Pouch bacteria: an understudied and potentially important facet of marsupial reproduction
Toby Maidment A * and Raphael Eisenhofer B *A Queensland University of Technology, Centre for Immunology and Infection Control, Queensland Institute of Medical Research, 300 Herston Road, Brisbane, Qld 4001, Australia.
B Center for Evolutionary Hologenomics, Globe Institute, University of Copenhagen, DK-1353 Copenhagen, Denmark.

Toby Maidment is a research assistant at the Centre for Immunology and Infection Control, Queensland University of Technology. His current research focuses on koala reproductive diseases, host–microbiome interactions in the human FRT, and vaccine development. His major interests include Australian mammal conservation and microbial ecology. |

Dr Raphael Eisenhofer is a postdoctoral researcher at the Globe Institute, University of Copenhagen, Denmark, and an adjunct assistant lecturer at the University of Adelaide. His research interest is studying the microbiomes of native Australian mammals, with the long-term goal of applying microorganisms to improve mammal conservation in Australia. |
Microbiology Australia 44(1) 41-44 https://doi.org/10.1071/MA23010
Submitted: 16 January 2023 Accepted: 10 February 2023 Published: 3 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Australia is home to a rich biodiversity of marsupials that are found nowhere else. Unfortunately, many of these species are currently threatened with extinction due to introduced feral predators and other anthropogenic factors. There is growing recognition that host-associated microorganisms can play important roles for animal health, with billions of dollars currently being invested into human gut microbiome research and the development of microbiome-based therapeutics to improve human health. Can microorganisms also be harnessed to stem the tide of marsupial extinctions? In this review, we provide an overview of some of the challenges facing Australia’s marsupials, and our current understanding of the microbiology of the marsupial pouch. We also propose outstanding research questions pertaining to the marsupial pouch, which, if addressed, may provide actionable knowledge and novel microbial therapies that could help stem the tide of marsupial extinctions in Australia.
Introduction
The island continent of Australia is globally significant for its unique biodiversity, and is home to the most distinctive terrestrial mammal fauna on Earth. Australia is particularly notable for harbouring the world’s richest diversity of extant marsupials, with over 200 species found only in Australia, across habitats ranging from arid deserts to tropical rainforests.1 These include several iconic taxa such as kangaroos, koalas, and wombats, many of which hold great cultural significance to Australians.
Marsupials diverged from eutherian (‘placental’) mammals c. 160 Ma during the early Jurassic period,2 with Australian lineages evolving largely in geographic isolation following separation from the Gondwanan supercontinent c. 40 Ma. Unsurprisingly given this divergence time, several anatomical and physiological differences exist between marsupials and eutherians – the most notable being reproductive strategy. Marsupials give birth after a short gestation period to undeveloped young (hereafter ‘joeys’), which crawl toward and latch onto a teat located in a maternal pouch (marsupium) and continue development ex utero through lactation. This means that, unlike eutherian neonates, marsupial joeys are exposed to the external environment prior to developing a functional immune system and are thus highly vulnerable to microbial infections.3 Despite this risk, however, ex utero development is highly advantageous for survival in Australia’s adverse and often unpredictable environments, as it allows for increased maternal control of reproductive effort during unfavourable conditions.4
Challenges facing Australia’s marsupials
Despite harbouring much of the earth’s mammalian diversity, Australia currently reports the highest mammalian extinction rate on earth, with 39 mammal species confirmed to have become extinct in the ~200 years since European colonisation.5 These extinctions represent >10% of all global mammal extinctions during this period, and amount to a major loss of global marsupial diversity.1 With 52 Australian mammals currently listed as Endangered (incl. 9 Critically Endangered) and 58 listed as Vulnerable, further mammalian extinctions are likely in coming decades without substantial intervention.5 The primary drivers of population declines among Australian mammals are predation by introduced species such as feral cats (Felis catus) and foxes (Vulpes vulpes), and habitat loss due to extensive land clearing.1 Several endangered marsupial species, including quolls, koalas and Tasmanian devils, have also experienced significant population declines due to disease, the spread of which is exacerbated by increased habitat fragmentation.6–8 Additionally, Australia’s mammals are vulnerable to increased frequency of extreme weather events such as bushfire, floods and drought resulting from climate change.9
