Tropical fever in remote tropics: tuberculosis or melioidosis, it depends on the lab
Jeffrey Warner A B and Catherine Rush A CA College of Public Health, Medical and Veterinary Sciences and Australian Institute of Tropical Health and Medicine, James Cook University, Townsville, Qld, Australia
B Email: jeffrey.warner@jcu.edu.au
C Email: catherine.rush@jcu.edu.au
Microbiology Australia 42(4) 173-178 https://doi.org/10.1071/MA21049
Submitted: 16 August 2021 Accepted: 22 September 2021 Published: 12 November 2021
Journal Compilation © The Authors 2021 Open Access CC BY-NC-ND, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Diagnostics tests used to identify the cause of infection using proteomics and genomics have revolutionised microbiology laboratories in recent times. However, approaches to build the capacity of clinical microbiology services in the rural tropics by simply transplanting these approaches have proven difficult to sustain. Tropical fever in the remote tropics is, by definition, a clinical diagnosis where the aetiology of fever is not known, treatment is empirical, guided by clinical suspicion with treatment failure often attributed to incorrect diagnosis or antimicrobial resistance. Tuberculosis (TB) in rural Papua New Guinea (PNG) is mostly diagnosed clinically, perhaps supported by microscopy. In fact, a ‘tuberculosis patient’ in rural PNG is included in the TB register upon commencement of TB treatment with or without any laboratory-based evidence of infection. The roll-out of GeneXpert is continuing to transform TB diagnostic certainty in TB endemic communities. Melioidosis is endemic in tropical regions and is increasingly reported to mimic TB. Isolation and identification of the causative agent Burkholderia pseudomallei remains the gold standard. Here, we discuss the increasing divide between rural and urban approaches to laboratory-based infection diagnosis using these two enigmatic tropical infectious diseases, in rural PNG, as examples.
Introduction
The disparity in rural/urban health outcomes in Australia is well documented and is being addressed by the expansion and specialisation of the clinical health workforce in regional Australia1. In contrast to this investment in and focus on regional clinical services, microbiology laboratories and skilled staff, that are critical in providing evidence for the accurate diagnosis and management of infectious disease, are retreating from regional locations. In their place are internet connected high throughput centralised labs in urban centres with regional labs becoming specimen referral centres2,3. Communities in the rural tropics, in the Asia Pacific outside Australia are different places to regional Australia. Urban/rural health systems in the Asia Pacific are more disconnected, they are often poorly resourced, and capacity is stretched to the extent that morbidity and mortality in the communities they serve is unacceptably high4. Also, the rates of infection in regional tropics and types of infection are different, antimicrobial resistance profiles are not well characterised and treatment failure rates are high. In PNG up to 80% of the population live in the rural districts of regional provinces where less than 50% of the national health resources exist5.
Melioidosis is an example of tropical fever that results in high case fatality rates when diagnosis is delayed. The infection is sporadic and uncommon and resistant to standard treatment used for pneumonia and sepsis6. Accurate and early diagnosis, leading to directed therapy, is linked to the extent of clinical suspicion and the laboratory capacity to isolate and identify the causative organism, the saprophytic Gram-negative bacillus, B. pseudomallei. Classical bacteriology, which remains the gold standard, is rarely available in regional PNG7. Tuberculosis on the other hand is an ancient human disease with global public health significance and is the leading cause of antimicrobial resistant (AMR) associated mortality8,9. The causative bacterium Mycobacterium tuberculosis is notoriously difficult and dangerous to manipulate using basic bacteriology and therefore these functions have been centralised to reference laboratories. In rural regions where TB is endemic, GeneXpert is being rolled out to support clinical diagnosis and is proving important in quick turnaround and AMR strain identification10,11. There is emerging evidence that melioidosis mimics TB and that the capacity of the laboratory is critical in the differential diagnosis. As centralised technological-based solutions to lab-based infectious disease diagnosis become the norm, are we paying enough attention to building and sustaining basic bacteriological services in the rural tropics and does this matter?
