the former refers to infections of less than three episodes per year, and the latter includes no less than three relapses or re-infections in a 12-month period, with entirely asymptomatic intervals between these episodes.3 It has been estimated that up to 75% of reproductive women experience VVC and ~5–8% suffer from RVVC.4 C. albicans remains the leading causative agent for both sporadic VVC and RVVC.5 Other non-albicans Candida species account for 10–20% of RVVC, with a reported upward trend and an emerging importance.5
Several studies evaluated antifungal susceptibility of Candida yeast cells isolated from VVC patients and reported very low percentage of azole resistance among these clinical isolates.6,7 Despite the fact that most Candida clinical isolates from VVC patients, including those with RVVC remain sensitive to azoles, long-term maintenance therapy using fluconazole often fails to eradicate Candida cells or completely cure the infection, suggesting that microbial strategies other than intrinsic resistance, such as biofilm formation may contribute to the suboptimal treatment outcome and infection recurrence.8,9 This review will discuss insights into the roles of biofilm formation of Candida, including but not limited to the albicans species, in the pathogenesis and persistence of RVVC.
The current debate: with or without Candida biofilms in the human vagina?
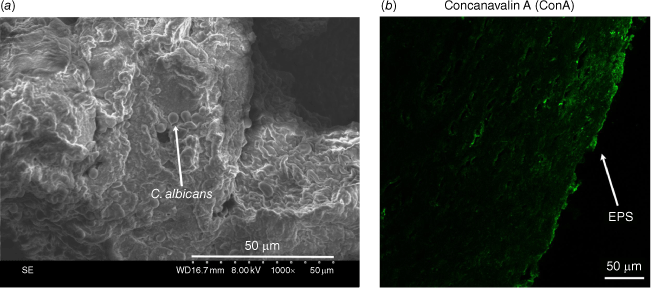
Biofilm formation has been proposed as one of the most important virulence factors of Candida spp. contributing to the occurrence of RVVC and its tolerance to antifungal treatments.9,10 Supporting this theory are the clinical pattern of RVVC that coincides with the model of relapsing infections proposed by Lewis, in which biofilm formation is the main culprit,11 and experimental findings from in vivo studies using mice as the host.9,10 Murine models of this disease, however, differ from human VVC and RVVC in several physiological aspects of the vaginal environment, including the absence of C. albicans in the vaginal microbiota, neutral vaginal pH, and dependence on exogenous estrogen to initiate fungal colonisation.10,12 Such differences have led researchers to question whether the importance of Candida biofilms in the pathogenesis of VVC and RVVC established in rodents could be transferred to humans.12 Recently, Swidsinski et al. examined vaginal tissues from patients with sporadic VVC or RVVC using fluorescence in situ hybridisation (FISH) and Candida-specific 18S rRNA probes. Based on cytogenetic findings, these authors disputed the presence of Candida biofilms in the human vagina, and concluded that VVC and RVVC was a result of polymicrobial invasion of vaginal tissues.13 In our preliminary clinical study, we observed biofilm-like Candida growths on the vaginal epithelium of RVVC patients, using scanning electron microscopy to visualise adherent Candida cells (Fig. 1a), and confocal laser scanning microscopy in combination with Concanavalin A (ConA) staining for the presence of Candida biofilm extracellular polymeric substances (EPS) (Fig. 1b). Such high-resolution visual evidence supports the presence of Candida biofilms in the vagina of RVVC patients and its possible association with the disease.
(a) Scanning electron microscopy (SEM) of human vaginal tissue showing Candida albicans monolayer biofilms grown on the vaginal epithelium of a recurrent vulvovaginal candidiasis (RVVC) patient. (b) Confocal laser scanning microscopy (CLSM) of a vaginal biopsy section stained with ConA supported the presence of Candida biofilm extracellular polymeric substance (EPS) matrix (green).
