Melioidosis in northern Australia
Josh Hanson A B * and Simon Smith AA Department of Medicine, Cairns Hospital, Cairns, Qld, Australia.
B The Kirby Institute, University of New South Wales, Sydney, NSW, Australia.

Dr Josh Hanson is a general and infectious diseases physician based in Cairns. He is interested in the clinical management of infectious diseases in resource poor and remote settings. |

Dr Simon Smith is an infectious diseases and general internal medicine physician in Cairns. His research interests include melioidosis, leptospirosis, and management of severe clinical manifestations of tropical diseases. |
Microbiology Australia 43(3) 120-124 https://doi.org/10.1071/MA22038
Submitted: 30 May 2022 Accepted: 9 July 2022 Published: 2 September 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Burkholderia pseudomallei, the environmental bacterium that causes melioidosis, is endemic to northern Australia. Melioidosis is a strongly seasonal disease, occurring predominantly in individuals with specific comorbidities that include diabetes mellitus, chronic kidney disease, chronic lung disease, immunosuppresion, malignancy and hazardous alcohol use. Most patients are bacteraemic and the majority have pneumonia, however, the infection can involve almost any organ, with the skin, soft tissues, genitourinary system, bones, and joints frequently affected; multi-organ involvement is also common. Central nervous system involvement is less frequent but is more likely to cause death and long-term disability. The incidence of melioidosis is increasing in Australia, but improvements in management have resulted in the local case-fatality rate declining to approximately 10%. Further progress requires greater awareness of the disease and the development of technologies that might expedite diagnosis. A deeper understanding of the disease’s pathophysiology – particularly the role of virulence factors – may also help define optimal management strategies, including the duration of antimicrobial therapy and the role of adjunctive treatments. Public health strategies that address the risk factors for this opportunistic infection – and the social inequity that drives them – would also reduce the morbidity and mortality of this life-threatening disease.
Keywords: bacterial infection, Burkholderia pseudomallei, clinical medicine, epidemiology, indigenous health, melioidosis, microbiology, public health, sepsis, tropical Australia.
Background
Melioidosis, caused by the environmental, saprophytic bacterium Burkholderia pseudomallei, is one of the most common causes of sepsis in tropical Australia.1,2 Even with optimal supportive care, the case-fatality rate is approximately 10%, but outside endemic areas, it remains a neglected disease.3 B. pseudomallei lives in the soil and surface water across the tropical north of Australia (above latitude 20°S), but outbreaks due to contaminated water have occurred further south (latitude 25.5°S) and focal areas of endemicity have even been described in temperate regions (latitude 31°S).4 In humans, melioidosis can involve almost any organ, with the clinical presentation a result of the complex interplay between host, pathogen, and the environment.5,6 B. pseudomallei can also infect animals, with pigs, goats, sheep, and camels among the most susceptible.4
Epidemiology
In tropical Australia melioidosis is strongly seasonal, with most human cases occurring during the summer wet season when moist soil provides optimal conditions for the growth and survival of B. pseudomallei. Heavy rainfall also disturbs the topsoil and clustering after severe weather events is documented.7 The incidence of melioidosis is increasing in Australia and is highest in the Top End of the Northern Territory, where an annual rate of up to 50.2/100 000 population has been reported.8,9 However, while anthropogenic climate change is anticipated to increase the future incidence of many seasonal infectious diseases, environmental disruption resulting from urban expansion appears to be having a greater impact on the incidence of melioidosis in Australia presently.10,11
The predominant mode of exposure is thought to be percutaneous, although inhalation and ingestion are also documented.12 Very few of these exposures lead to clinical symptoms; however, patients with specific comorbidities – particularly diabetes mellitus, chronic lung disease, chronic kidney disease, immunosuppression, malignancy, and hazardous alcohol consumption – are more likely to develop disease.8,13,14 Many of these comorbidities are more common among socioeconomically disadvantaged communities and this is one of the reasons why Aboriginal and Torres Strait Islander Australians bear a disproportionate burden of the disease.8,12,15 As tropical Australia is a popular tourist destination, melioidosis should also be considered in patients who have travelled to the region and have predisposing comorbidities and a compatible clinical syndrome. Person-to-person transmission is extremely uncommon, and no special precautions are required for health workers caring for these patients.