Both tropical but for different reasons
Burkholderia pseudomallei is mostly, biogeographically bound to the tropics. However, the distribution of the organism in the environment is not random and this contributes to the spatial clustering of melioidosis where it is considered endemic6. The incidence of infection in the tropics is therefore unpredictable. Acquisition of the organism more readily occurs in individuals with a robust association with the environment, such as occurs in subsistence communities, first nations peoples who live rurally and those exposed to extreme weather or other environmental disturbances12,13. As a consequence, melioidosis is primarily a disease of the rural tropics. Typically14, but not always15, case numbers are higher during wet seasons and this contributes to the temporal clustering of cases which often aids in clinical predictability. Comorbidities define the extent of the infection; melioidosis can present as a subacute localised, disseminated, or systemic disease. Given this protean mix of clinical manifestations, it is often described as the ‘great mimicker’16. In our region, melioidosis is endemic in Asia and northern Australia and reports are emerging across Oceania13,17. The gold standard laboratory-based diagnostic is still isolation and identification of the bacterium from clinical samples, which needs to be conducted safely as the organism, just like M. tuberculosis, is considered in some jurisdictions a risk group 3 pathogen6. Traditional bacteriology requires people who have been trained in and have maintained subjective, descriptive laboratory skills.
Tuberculosis is an ancient human infection that has been mostly controlled or eradicated in developed countries. It is a disease of poor socio-economic conditions and poor access to healthcare. The emergence of drug resistant TB (DR-TB) has awakened an interest in TB in the developed world. As a result, sophisticated, specific microbial diagnostic platforms for TB have been developed to provide clinically relevant turnaround times. Once established, these technologies do not require extensive laboratory skills. However, they do require consistent and reliable power supply and are costly per test which contributes to the complexity of the roll-out10. However, for health program leaders they are an addictively simple and very effective laboratory-based diagnostic for a global public health problem.
Epidemiologically, melioidosis and TB are very different, yet in some parts of the rural Asia Pacific, their incidence and clinical presentation overlap. The question is, are these two diseases able to be diagnosed with equal confidence and does that matter?
TB in rural PNG: is it true?
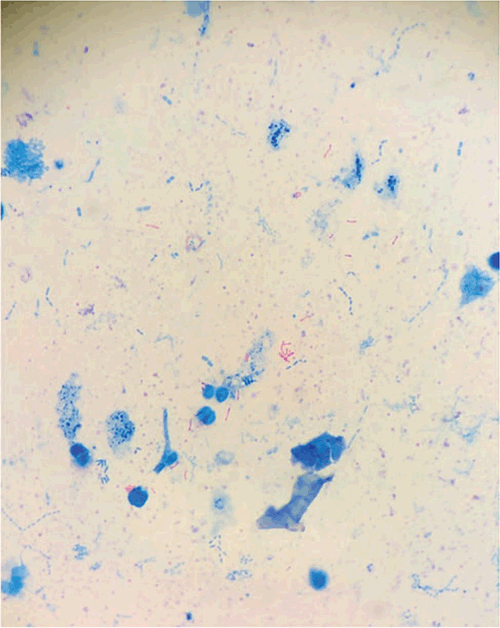
In the district hospital at Balimo in the rural remote region of Middle Fly, Western Province, there are no doctors, with hardworking nurses, community health workers and health extension officers forming the health workforce and power from diesel generators is sporadic (Figure 1). The laboratory is rudimentary, has one dedicated full-time staff member and functions are limited to malaria blood film examination, ZN stain for TB (Figure 2), basic haematology and blood transfusion – typical of a remote district hospital in the Asia Pacific. The population of this region is approximately 40,000 with most people living a subsistence lifestyle in villages where access to health care is difficult – also typical of a remote tropical community18. In this community the reported rate of TB is close to twice the national annual incidence at approximately 700–900/100 000 population, a significant proportion are children, gender equal and evidence of drug resistance is emerging19,20. A particular feature of the disease in Balimo is the high rates of extrapulmonary (EP-TB) disease, approaching 75% of all cases. In a cross sectional, retrospective snapshot of TB patients at Balimo, approximately 40% of pulmonary TB (P-TB) patients were commenced on TB treatment with AFB negative microscopy or when no smear was available. Also, approximately 98% of the EP-TB cases on treatment, had no laboratory evidence of TB. Hence, a significant feature of the TB epidemic in this region is diagnostic uncertainty19.
|
Melioidosis and TB mimicry
Early reports of melioidosis described the protean manifestation of the disease and its mimicry of well-known causes of tropical fever including TB21. Salient messages emerged in the 1950s that high case fatality resulted without early and appropriate treatment. This was particularly true where a ‘provisional diagnosis of pulmonary tuberculosis was made’ in the repeatedly AFB negative patient22.