Given the complexities associated with managing these threats, establishing healthy insurance populations ex situ through captive breeding is essential for preventing further mammal extinctions in Australia. These captive populations are an invaluable resource for reintroduction and repopulation programmes, as well as furthering research into threat and disease adaptation. Captive breeding is also beneficial for expanding and maintaining genetic diversity through the application of selective breeding and recent advances in artificial insemination.10 This is particularly useful for the genetic rescue of increasingly fragmented mammal populations by targeted translocation.11
Despite these benefits, however, the successful management and breeding of endangered marsupials in captivity presents several challenges. For instance, the reproductive success of several species can be hindered by low fertility and conception rates. This can occur because of several factors, including breeding incompatibility among captive stock, behavioural or social shifts in captivity, and dietary changes.12 Overall breeding outputs in some species are also affected by high rates of neonatal and juvenile mortality, which can occur because of infections, behavioural stress caused by environmental modification in captivity and other environmental factors that remain poorly understood.13–15 The issue of neonatal mortality during early development is of particular concern in captive koala colonies, where seasonal mortality rates among pouch young can exceed 50%, predominantly due to bacterial infections.13
Recent research into host–microbiome associations has shown that microbes play important roles in animal health and evolution.16 Monitoring and modulating the gut microbiome is being proposed as a tool for improving conservation outcomes for endangered animals.17 Comparatively less is known about host-microbiome associations in the context of the mammalian female reproductive tract (FRT; e.g. vagina, urogenital tract, milk, pouch), especially for endangered species. However, growing evidence suggests that FRT-associated microbial communities may play important roles in several host functions essential for reproductive and developmental success.18 Extending this logic to marsupials, we propose that microbial communities in the maternal pouch may represent an important and overlooked factor of successful reproduction (Fig. 1).
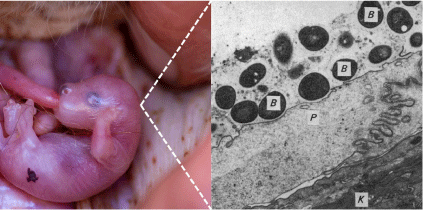
|
Pouch microbiology – what is known
To date, microbiological research into the marsupial pouch has received little attention, with fewer than ten studies being published since the first in 1972 by Yadav, Stanley and Waring on the quokka (Setonix brachyurus).19 This and the three other cultivation-dependent studies found substantial reductions in, or a complete lack of, culturable bacteria from koala, tammar wallaby, brushtail possum and quokka pouches prior to and immediately following birth.19–22 The first use of cultivation-independent techniques to study microbes in the marsupial pouch was in 2004 by Deakin and Cooper.23 Using a mixture of cultivation-dependent and -independent methods on common brushtail possums (Trichosurus vulpecula), they found similar trends to the prior cultivation-dependent studies.23 Similar trends were also observed in a cultivation-independent study on tammar wallaby (Macropus eugenii) pouches.24 Two further cultivation-independent studies using high-throughput 16S rRNA gene sequencing on Tasmanian devil pouches (Sarcophilus harrisii) found differences in composition, but not diversity, between lactating and non-lactating females.25,26 Overall, the trend in these pouch microbiology studies is a shift in diversity or composition associated with the reproductive status of the host, generally with a reduction in bacterial diversity close to and immediately following joey birth.
However, it is increasingly recognised that DNA contamination can compromise 16S rRNA gene studies – particularly those targeting samples with low microbial biomass.27 Recently, Weiss et al. applied a robust experimental framework, including the collection and sequencing of numerous negative control samples and the quantitative estimation of sample biomass using qPCR, to demonstrate that the southern hairy-nosed wombat (SHNW) (Lasiorhinus latifrons) pouch does indeed harbour resident bacteria.28 Weiss et al. analysed multiple sample types from 26 wild SHNWs to show that the pouch of reproductively active females is compositionally distinct from other body sites and is dominated by a handful of Gram-positive bacteria.28 The closest match for three of the five dominant pouch bacteria were to pouch bacterial isolates from tammar wallabies,24 with 16S rRNA gene sequence divergence consistent with the estimated divergence time between tammar wallabies and wombats – offering tantalising (albeit preliminary) evidence for co-speciation between pouch bacteria and marsupials.