Clinical presentation
Approximately 85% of melioidosis cases have an acute presentation although more subacute presentations – with symptoms present for longer than 2 months – are also seen; reactivation from latent infection occurs but it is less frequent than previously believed, representing approximately 3% of all cases.8
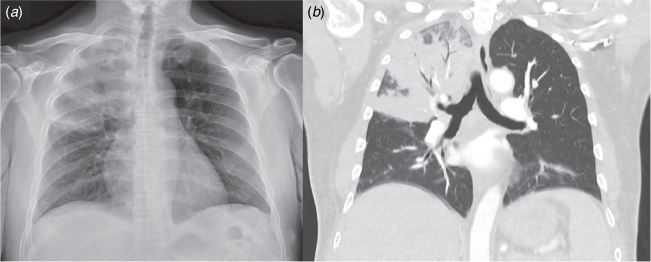
Melioidosis can involve almost any organ, but pneumonia is present in most cases8,13,14 (Fig. 1). Lung involvement often resembles a typical community-acquired pneumonia with a short history of fever, rigors, cough, and dyspnoea. However, patients may also present with respiratory symptoms that have failed to respond to weeks of usual empirical outpatient therapies. This subacute presentation can bear some resemblance to tuberculosis, but in Northern Australia, pulmonary melioidosis is the more likely diagnosis.
|
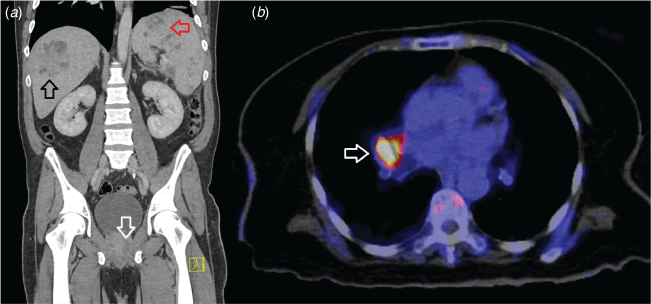
Bacteraemia is present in 56–70% of Australian cases and up to a quarter present with septic shock.16,17 Other typical presentations include skin and soft tissue infections, abscesses of the liver and spleen and infections of the bones and joints. Prostatic involvement, which occurs in ~20% of affected men, is a characteristic manifestation.8 Multi-organ involvement is also common (Fig. 2). Central nervous system involvement occurs in only 4% of cases, but is one of the most feared manifestations and may present as encephalomyelitis, brain abscess, meningitis or as an extradural collection.18 B. pseudomallei may also cause lymphadenopathy, nodules or masses that may be mistaken for neoplastic lesions, emphasising the importance of sending biopsies for culture in the appropriate clinical context (Fig. 2).