Whilst melioidosis endemicity in some parts of Asia and northern Australia is well documented, evidence from Oceania is sketchy, the disease is not notifiable and most reports are from periodic rounds of active case finding (reviewed in 13). The first human case report in PNG was in 1965 from a patient referred to Port Moresby Hospital from Gemo island, the then ‘leprosarium’. Since independence in 1975 sporadic reports have emerged from the urban capital, some of which had a history of TB investigation or the presentation was said to resemble TB (reviewed in 17). These rare reports have led to the speculation that melioidosis is less common in PNG than northern Australia23.
The latest active case detection study in Port Moresby during 2000 and 2002, included screening sputa presented for TB investigation at the Central Public Health Laboratory. Of the 417 MTB smear negative patients, one was melioidosis culture confirmed, confirming that melioidosis, although rare, was present in the clinically diagnosed TB patient cohort17.
In contrast to these reports from urban Port Moresby, Balimo was revealed as a regional melioidosis hot spot. During the 1990s, sporadic yet specific laboratory-based, active case finding for melioidosis was conducted over a 12–18-month period. Presenting patients were assessed as per local clinical protocols with the addition of basic bacteriology including Ashdown agar-based selective culture techniques. Laboratory staff were trained in recognising B. pseudomallei colonial morphology (Figure 3) and simple phenotypic features such as Gram stain (GNB), oxidase (positive), gentamicin resistance and amoxicillin/clavulanate susceptibility, for identification. During this time, nine cases of culture confirmed melioidosis were revealed in a patient cohort of tropical fever where standard treatment had previously failed. A feature of the disease was childhood predilection, chronic localised pulmonary disease and over half were initially diagnosed with TB and had commenced TB treatment. These case numbers, revealed in less than 2 years, from this isolated rural community, outnumbered all the previous reported culture confirmed cases of melioidosis from PNG24,25.

|
The TB and Melioidosis Nexus: the same host factors define severity of disease
Not only can these two diseases be similar in clinical presentation and be prevalent in the same region, both causative organisms are intracellular pathogens, capable of chronic disease and latency and for both, cell-mediated T-cell responses (CMI) and interferon-mediated signalling pathways are important for protective immunity26,27. Furthermore, they share common comorbid risk factors which can affect clinical presentation. In both, failure to develop adequate T-cell responses following infection leads to disease progression, dissemination and individuals often succumb to infection.
In the Balimo region, TB and melioidosis are commonly found in children19,24. Children with localised pulmonary melioidosis had poor CMI function compared to those family members with similar evidence of environmental exposure but did not present with disease28. These children would typically progress to more severe disease due to delay in diagnosis and commencement of direct therapy. Our findings using mitogen-stimulated peripheral blood T cells from individuals from Balimo provide evidence for an age-related defect in the ability of CD4 (helper) and CD8 (cytotoxic) T cells of otherwise healthy children to produce the TB-protective cytokine, interferon-gamma, whilst the production of other cytokines including tumour necrosis factor-alpha remained intact (Figure 4). Hence, dysregulation of cellular immunity may be responsible for acquisition and development of TB and melioidosis in this and other parts of the rural tropics where exposure to both, for different reasons, is possible.
Conclusion: it’s still happening
In some parts of the rural tropics TB and melioidosis overlap and share many features, but the capacity to identify these two diseases is not equal: this may result in an under recognition of melioidosis in rural communities. Since the late 1990s, the basic bacteriological capacity at Balimo has effectively ceased. A GeneXpert was recently installed in Balimo to aid in the management of the emerging DR-TB epidemic; however, its utility has stalled due to lack of reagents. Just before COVID broke in January 2020, our group delivered melioidosis specific reagents as part of our continuing collaborative TB program with the Balimo District Hospital. As a result, during late 2020 and early 2021 three more cases of culture confirmed melioidosis were identified, all were children and all were initially admitted to the TB ward.
Melioidosis is sporadic, often seasonal and case incidence is non-randomly spatially clustered within the tropics. Clinical presentation can mimic TB and without basic bacteriology services, which are rare and retreating in rural regions, diagnosis can be delayed resulting in high case fatality rates. If we want to help build microbiological capacity in the regional tropics of the Asia Pacific, we must embrace both basic and advanced technology to ensure the services are sustainable and relevant and understand that the challenges of the urban and rural microbiologists are not the same.
