Development of container laboratories in response to COVID-19
Russell Cole A BA Consultant Medical Laboratory Scientist, Pacific Pathology Training Centre, Wellington, New Zealand
B Email: pptc@pptc.org.nz; Russellc@pptc.org.nz
Microbiology Australia 42(4) 187-191 https://doi.org/10.1071/MA21052
Submitted: 9 August 2021 Accepted: 27 September 2021 Published: 29 October 2021
Journal Compilation © The Authors 2021 Open Access CC BY, published (by CSIRO Publishing) on behalf of the ASM
Abstract
A silver lining has emerged from the current COVID-19 pandemic that medical laboratory services across the Pacific have benefitted by. The pressures of COVID-19 exposed numerous deficiencies in our healthcare systems with a particular spotlight beamed onto national diagnostic testing capabilities. Consequently, laboratory services have been thrust into the centre of attention in biosecurity issues and become a high priority focus in the drive to ensure performance accuracy and diagnostic capacity is realised. Resources have been directed to ensure laboratories are prepared and responsive to early detection, but also prepared and ready for the recovery stages when borders re-open and new generation tourists are screened. An exciting development to come out of these uncertain times is the growing popularity of mobile laboratory facilities housed in shipping containers. They provide a convenient, cheap and timely solution to smaller Pacific Island nations by greatly enhancing their molecular technology platforms and containment facilities all in one box. A collaborated effort from Pacific partnership organisations has been instrumental in accelerating the establishment of these novel laboratory units and help address the need for testing facilities in geographically isolated sites.
In February 2021, the Pacific Pathology Training Centre (PPTC) won its fourth tender contract with international funders to supply medical testing shipping containers to designated and in need Pacific island nations of the South Pacific. In response to the growing threat of SARS-CoV-2 in the region, the PPTC was called on to project manage the novel container laboratory concept, train the local staff in the new technologies and finally deliver the facilities unit to new locations in Tokelau, Niue, Kiribati and Fiji. The containers primarily cater for SARS-CoV-2 testing through GeneXpert or RT-PCR platforms but have also been equipped with biochemistry, haematology and microbiology essentials to greatly enhance the whole diagnostic testing capacity. This aspect of diagnostic testing is of particular benefit for the communities as infectious diseases and non-communicable diseases are still rising in the shadows of COVID-19.
So who are these people? Why are they so valuable in driving laboratory developments in the Pacific region? How much expertise do they have in microbiology and the clinical sciences?
Established in 1980, the PPTC is a non-government organisation operating out of the Wellington Hospital campus in New Zealand1. For over 40 years it has advanced and strengthened clinical laboratory services in Pacific Island countries through its delivery of scientific teaching programmes, regional quality assurance programmes and workforce development courses for the laboratory science professional2.
The PPTC has had the privilege of working alongside partner organisations in Australia and the Pacific in helping to establish better health outcomes, raise quality standards and strengthen laboratory surveillance through the existing national networks. In the past few years it has collaborated extensively with the Peter Doherty Institute for Infection and Immunity, the Burnet Institute and the Pacific Region Infectious Disease Association (PRIDA) on strategic laboratory strengthening projects in Solomon Islands, Samoa, Papua New Guinea and Fiji. PPTC has a long history of development work in Tonga, Vanuatu, Tuvalu, Niue, Cook Islands, Nauru, Marshall Islands, Kiribati and Federated States of Micronesia to name a few island nations.
Staffed by specialist scientists, all of whom are previous managers and team leaders, the PPTC team has positively impacted many diagnostic laboratories through management consultation visits, provision of technical automation advice, staff training workshops and in the progression of operational Quality Management systems towards recognised accreditation standards of practice3 (Figure 1).

|
All of the staff are travelling laboratory consultants, ready to lend assistance in each of the technical sciences including haematology and blood transfusion but also have wide experience in laboratory information systems, workflow design concepts and laboratory management.