Characterisation of pouch-associated microbial communities has also highlighted a potential link between bacterial composition and reproductive failure. This was demonstrated in a recent study (Maidment) of 38 captive koalas (Phascolarctos cinereus), where females who lost pouch young exhibited a significantly different pouch microbial compositional profile to females rearing to full term. Interestingly, although both animal groups showed similar decreases in microbial richness between mating and parturition, the pouch microbiota of successful mothers re-diversified in the months following parturition, whereas unsuccessful mothers remained dominated by Enterobacteriaceae until loss of young occurred 5–7 months post-partum.29 Taken together with similar findings in koalas by Osawa et al.21 and O’Callaghan,13 these recent findings add further weight to the hypothesis that dysbiosis of microbial communities in the marsupial pouch may be associated with neonatal mortality.
Unknowns and future directions
The past decade has seen a rapid accumulation of evidence demonstrating that the gut microbiome can play important roles in host health,16 and some have suggested that the gut microbiome should be considered for animal conservation.17 We wish to extend this idea and hypothesise that some pouch bacteria are beneficial to the reproductive success of marsupials. One beneficial function that pouch bacteria could bring to their hosts is the competitive exclusion of potentially harmful microorganisms from the pouch. The exact mechanisms are unknown, but bacteria are known to use a range of antagonistic tools to gain an advantage over heterospecific bacteria.30 Such bacteria could have co-speciated with their marsupial hosts and are thus likely well adapted to surviving endogenous host antimicrobial defences. Perturbations of the ‘natural’ pouch microbiome could therefore have detrimental impacts to the reproductive success of marsupials by increased inflammation or joey mortality caused by opportunistic microorganisms. Such disruptions to the pouch microbiome could be caused by various factors present in captivity (which can affect the gut microbiome31), such as horizontal transfer of pathogens from humans, antibiotic treatment, or the lack of vertical transmission of pouch bacteria from mother to joey (in cases where an underdeveloped joey is rescued from a dead mother’s pouch).
Our current understanding of marsupial pouch microbiology is limited. More experiments are needed to confirm a link between pouch bacteria and the reproductive success of marsupials, and to identify factors that may influence the composition of the pouch microbiome across marsupials. Some key outstanding questions that we think should be addressed are:
Are captive pouch microbiomes different from wild?
For a given marsupial species, is there a ‘healthy’ pouch microbiome? If so, can diagnostic tests be developed to aid breeding programs?
Can pouch bacteria competitively exclude opportunistic pathogens? If so, by which mechanisms?
How do pouch bacteria evade endogenous host defences?
Can pouch bacteria influence the immunological profile of the pouch? If so, how?
Do pouch bacteria influence the development of the joey’s immune system? If so, what are the implications for the joey’s future health?
Have pouch bacteria been co-speciating with their marsupial hosts?
Can the pouch microbiome be manipulated by probiotics or pouch-microbiome transplants?
Such knowledge could be applied in a conservation management context, leading to the development of novel tools and therapies to improve the captive breeding success of marsupials and help stem the tide of marsupial extinctions in Australia.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] Woinarski, JCZ et al. (2015) Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proc Natl Acad Sci USA 112, 4531–4540.| Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement.Crossref | GoogleScholarGoogle Scholar |
[2] dos Reis, M et al. (2012) Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc R Soc B Biol Sci 279, 3491–3500.
| Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny.Crossref | GoogleScholarGoogle Scholar |
[3] Krause, WJ et al. (1978) Postnatal development of the epidermis in a marsupial, Didelphis virginiana. J Anat 125, 85–99.
[4] Tyndale-Biscoe H, Renfree M (1987) Breeding biology of marsupials by family. In Reproductive Physiology of Marsupials. pp. 14–94. Cambridge University Press.