Biotic and abiotic Candida biofilms associated with RVVC
Two types of clinical biofilms may be linked to RVVC depending on their supporting substratum, including abiotic biofilms that grow on the surface of intrauterine devices (IUDs), and tissue-based biotic biofilms that are formed directly on the vaginal epithelium.10,14 IUD-based biofilms resemble in vitro biofilms cultured in 96-well microplate or on silicone pads and present either as a complex three-dimensional structure comprising of multilayer aggregates of yeast cells encased within a matrix of EPS,14 or yeast-dominated adherent monolayers.15 Many molecular regulators of Candida biofilm formation on abiotic surfaces have been identified, comprising mainly transcription factors such as Bcr1, Tec1, Efg1, Ndt80, Rob1, Med31 and Ccr4.16,17 Epithelium-based biotic Candida biofilms differ significantly from abiotic biofilms in morphology, often presenting as monolayers or microcolony biofilms in the vagina.9,10 Epithelium-based biofilms may play a more important role in RVVC than IUD-based abiotic biofilms, as many patients still suffer from RVVC without an IUD or after removal of the infected IUD.18
The possible important role of biofilm formation in the pathogenesis of RVVC
Biofilm formation of Candida has been long proposed as an important microbial strategy that contributes to the pathogenesis of VCC and RVVC. Most Candida clinical isolates from VVC patients were found to be able to form in vitro biofilms on abiotic surfaces,19 though individual clinical isolates may vary greatly in their abilities to produce biofilms in 96-well microplates.20 In the general model of Candida mucosal infections, C. albicans express multiple surface proteins (adhesins) upon immediate contact with the host epithelium, mediating microbial adherence to host cells and formation of biotic biofilms. These adhesins include the ALS family, Ece1, Hwp1, Phr1 and Phr2.21,22 The importance of Als3, Hwp1 and Phr1 in the initiation of VVC remains to be questioned. The unique acidic vaginal environment (pH < 4.2) of VVC patients represses the expression of phr123 and does not support filamentous growth of Candida that is essential for the expression of hwp1 and als3.24 The importance of Candida biofilm formation in the pathogenesis of VVC was also supported by our recent work that compared the histopathological changes of vaginal mucosa infected with wild type Candida and their bcr1 and med31 mutants.9 These mutant strains were deficient in early fungal adherence in vitro and in vivo and caused less severe vaginal infections in mice, characterised by lower fungal colonisation of the vaginal mucosa and milder host histopathological responses.9
Antifungal resistance of epithelium-associated biofilms contributes to the relapse of RVVC
In addition to its involvement in the pathogenesis of RVVC, more recently, biofilm formation of Candida has been implicated in the persistence or relapses of RVVC. Numerous in vitro studies have showed increased resistance of Candida biofilms formed in 96-well microplates to antifungal agents, in particular azoles.9,25 The elevated antifungal resistance of in vitro Candida biofilms is at least partially due to the retarded penetration of azoles into the biofilm EPS matrix.26 Biofilm-associated Candida cells contain more β-1,3-glucans and other carbohydrates than their planktonic counterpart and bind to 4–5-fold more fluconazole. Another possible mechanism of biofilms resistance is the up-regulation of efflux pumps in biofilm-embedded Candida cells, including the ABC (ATP binding cassette) transporters encoded by Cdr genes and MFS (major facilitator superfamily) transporters encoded by Mdr genes.27 Overexpression of these efflux pumps induce Candida resistance to various antifungal drugs such as azoles, terbinafine and amorolfine.28–30 The high antifungal resistance and underlying mechanisms found in abiotic Candida biofilms may not be applicable to biotic biofilms formed on the vaginal epithelium, as short-term application of antifungal drugs such as azoles remains highly effective in managing acute episodes of RVVC, suggesting transiently effective killing of biofilm cells at the infection site.31 Clinical studies evaluating the efficacy of prolonged antifungal maintenance therapies against RVVC also found a persister-based biphasic killing kinetics, supported by the relapse of infection once the treatment was discontinued.8 High tolerance of Candida biofilms to fluconazole in RVVC patients may explain why suppressive maintenance therapies, even used for a prolonged period, are effective in controlling symptomatic RVVC but are rarely curative, despite the general in vitro sensitivity of causative Candida to fluconazole.8 Our recent animal studies showed the presence of persister cells in epithelium-based biofilms that might result in a new episode after resuscitation.9,25 Persister cells are a small population equipped with delicate metabolic control and a coordinated stress response, showing subdued major energy-generating pathways, down-regulated glycolysis and protein synthesis, and tolerance to numerous antifungal drugs at high concentrations.32
Limitations of the current VVC animal model
The current murine model of VVC has been widely used to study in vivo interactions between pathogenic Candida strains and the vagina.10 The disease state of infected animals is characterised by two histopathological changes that are often associated with acute mucosal infections, sub-epithelial micro-abscesses and neutrophil infiltration.9,10 It is deduced that the current mouse VVC model may adequately represent the acute phase of VVC and RVVC, but not the chronic nature of RVVC. A new animal model is urgently needed for the comprehensive understanding of the disease pathogenesis and development of more effective therapies. Using vaginal isolates rather than laboratory reference strain with a bloodstream origin such C. albicans DAY185 or C. albicans SC5314, extending the use of exogenous estrogen and the incubation period from 48–72 h to 1–2 weeks may re-direct the infection from neutrophil-dominated acute infections to persistent and asymptomatic chronic infections.