Prior to the COVID-19 imposed lockdowns, PPTC regularly held centre-based courses in each of the scientific disciplines Biochemistry, Haematology, Microbiology, Blood Transfusion, Quality Management and Health and Safety. It also delivers a 2-year distance learning Diploma for Technicians, which has been widely recognised throughout the Pacific as the baseline qualification to hold if pursuing a laboratory career. Established in 2006, 116 students have successfully graduated with this Technician level Diploma and gone on to fill senior leadership positions in their home laboratories. The Microbiology section is well represented with nine modules including antibiotics, parasitology and immunology, numerous assignments and student practical logbooks requiring completion. This Technician qualification has proved extremely valuable in the geographically isolated North Pacific laboratories of Micronesia, Marshall Islands and Kiribati along with the southern neighbours Samoa, Vanuatu, Tuvalu, Tonga and Cook Islands.
Who we are
There is a big emphasis to promote professional competency, workforce skill levels and effectively mentor Pacific Island laboratory workers in management and leadership. A valuable tool amongst the PPTC’s arsenal is the delivery and provision of a comprehensive External Quality Assurance programme to 90 Asian and Pacific Island laboratories. Recently a molecular diagnostics module for SARS-CoV-2 was added to supplement the scientific programme to now include Haematology, Clinical Biochemistry, Microbiology, Transfusion Science, Infectious Disease Serology, Anatomical Pathology and Molecular Diagnostics (SARS-CoV-2).
These programmes are dispatched to Pacific Island laboratories three times a year and help PPTC consultants to monitor technical performance and accuracy in relation to test reports when there is opportunity to visit home laboratories. Additional auditing activities, skill training and operational aspects of management are built into the consultant visits designed to cover each laboratory department and service.
Since early 2020, the COVID-19 pandemic has seriously impacted all hospitals and healthcare providers across the globe to which the Pacific region was no exception. Attention and resources were immediately diverted towards frontline diagnostic services and highlighted the need for public health preparedness among local communities. Not surprisingly it exposed many inadequate resource issues around diagnostic systems, reference laboratory networks and contained testing facilities. For the Pacific region it accelerated the introduction of molecular diagnostics and RT-PCR technologies into small, test restrictive laboratories.
This prompted the NZ Ministry of Foreign Affairs and Trade (MFAT) to engage the PPTC in a project to procure and establish a container laboratory system for Tokelau that would accommodate SARs-CoV-2 testing, provide essential laboratory test instrumentation and deliver the associated training and support for Tokelau’s laboratory personnel. The PPTC was able to draw on a previous mini testing laboratory experience from 2012 when a laboratory container facility was established at the Marine Training Centre in Kiribati. A remodelled version, customised for Tokelau was designed and orchestrated by Navin Karan, PPTC’s Programme Manager and Molecular specialist, who worked collectively with WHO and the SPC advisors, alongside shipping container outfitters Boxman Ltd, who operate out of Wellington.
Essential diagnostic testing equipment was purchased and installed in the facility, which has now been transported to Tokelau and has been fully operational since December 2020. Comprehensive training sessions were initiated with Mr Orisi Matatolu, Tokelau’s sole laboratory scientist, via Zoom as soon as the laboratory instrumentation and internet connections were established. Although this was not the most ideal way to introduce and teach new technology concepts, it was unfortunately the only option due to the international travel restrictions in place.
It is not only in the delivery and implementation of the facility that PPTC’s expertise are utilised but also in the building up of Quality Management documentation, departmental operational procedures and essential supply chain pathways for analyser reagents and diagnostic testing consumables. The container laboratories now developed have to be sustainable long term and provide an effective operational service for the island communities.
Niue: May 2021
Following on from the successful Tokelau project, WHO commissioned the PPTC to design, implement and deliver a comprehensive medical laboratory testing facility to South Pacific neighbours Niue, in response to a request by the Niue Government. Unlike Tokelau, Niue’s Foou Hospital already contained a small laboratory facility but needed to expand its general diagnostic testing scope and be COVID-19 capable.