[5] Australian Government. EPBC Act List of Threatened Fauna. https://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl (accessed 14 January 2023)
[6] Madden, D et al. (2018) Koala immunology and infectious diseases: how much can the koala bear? Dev Comp Immunol 82, 177–185.
| Koala immunology and infectious diseases: how much can the koala bear?Crossref | GoogleScholarGoogle Scholar |
[7] Peacock, D and Abbott, I (2014) When the ‘native cat’ would ‘plague’: historical hyperabundance in the quoll (Marsupialia : Dasyuridae) and an assessment of the role of disease, cats and foxes in its curtailment. Aust J Zool 62, 294–344.
| When the ‘native cat’ would ‘plague’: historical hyperabundance in the quoll (Marsupialia : Dasyuridae) and an assessment of the role of disease, cats and foxes in its curtailment.Crossref | GoogleScholarGoogle Scholar |
[8] Pye, RJ et al. (2016) Devil facial tumor disease. Vet Pathol 53, 726–736.
| Devil facial tumor disease.Crossref | GoogleScholarGoogle Scholar |
[9] Hughes, L (2011) Climate change and Australia: key vulnerable regions. Reg Environ Change 11, 189–195.
| Climate change and Australia: key vulnerable regions.Crossref | GoogleScholarGoogle Scholar |
[10] Johnston, S (2019) Challenges associated with the development and transfer of assisted breeding technology in marsupials and monotremes: lessons from the koala, wombat and short-beaked echidna. Reprod Fertil Dev 31, 1305–1314.
| Challenges associated with the development and transfer of assisted breeding technology in marsupials and monotremes: lessons from the koala, wombat and short-beaked echidna.Crossref | GoogleScholarGoogle Scholar |
[11] Seddon JM, Schultz B (2020) Koala conservation in Queensland, Australia: a role for assisted gene flow for genetic rescue? In Conservation Genetics in Mammals: Integrative Research Using Novel Approaches (Ortega J, Maldonado JE, eds). pp. 331–349. Springer International Publishing.
[12] Hogan, LA et al. (2013) Wombat reproduction (Marsupialia; Vombatidae): an update and future directions for the development of artificial breeding technology. Reproduction 145, R157–R173.
| Wombat reproduction (Marsupialia; Vombatidae): an update and future directions for the development of artificial breeding technology.Crossref | GoogleScholarGoogle Scholar |
[13] O’Callaghan P (1996) Growth and mortality of koala pouch and back young. In Proceedings of the Australian Koala Foundation Annual Conference, Coolangatta, Qld, Australia. pp. 101–109. Australian Koala Foundation, Brisbane, Qld, Australia.
[14] Hayward, MW et al. (2005) Mortality and survivorship of the quokka (Setonix brachyurus) (Macropodidae : Marsupialia) in the northern jarrah forest of Western Australia. Wildl Res 32, 715–722.
| Mortality and survivorship of the quokka (Setonix brachyurus) (Macropodidae : Marsupialia) in the northern jarrah forest of Western Australia.Crossref | GoogleScholarGoogle Scholar |
[15] Thompson, CK et al. (2015) Survival, age estimation and sexual maturity of pouch young of the brush-tailed bettong (Bettongia penicillata) in captivity. Aust Mammal 37, 29–38.
| Survival, age estimation and sexual maturity of pouch young of the brush-tailed bettong (Bettongia penicillata) in captivity.Crossref | GoogleScholarGoogle Scholar |
[16] McFall-Ngai, M et al. (2013) Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci 110, 3229–3236.
| Animals in a bacterial world, a new imperative for the life sciences.Crossref | GoogleScholarGoogle Scholar |
[17] Trevelline, BK et al. (2019) Conservation biology needs a microbial renaissance: a call for the consideration of host-associated microbiota in wildlife management practices. Proc R Soc B Biol Sci 286, 20182448.
| Conservation biology needs a microbial renaissance: a call for the consideration of host-associated microbiota in wildlife management practices.Crossref | GoogleScholarGoogle Scholar |
[18] Comizzoli, P et al. (2021) Interactions between reproductive biology and microbiomes in wild animal species. Anim Microbiome 3, 87.
| Interactions between reproductive biology and microbiomes in wild animal species.Crossref | GoogleScholarGoogle Scholar |
[19] Yadav, M et al. (1972) The microbial flora of the gut of the pouch-young and the pouch of a marsupial, Setonix brachyurus. J Gen Microbiol 70, 437–442.