Less than 5% of cases occur in children, which is likely to be explained by a lower prevalence of predisposing comorbidities in this population. Affected children usually have milder disease, with localised skin infection the most common manifestation.19 However, disseminated disease is also seen and fatalities have been reported in previously well children despite optimal supportive care.20,21 Fatal paediatric cases may be due to the presence of virulence factors, which have been linked to clinical phenotype and prognosis in specific clinical situations, or the result of a larger inoculum.18,22
Microbiology
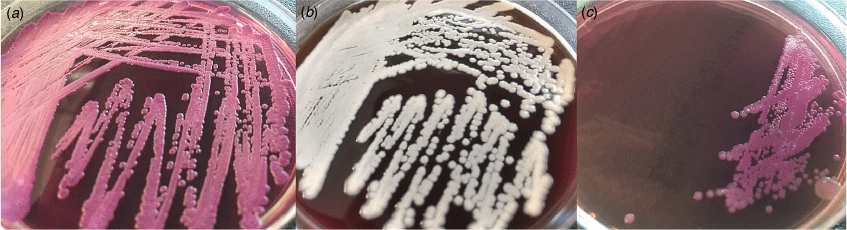
The diagnosis of melioidosis requires the culture of B. pseudomallei – a small, Gram-negative, oxidase-positive, motile, aerobic bacillus – from blood, sputum, urine, pus, or other clinical specimens.23 Although the use of selective media can facilitate diagnosis, the organism also grows well on traditional media24 (Fig. 3). True colonisation is extremely uncommon, therefore isolation of B. pseudomallei almost always warrants treatment. In high-volume centres, laboratory scientists are adept at identifying B. pseudomallei; however, laboratories unfamiliar with the organism may disregard it as an environmental contaminant.5
|
There is interest in the use of lateral flow immunoassay testing, PCR and MALDI-TOF to expedite the diagnosis of melioidosis, however PCR has not been sufficiently sensitive or specific for direct detection from clinical samples, and while the lateral flow immunoassay shows promise for the rapid diagnosis of melioidosis directly from pus and urine, it is not yet widely available, nor incorporated into validated diagnostic algorithms.25–27 Serology has very limited utility in the diagnosis of the disease as it has poor sensitivity and may be positive in healthy individuals in endemic areas.26
Therapy
There are two phases of antimicrobial therapy for melioidosis: the initial intravenous intensive phase aims to prevent death, while the subsequent oral eradication therapy (beginning immediately after the initial intensive phase) aims to prevent disease relapse.
B. pseudomallei is intrinsically resistant to penicillin, ampicillin, first- and second-generation cephalosporins, gentamicin, tobramycin, and streptomycin, while in vitro testing of the organism against quinolones usually shows resistance or intermediate susceptibility. Therefore, during the initial intensive phase it is recommended that patients receive ceftazidime or meropenem; the duration of therapy varies with the clinical phenotype, although a minimum of 2 weeks is recommended and source control is essential.28 Adjunctive trimethoprim-sulfamethoxazole (TMP-SMZ) is often added to patients with extrapulmonary infection due to its intracellular activity and ability to penetrate tissues, although the clinical benefit of this approach is uncertain.28 Granulocyte colony-stimulating factor is used by some clinicians in the management of critically ill patients; however, there are no data from randomised controlled trials to support its routine use.17
Recrudescence and relapse – defined as return of clinical disease during and after the eradication phase respectively – can be fatal. Recrudescence occurs in approximately 5% of Australian patients while relapse occurs in up to 4%, and both are more common in patients who are unable to adhere to prescribed therapies.8,29 However, adherence to currently recommended treatment regimens can be challenging: Australian treatment guidelines recommend a minimum of 3 months of high-dose TMP-SMZ during the eradication phase.28 Even with the addition of daily folic acid, up to 30% of patients receiving high-dose TMP-SMZ have side effects that necessitate cessation, dose reduction or substitution of doxycycline or amoxycillin-clavulanate, agents that are less effective.28,30 Accordingly, there has been a gradual evolution in clinical practice to prescribe longer courses of intravenous therapy and there is interest in high volume centres in abbreviating the duration of the oral eradication phase of therapy.31
Outcomes
The case-fatality rate of melioidosis has declined significantly in Australia over recent decades; this is likely to be explained, predominantly, by earlier recognition of the patient with sepsis and advances in critical care. Prompt administration of antibiotics with activity against B. pseudomallei has been facilitated by the electronic promulgation of national guidelines for melioidosis management, which also recommend empirical meropenem when melioidosis is a possible diagnosis.28 However, even in Australia’s well resourced health system, the case-fatality rate remains approximately 10%. This is frequently linked to underlying comorbidities and the social determinants of health that drive them.8,15 Furthermore, premature death in survivors is common, with approximately one-quarter of survivors dying within 5 years, frequently from the comorbidity that predisposed them to their melioidosis.32 These deaths are often preventable; the episode of melioidosis is therefore also an opportunity for clinicians to optimise the management of these comorbidities to improve patients’ overall long-term outcomes.