The container laboratory was seen as a significant expansion of services and located adjacent to the Hospital Laboratory site. The robust 6-m double container was designed to accommodate phlebotomy collection, biochemistry, haematology and molecular PCR testing with the inclusion of GeneXpert and Biofire instruments. Microbiology remains in the primary hospital facility alongside serology and blood transfusion donor testing (Figure 2).

|
Programme Manager Navin Karan was adamant that the container be built to withstand natural disasters and prove to be cyclone safe. The containers also needed to be of appropriate size so as to be lifted and moved around if needed. Consequently, it has fitted metal shutters on the exterior to provide hurricane protection for the windows and help when transporting the unit. The increased capacity to test for both communicable diseases and non-communicable diseases is of immense benefit to the people of Niue. Niue High Commissioner to New Zealand, Mr Fisa Pihigia expressed his sincere appreciation and thanks to the PPTC along with others playing their part in the laboratory construction during a commissioning service in Wellington in April. Since then, the container laboratory arrived on its new site and has been fully operational since May 2021.
Kiribati: May 2021
Running concurrent with the Niue project was an approach from The Pacific Community (SPC) and the Government of Kiribati to oversee the refurbishment of container units to be used as a PCR laboratory for Kiribati Medical services. This was to ensure that COVID-19 testing could commence as soon as possible while the existing medical laboratory facilities received an upgrade and site modifications. As part of this project, the PPTC worked with the very same NZ container outfitter to ensure that the testing laboratory was fit for purpose. The Kiribati facility had a more specialised purpose in being a molecular PCR testing laboratory and therefore required stringent design modifications to enable separate extraction room, clean room and amplification room requirements. Detailed specifications were discussed with the Kiribati Medical Services team, the SPC and experts from the Doherty Institute before the final design was approved and initiated (Figure 3a).
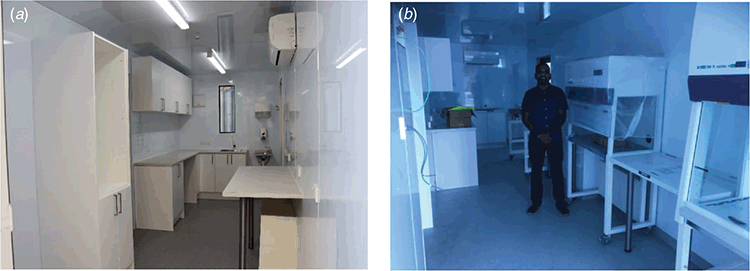
|
This molecular laboratory is currently fully operational and working well in Kiribati being located on the outskirts of Tarawa as a community based COVID-19 testing facility. Further training for laboratory personal was carried out by the Doherty Institute and the SPC to validate the introduced technologies and ensure staff competency.
Cook Islands: July 2021
The PPTC was called upon on behalf of the Cook Islands Health Department and New Zealand Ministry of Foreign Affairs and Trade to provide technical training and expertise towards setting up the newly built COVID-19 RT-PCR testing facility in the Rarotonga Hospital Laboratory. Although this was a refurbishment build and an additional sector for the existing laboratory, it had numerous similarities to the container facilities we had been previously involved with giving the team an insightful appreciation of the work specifications required.
A PPTC consultant worked with the SPC representatives, Doherty Institute experts and the friendly Rarotonga laboratory staff to implement and install appropriate technical equipment through planned practical training sessions. The bonus was that a travel bubble had been established during this time that enabled face to face training and quarantine free visits for our PPTC consultant, who was able to visit the island on two occasions prior to the borders being re-opened for tourists.
Introducing RT- PCR technology to the Cook Islands Laboratory brought its own set of challenges. The facilities were ideal in layout and design. The equipment and essential reagents had arrived and were validated for use. All that remained was the availability of staff to go through the detailed teaching programme, the online training materials and become familiar with the delicate and complex pathways of RT-PCR assays. Four of the ten staff members participated in training sessions spanning over a 4-week period to learn the concepts of contamination, reagent mixes, extraction and measurement. Numerous practise runs were carried out and critical process control points identified before the laboratory was ready to go live.