Conclusions and future perspectives
A comprehensive understanding of the important role of Candida biofilm formation in RVVC, is crucial for the discovery of more effective antifungal therapies for this troublesome disease. Current experimental and clinical data, including that from our preliminary study, suggest a possible association between Candida biofilms and the pathogenesis of RVVC. A clinical study that recruits a substantial number of patients with accurately diagnosed RVVC or sporadic VVC, collecting vaginal tissue samples from patients at an asymptomatic period, and quantitatively and qualitatively analysing tissue samples is required to validate the presence of Candida biofilms in human RVVC. Further investigation is also needed to confirm the important role of Candida biofilm formation in the pathogenesis and persistence of RVVC, using an optimised animal model that properly represents the chronic nature of RVVC, clinical isolates from the vagina, and their genetically manipulated strains varying in biofilm formation, such as bcr1-knockout mutant strains, complementary strains, or over-expression strains.
References
[1] Cauchie, M et al. (2017) Candida and its dual lifestyle as a commensal and a pathogen. Res Microbiol 168, 802–10.| Candida and its dual lifestyle as a commensal and a pathogen.Crossref | GoogleScholarGoogle Scholar |
[2] Achkar, JM and Fries, BC (2010) Candida infections of the genitourinary tract. Clin Microbiol Rev 23, 253–73.
| Candida infections of the genitourinary tract.Crossref | GoogleScholarGoogle Scholar |
[3] Sobel, JD et al. (1998) Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 178, 203–11.
| Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations.Crossref | GoogleScholarGoogle Scholar |
[4] Sobel, JD (1992) Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis 14, S148–53.
| Pathogenesis and treatment of recurrent vulvovaginal candidiasis.Crossref | GoogleScholarGoogle Scholar |
[5] Gonçalves, B et al. (2016) Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol 42, 905–27.
| Vulvovaginal candidiasis: epidemiology, microbiology and risk factors.Crossref | GoogleScholarGoogle Scholar |
[6] Richter, SS et al. (2005) Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol 43, 2155–62.
| Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases.Crossref | GoogleScholarGoogle Scholar |
[7] Kalkanci, A et al. (2013) Candida vaginitis in non-pregnant patients: a study of antifungal susceptibility testing and virulence factors. J Obstet Gynaecol 33, 378–83.
| Candida vaginitis in non-pregnant patients: a study of antifungal susceptibility testing and virulence factors.Crossref | GoogleScholarGoogle Scholar |
[8] Collins, LM et al. (2020) Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis 24, 48–52.
| Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans.Crossref | GoogleScholarGoogle Scholar |
[9] Wu, X et al. (2020) Biofilm formation of Candida albicans facilitates fungal infiltration and persister cell formation in vaginal candidiasis. Front Microbiol 11, 1117.
| Biofilm formation of Candida albicans facilitates fungal infiltration and persister cell formation in vaginal candidiasis.Crossref | GoogleScholarGoogle Scholar |
[10] Harriott, MM et al. (2010) Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156, 3635–44.
| Candida albicans forms biofilms on the vaginal mucosa.Crossref | GoogleScholarGoogle Scholar |
[11] Lewis, K (2010) Persister cells. Annu Rev Microbiol 64, 357–72.
| Persister cells.Crossref | GoogleScholarGoogle Scholar |
[12] Cassone, A and Sobel, JD (2016) Experimental models of vaginal candidiasis and their relevance to human candidiasis. Infect Immun 84, 1255–61.
| Experimental models of vaginal candidiasis and their relevance to human candidiasis.Crossref | GoogleScholarGoogle Scholar |
[13] Swidsinski, A et al. (2019) Vulvovaginal candidiasis: histologic lesions are primarily polymicrobial and invasive and do not contain biofilms. Am J Obstet Gynecol 220, 91.e1–8.
| Vulvovaginal candidiasis: histologic lesions are primarily polymicrobial and invasive and do not contain biofilms.Crossref | GoogleScholarGoogle Scholar |
[14] Auler, ME et al. (2010) Biofilm formation on intrauterine devices in patients with recurrent vulvovaginal candidiasis. Med Mycol 48, 211–6.
| Biofilm formation on intrauterine devices in patients with recurrent vulvovaginal candidiasis.Crossref | GoogleScholarGoogle Scholar |
[15] Chassot, F et al. (2008) Can intrauterine contraceptive devices be a Candida albicans reservoir? Contraception 77, 355–9.
| Can intrauterine contraceptive devices be a Candida albicans reservoir?Crossref | GoogleScholarGoogle Scholar |
[16] Nett, JE et al. (2009) Time course global gene expression analysis of an in vivo Candida biofilm. J Infect Dis 200, 307–13.