Prevention
In endemic areas public health strategies to prevent melioidosis largely revolve around advising individuals with predisposing comorbidities to minimise exposure to soil and surface water during the wet season and to stay indoors during heavy rains. However, it is recognised that adherence to these recommendations is challenging. There are data to support chemoprophylaxis in selected populations, but the relatively low incidence in even high-risk populations and the potential life-threatening side-effects of TMP-SMZ means that this is likely to have a very limited role in combating the disease.33,34 Vaccines are under development, although none have yet undergone human trials.35 Public health strategies to reduce the incidence of predisposing risk factors – or at least optimise their management – might reduce the burden of melioidosis as well as other complications from these comorbidities.
Conclusions
Even with optimal supportive care approximately 10% of Australian patients with melioidosis will die. A greater understanding of the disease’s pathophysiology will inform management strategies, including the optimal duration of antimicrobial therapy and the utility of adjunctive treatments. The development of effective public health strategies to prevent melioidosis would also be expected to reduce the significant burden of this life-threatening disease, which, despite a rising incidence, remains under-recognised outside of tropical Australia.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The authors acknowledge the assistance of Ms Shannon Clarke in the preparation of Fig. 3.
References
[1] Davis, JS et al.. (2011) Sepsis in the tropical Top End of Australia’s Northern Territory: disease burden and impact on Indigenous Australians. Med J Aust 194, 519–24.| Sepsis in the tropical Top End of Australia’s Northern Territory: disease burden and impact on Indigenous Australians.Crossref | GoogleScholarGoogle Scholar |
[2] Hanson, J et al.. (2020) The applicability of commonly used predictive scoring systems in Indigenous Australians with sepsis: an observational study. PLoS One 15, e0236339.
| The applicability of commonly used predictive scoring systems in Indigenous Australians with sepsis: an observational study.Crossref | GoogleScholarGoogle Scholar |
[3] Savelkoel, J et al.. (2021) A call to action: time to recognise melioidosis as a neglected tropical disease. Lancet Infect Dis 22, e176–82.
| A call to action: time to recognise melioidosis as a neglected tropical disease.Crossref | GoogleScholarGoogle Scholar |
[4] Smith, S et al.. (2018) Melioidosis: an Australian perspective. Trop Med Infect Dis 3, 27.
| Melioidosis: an Australian perspective.Crossref | GoogleScholarGoogle Scholar |
[5] Inglis, TJJ (2021) Melioidosis in Australia. Microbiol Aust 42, 96–9.
| Melioidosis in Australia.Crossref | GoogleScholarGoogle Scholar |
[6] Hempenstall, AJ et al.. (2019) Melioidosis in the Torres Strait Islands, Australia: exquisite interplay between pathogen, host, and environment. Am J Trop Med Hyg 100, 517–21.
| Melioidosis in the Torres Strait Islands, Australia: exquisite interplay between pathogen, host, and environment.Crossref | GoogleScholarGoogle Scholar |
[7] Kaestli, M et al.. (2016) The association of melioidosis with climatic factors in Darwin, Australia: a 23-year time-series analysis. J Infect 72, 687–97.
| The association of melioidosis with climatic factors in Darwin, Australia: a 23-year time-series analysis.Crossref | GoogleScholarGoogle Scholar |
[8] Currie, BJ et al.. (2021) The Darwin Prospective Melioidosis Study: a 30-year prospective, observational investigation. Lancet Infect Dis 21, 1737–46.
| The Darwin Prospective Melioidosis Study: a 30-year prospective, observational investigation.Crossref | GoogleScholarGoogle Scholar |
[9] Smith, S et al.. (2021) Increased incidence of melioidosis in Far North Queensland, Queensland, Australia, 1998–2019. Emerg Infect Dis 27, 3119–23.