Fiji: September 2021
In recognising a growing demand for COVID-19 testing, the Ministry of Health and Medical Services in Fiji (MOHMS) expressed an urgent need to establish a border health protection unit close to the International airport in Nadi. It was proposed to strengthen border control systems and serve as a major testing centre for people transitioning through Fiji to other parts of the Pacific. The PPTC was successful in being awarded this contract by WHO and promptly initiated our most ambitious project to date. It was on a scale far larger than we initially anticipated and incurred numerous supply chain delays for both the container construction and delivery of laboratory products.
Four 12-m container units were proposed in a cluster style Laboratory complex to deliver RT-PCR testing in Nadi. It would enhance and complement testing capabilities already operating out of the main national laboratory facilities in Suva and include thermocyclers, a semi-automated RNA extraction analyser, biohazard safety cabinets and appropriate equipment associated with a level 2 containment facility. Initially the RT-PCR laboratory would be set up for COVID-19 testing but have sufficient scope to expand on the molecular diagnostic capacity into the future. The newly constructed facility is expected to process up to 200 SARS-CoV-2 tests per day on the RT-PCR platforms and is equipped with appropriate insulation, air conditioning units, solar panels with generator back-up, plumbing and electrical service ducts, designated rooms in the PCR suite, staff facilities and storage areas. General biochemistry, haematology and microbiology tests can be incorporated into the container complex if considered necessary after the molecular testing platforms are established and operating to full capacity (Figure 3b).
The need for this facility escalated immensely as Fiji found itself facing the onslaught of a COVID-19 Delta variant outbreak. As the number of cases and screen tests skyrocketed, the container laboratories were deep into construction phases, building supply pressures and global shipping delays. The network of supply companies providing essential laboratory items for the fit out were also subject to lockdown conditions and frustrating border restrictions, all of which contributed to an extended timeline to PPTC’s project completion dates.
The four-unit laboratory complex left New Zealand shores in early September so as to be established and fully operational in Nadi by late October. Not a moment too soon for Fiji as it looks to restore the tourism industry to its economy and be ready for large scale laboratory screening for holiday makers. The PPTC will continue to deliver molecular training for the staff along with lending assistance in operational quality procedures and Health and Safety risk management, for some time after the new complex is established on Fijian soil. Local scientists from Fiji CDC will also play a prominent role in implementing training and procedures in the new facility.
Huge credit in coordinating this project needs to go to Mr Navin Karan, PPTC’s Molecular specialist and Programme coordinator, for his tireless efforts and dedication in bringing project technical details together and seeing a phenomenal task come to fruition (Figure 3b).
The concept of a container laboratory has been around for a number of years particularly in deploying Military Medical units and mobile Emergency Response teams. For the Pacific, they have been received as a welcomed solution and godsend in addressing the deficiencies in the health sector’s that have been exposed by COVID-19. The new facilities have greatly enhanced and boosted capacity for the national laboratory services they have been located in.
For the PPTC, it has been a privilege to be involved in development opportunities where our collective expertise in general medical laboratory services has been used to benefit the regions health status and help protect from infectious disease outbreaks. There are more projects on the horizon and a perceived abundance of shipping containers waiting in dockyards for new homes. Watch this space as we head into 2022.
Conflicts of interest
The author declares no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] Mackenzie R, Elliot J. (2018) Pacific Paramedical Training Centre – the first 30 years 1980-2010, Ngiao Press, Martinborough.[2] Pacific Pathology Training Centre Websitewww.pptc.org.nz
[3] International Organisation for Standardization (2014) Medical Laboratories-Requirements for quality and competence. 3rd Edition. ISO15189:2012. Geneva.
Biography
Russell Cole, NZCS, Dip MLSc, MNZIMLS, is Laboratory Consultant and Quality Manager at Pacific Pathology Training Centre, Wellington. He is a previous Laboratory Manager with specialties in Microbiology and core Laboratory disciplines. He currently enjoys his role as a Consultant and Lecturer at the PPTC where he has the opportunity to mentor and train Pacific Island laboratory staff in the complex world of laboratory management and leadership.