| Time course global gene expression analysis of an in vivo Candida biofilm.Crossref | GoogleScholarGoogle Scholar |
[17] Nobile, CJ et al. (2012) A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 148, 126–38.
| A recently evolved transcriptional network controls biofilm development in Candida albicans.Crossref | GoogleScholarGoogle Scholar |
[18] Cakiroglu, Y et al. (2015) Does removal of CU-IUD in patients with biofilm forming candida really maintain regression of clinical symptoms? J Obstet Gynaecol 35, 600–3.
| Does removal of CU-IUD in patients with biofilm forming candida really maintain regression of clinical symptoms?Crossref | GoogleScholarGoogle Scholar |
[19] Paiva, LCF et al. (2012) Assessment of in vitro biofilm formation by Candida species isolates from vulvovaginal candidiasis and ultrastructural characteristics. Micron 43, 497–502.
| Assessment of in vitro biofilm formation by Candida species isolates from vulvovaginal candidiasis and ultrastructural characteristics.Crossref | GoogleScholarGoogle Scholar |
[20] Araújo Paulo de Medeiros, M et al. (2017) Characterization of virulence factors of vaginal and anal isolates of Candida albicans sequentially obtained from patients with vulvovaginal candidiasis in north-east Brazil. J Mycol Med 27, 567–72.
| Characterization of virulence factors of vaginal and anal isolates of Candida albicans sequentially obtained from patients with vulvovaginal candidiasis in north-east Brazil.Crossref | GoogleScholarGoogle Scholar |
[21] Sheppard, DC et al. (2004) Functional and structural diversity in the Als protein family of Candida albicans. J Biol Chem 279, 30480–9.
| Functional and structural diversity in the Als protein family of Candida albicans.Crossref | GoogleScholarGoogle Scholar |
[22] Hoyer, LL et al. (2008) Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family — a sticky pursuit. Med Mycol 46, 1–15.
| Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family — a sticky pursuit.Crossref | GoogleScholarGoogle Scholar |
[23] De Bernardis, F et al. (1998) The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun 66, 3317–25.
| The pH of the host niche controls gene expression in and virulence of Candida albicans.Crossref | GoogleScholarGoogle Scholar |
[24] Ponniah, G et al. (2007) State of differentiation defines buccal epithelial cell affinity for cross-linking to Candida albicans Hwp1. J Oral Pathol Med 36, 456–67.
| State of differentiation defines buccal epithelial cell affinity for cross-linking to Candida albicans Hwp1.Crossref | GoogleScholarGoogle Scholar |
[25] Wu, X et al. (2019) RAFT-derived polymethacrylates as a superior treatment for recurrent vulvovaginal candidiasis by targeting biotic biofilms and persister cells. Front Microbiol 10, 2592.
| RAFT-derived polymethacrylates as a superior treatment for recurrent vulvovaginal candidiasis by targeting biotic biofilms and persister cells.Crossref | GoogleScholarGoogle Scholar |
[26] Al-Fattani, MA and Douglas, LJ (2006) Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55, 999–1008.
| Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance.Crossref | GoogleScholarGoogle Scholar |
[27] White, TC et al. (2002) Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother 46, 1704–13.
| Resistance mechanisms in clinical isolates of Candida albicans.Crossref | GoogleScholarGoogle Scholar |
[28] Mukherjee, PK et al. (2003) Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun 71, 4333–40.
| Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols.Crossref | GoogleScholarGoogle Scholar |
[29] Prasad, R and Goffeau, A (2012) Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu Rev Microbiol 66, 39–63.
| Yeast ATP-binding cassette transporters conferring multidrug resistance.Crossref | GoogleScholarGoogle Scholar |
[30] Niimi, M et al. (2004) Regulated overexpression of CDR1 in Candida albicans confers multidrug resistance. J Antimicrob Chemother 54, 999–1006.
| Regulated overexpression of CDR1 in Candida albicans confers multidrug resistance.Crossref | GoogleScholarGoogle Scholar |
[31] Donders, G et al. (2022) Management of recurrent vulvovaginal candidosis: Narrative review of the literature and European expert panel opinion. Front Cell Infect Microbiol 12, 934353.
| Management of recurrent vulvovaginal candidosis: Narrative review of the literature and European expert panel opinion.Crossref | GoogleScholarGoogle Scholar |
[32] Li, P et al. (2015) Delicate metabolic control and coordinated stress response critically determine antifungal tolerance of Candida albicans biofilm persisters. Antimicrob Agents Chemother 59, 6101–12.
| Delicate metabolic control and coordinated stress response critically determine antifungal tolerance of Candida albicans biofilm persisters.Crossref | GoogleScholarGoogle Scholar |