| Increased incidence of melioidosis in Far North Queensland, Queensland, Australia, 1998–2019.Crossref | GoogleScholarGoogle Scholar |
[10] Kaestli, M et al.. (2009) Landscape changes influence the occurrence of the melioidosis bacterium Burkholderia pseudomallei in soil in northern Australia. PLoS Negl Trop Dis 3, e364.
| Landscape changes influence the occurrence of the melioidosis bacterium Burkholderia pseudomallei in soil in northern Australia.Crossref | GoogleScholarGoogle Scholar |
[11] Fairhead, LJ et al.. (2022) The seasonality of infections in tropical Far North Queensland, Australia: a 21-year retrospective evaluation of the seasonal patterns of six endemic pathogens. PLOS Glob Public Health 2, e0000506.
| The seasonality of infections in tropical Far North Queensland, Australia: a 21-year retrospective evaluation of the seasonal patterns of six endemic pathogens.Crossref | GoogleScholarGoogle Scholar |
[12] Cheng, AC and Currie, BJ (2005) Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18, 383–416.
| Melioidosis: epidemiology, pathophysiology, and management.Crossref | GoogleScholarGoogle Scholar |
[13] Stewart, JD et al.. (2017) The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management. PLoS Negl Trop Dis 11, e0005411.
| The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management.Crossref | GoogleScholarGoogle Scholar |
[14] Gassiep, I et al.. (2022) The epidemiology of melioidosis in Townsville, Australia. Trans R Soc Trop Med Hyg 116, 328–35.
| The epidemiology of melioidosis in Townsville, Australia.Crossref | GoogleScholarGoogle Scholar |
[15] Hanson, J et al.. (2021) Melioidosis–a disease of socioeconomic disadvantage. PLoS Negl Trop Dis 15, e0009544.
| Melioidosis–a disease of socioeconomic disadvantage.Crossref | GoogleScholarGoogle Scholar |
[16] Salaveria, K et al.. (2021) The applicability of commonly used severity of illness scores to tropical infections in Australia. Am J Trop Med Hyg 106, 257–67.
| The applicability of commonly used severity of illness scores to tropical infections in Australia.Crossref | GoogleScholarGoogle Scholar |
[17] Stephens, DP et al.. (2016) Melioidosis causing critical illness: a review of 24 years of experience from the Royal Darwin Hospital ICU. Crit Care Med 44, 1500–5.
| Melioidosis causing critical illness: a review of 24 years of experience from the Royal Darwin Hospital ICU.Crossref | GoogleScholarGoogle Scholar |
[18] Gora, H et al.. (2022) Melioidosis of the central nervous system; impact of the bimABm allele on patient presentation and outcome. Clin Infect Dis , ciac111.
| Melioidosis of the central nervous system; impact of the bimABm allele on patient presentation and outcome.Crossref | GoogleScholarGoogle Scholar |
[19] McLeod, C et al.. (2015) Clinical presentation and medical management of melioidosis in children: a 24-year prospective study in the Northern Territory of Australia and review of the literature. Clin Infect Dis 60, 21–6.
| Clinical presentation and medical management of melioidosis in children: a 24-year prospective study in the Northern Territory of Australia and review of the literature.Crossref | GoogleScholarGoogle Scholar |
[20] Young, A et al.. (2017) Case report: fatal pediatric melioidosis despite optimal intensive care. Am J Trop Med Hyg 97, 1691–4.
| Case report: fatal pediatric melioidosis despite optimal intensive care.Crossref | GoogleScholarGoogle Scholar |
[21] Smith, S et al.. (2017) Children with melioidosis in Far North Queensland are commonly bacteraemic and have a high case fatality rate. Commun Dis Intell Q Rep 41, E318–21.