| The microbial flora of the gut of the pouch-young and the pouch of a marsupial, Setonix brachyurus.Crossref | GoogleScholarGoogle Scholar |
[20] Charlick, J et al. (1981) Quantitative alterations of the aerobic bacterial flora of the pouch of Setonix brachyurus (quokka) during oestrus, anoestrus, pregnancy and lactating anoestrus (pouch young). Aust J Exp Biol Med Sci 59, 743–751.
| Quantitative alterations of the aerobic bacterial flora of the pouch of Setonix brachyurus (quokka) during oestrus, anoestrus, pregnancy and lactating anoestrus (pouch young).Crossref | GoogleScholarGoogle Scholar |
[21] Osawa, R et al. (1992) Microflora of the pouch of the koala (Phascolarctos cinereus). J Wildl Dis 28, 276–280.
| Microflora of the pouch of the koala (Phascolarctos cinereus).Crossref | GoogleScholarGoogle Scholar |
[22] Old, JM and Deane, EM (1998) The effect of oestrus and the presence of pouch young on aerobic bacteria isolated from the pouch of the tammar wallaby, Macropus eugenii. Comp Immunol Microbiol Infect Dis 21, 237–245.
| The effect of oestrus and the presence of pouch young on aerobic bacteria isolated from the pouch of the tammar wallaby, Macropus eugenii.Crossref | GoogleScholarGoogle Scholar |
[23] Deakin, JE and Cooper, DW (2004) Characterisation of and immunity to the aerobic bacteria found in the pouch of the brushtail possum Trichosurus vulpecula. Comp Immunol Microbiol Infect Dis 27, 33–46.
| Characterisation of and immunity to the aerobic bacteria found in the pouch of the brushtail possum Trichosurus vulpecula.Crossref | GoogleScholarGoogle Scholar |
[24] Chhour, K-L et al. (2010) An observational study of the microbiome of the maternal pouch and saliva of the tammar wallaby, Macropus eugenii, and of the gastrointestinal tract of the pouch young. Microbiology 156, 798–808.
| An observational study of the microbiome of the maternal pouch and saliva of the tammar wallaby, Macropus eugenii, and of the gastrointestinal tract of the pouch young.Crossref | GoogleScholarGoogle Scholar |
[25] Cheng, Y et al. (2015) The Tasmanian devil microbiome—implications for conservation and management. Microbiome 3, 76.
| The Tasmanian devil microbiome—implications for conservation and management.Crossref | GoogleScholarGoogle Scholar |
[26] Peel, E et al. (2016) Cathelicidins in the Tasmanian devil (Sarcophilus harrisii). Sci Rep 6, 35019.
| Cathelicidins in the Tasmanian devil (Sarcophilus harrisii).Crossref | GoogleScholarGoogle Scholar |
[27] Eisenhofer, R et al. (2019) Contamination in low microbial biomass microbiome studies: issues and recommendations. Trends Microbiol 27, 105–117.
| Contamination in low microbial biomass microbiome studies: issues and recommendations.Crossref | GoogleScholarGoogle Scholar |
[28] Weiss, S et al. (2021) Host reproductive cycle influences the pouch microbiota of wild southern hairy-nosed wombats (Lasiorhinus latifrons). Anim Microbiome 3, 13.
| Host reproductive cycle influences the pouch microbiota of wild southern hairy-nosed wombats (Lasiorhinus latifrons).Crossref | GoogleScholarGoogle Scholar |
[29] Maidment T (2022) Characterisation of the pouch microbiome and association with reproductive outcomes in the koala (Phascolartos cinereus). MPh thesis, Queensland University of Technology.
[30] Granato, ET et al. (2019) The evolution and ecology of bacterial warfare. Curr Biol 29, R521–R537.
| The evolution and ecology of bacterial warfare.Crossref | GoogleScholarGoogle Scholar |
[31] McKenzie, VJ et al. (2017) The effects of captivity on the mammalian gut microbiome. Integr Comp Biol 57, 690–704.
| The effects of captivity on the mammalian gut microbiome.Crossref | GoogleScholarGoogle Scholar |