[22] Sarovich, DS et al.. (2014) Variable virulence factors in Burkholderia pseudomallei (melioidosis) associated with human disease. PLoS One 9, e91682.
| Variable virulence factors in Burkholderia pseudomallei (melioidosis) associated with human disease.Crossref | GoogleScholarGoogle Scholar |
[23] Fairley, L et al.. (2021) Systematic review and meta-analysis of diagnostic tests for diagnosis of melioidosis. Acta Trop 214, 105784.
| Systematic review and meta-analysis of diagnostic tests for diagnosis of melioidosis.Crossref | GoogleScholarGoogle Scholar |
[24] Ashdown, LR (1979) An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens. Pathology 11, 293–7.
| An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens.Crossref | GoogleScholarGoogle Scholar |
[25] Currie, BJ et al.. (2022) What is the role of lateral flow immunoassay for the diagnosis of melioidosis? Open Forum Infect Dis 9, ofac149.
| What is the role of lateral flow immunoassay for the diagnosis of melioidosis?Crossref | GoogleScholarGoogle Scholar |
[26] Gassiep, I et al.. (2021) Melioidosis: laboratory investigations and association with patient outcomes. Am J Trop Med Hyg 106, 54–9.
| Melioidosis: laboratory investigations and association with patient outcomes.Crossref | GoogleScholarGoogle Scholar |
[27] Meumann, EM et al.. (2006) Clinical evaluation of a type III secretion system real-time PCR assay for diagnosing melioidosis. J Clin Microbiol 44, 3028–30.
| Clinical evaluation of a type III secretion system real-time PCR assay for diagnosing melioidosis.Crossref | GoogleScholarGoogle Scholar |
[28] Therapeutic Guidelines Limited (2021) Melioidosis. https://tgldcdp.tg.org.au/viewTopic?etgAccess=true&guidelinePage=Antibiotic&topicfile=melioidosis&guidelinename=Antibiotic§ionId=toc_d1e62#toc_d1e62
[29] Pitman, MC et al.. (2015) Intravenous therapy duration and outcomes in melioidosis: a new treatment paradigm. PLoS Negl Trop Dis 9, e0003586.
| Intravenous therapy duration and outcomes in melioidosis: a new treatment paradigm.Crossref | GoogleScholarGoogle Scholar |
[30] Sullivan, RP et al.. (2019) Oral eradication therapy for melioidosis: important but not without risks. Int J Infect Dis 80, 111–4.
| Oral eradication therapy for melioidosis: important but not without risks.Crossref | GoogleScholarGoogle Scholar |
[31] Sullivan, RP et al.. (2020) 2020 review and revision of the 2015 Darwin melioidosis treatment guideline; paradigm drift not shift. PLoS Negl Trop Dis 14, e0008659.
| 2020 review and revision of the 2015 Darwin melioidosis treatment guideline; paradigm drift not shift.Crossref | GoogleScholarGoogle Scholar |
[32] Hanson, J and Smith, S (2019) High rates of premature and potentially preventable death among patients surviving melioidosis in tropical Australia. Am J Trop Med Hyg 101, 328–31.
| High rates of premature and potentially preventable death among patients surviving melioidosis in tropical Australia.Crossref | GoogleScholarGoogle Scholar |
[33] Majoni, SW et al.. (2018) Trimethoprim+sulfamethoxazole reduces rates of melioidosis in high-risk hemodialysis patients. Kidney Int Rep 3, 160–7.
| Trimethoprim+sulfamethoxazole reduces rates of melioidosis in high-risk hemodialysis patients.Crossref | GoogleScholarGoogle Scholar |
[34] Chau, KWT et al.. (2018) Antibiotic prophylaxis for melioidosis in patients receiving hemodialysis in the tropics? One size does not fit all. Am J Trop Med Hyg 99, 597–600.
| Antibiotic prophylaxis for melioidosis in patients receiving hemodialysis in the tropics? One size does not fit all.Crossref | GoogleScholarGoogle Scholar |
[35] Gassiep, I et al.. (2020) Human melioidosis. Clin Microbiol Rev 33, e00006-19.
| Human melioidosis.Crossref | GoogleScholarGoogle Scholar |