Conflicts of interest
The authors declare no conflicts of interest
Declaration of funding
Funded in part from NHMRC Hot North program and the Australian Institute Tropical Health and Medicine (AITHM).
Acknowledgements
The authors acknowledge the late Dr Evelyn Lavu, Director/Manager of the PNG Central Public Health Laboratory, who was leading and studying the National TB GeneXpert implementation program. Her dedication and leadership of Pathology in PNG will be greatly missed.
References
[1] Australian Institute of Health and Welfare (AIHW) (2020) Rural and remote health. Australian Government Canberra. https://www.aihw.gov.au/reports/australias-health/rural-and-remote-health[2] Dancer, S.J. et al. (2015) Microbiology service centralization: a step too far. J. Hosp. Infect. 91, 292–298.
| Microbiology service centralization: a step too far.Crossref | GoogleScholarGoogle Scholar | 26520590PubMed |
[3] Robinson, A. et al. (1999) Controversies affecting the future practice of clinical microbiology. J. Clin. Microbiol. 37, 883–889.
| Controversies affecting the future practice of clinical microbiology.Crossref | GoogleScholarGoogle Scholar | 10074496PubMed |
[4] The Lancet (2015) Rural health inequities: data and decisions. Lancet 385, 1803.
| 25987137PubMed |
[5] Grundy, J. et al. (2019) Independent State of Papua New Guinea Health System Review. World Health Organization. Regional Office for South-East Asia. https://apps.who.int/iris/handle/10665/280088
[6] Currie, B.J. (2015) Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin. Respir. Crit. Care Med. 36, 111–125.
| Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment.Crossref | GoogleScholarGoogle Scholar | 25643275PubMed |
[7] Ferguson, J.K. et al. (2020) Quality microbiological diagnostics and antimicrobial susceptibility testing, an essential component of antimicrobial resistance surveillance and control efforts in Pacific island nations. Western Pac. Surveill. Response J. 11, 41–46.
| Quality microbiological diagnostics and antimicrobial susceptibility testing, an essential component of antimicrobial resistance surveillance and control efforts in Pacific island nations.Crossref | GoogleScholarGoogle Scholar | 32963890PubMed |
[8] O’Neill, J. (2016) Tackling drug-resistant infections globally: Final report and recommendations. HM Government, London, UK. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf
[9] World Health Organization (2020) Global tuberculosis report 2020. World Health Organization, Geneva. https://www.who.int/publications/i/item/9789240013131
[10] Albert, H. et al. (2016) Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur. Respir. J. 48, 516.
| Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better?Crossref | GoogleScholarGoogle Scholar | 27418550PubMed |
[11] Lavu, E.K. et al. (2019) Drug-resistant tuberculosis diagnosis since Xpert® MTB/RIF introduction in Papua New Guinea, 2012-2017. Public Health Action 9, S12–S18.
| Drug-resistant tuberculosis diagnosis since Xpert® MTB/RIF introduction in Papua New Guinea, 2012-2017.Crossref | GoogleScholarGoogle Scholar | 31579644PubMed |
[12] Currie, B.J. et al. (2010) The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin Prospective Study. PLoS Negl. Trop. Dis. 4, e900.
| The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin Prospective Study.Crossref | GoogleScholarGoogle Scholar | 21152057PubMed |
[13] Warner, J.M. and Currie, B.J. (2018) Melioidosis in Papua New Guinea and Oceania. Trop. Med. Infect. Dis. 3, 34.
| Melioidosis in Papua New Guinea and Oceania.Crossref | GoogleScholarGoogle Scholar |
[14] Cheng, A.C. et al. (2006) Extreme weather events and environmental contamination are associated with case-clusters of melioidosis in the Northern Territory of Australia. Int. J. Epidemiol. 35, 323–329.
| Extreme weather events and environmental contamination are associated with case-clusters of melioidosis in the Northern Territory of Australia.Crossref | GoogleScholarGoogle Scholar | 16326823PubMed |
[15] Stewart, J.D. et al. (2017) Melioidosis in far north Queensland is not correlated with severe weather events. Med. J. Aust. 207, 394.
| Melioidosis in far north Queensland is not correlated with severe weather events.Crossref | GoogleScholarGoogle Scholar | 29092705PubMed |
[16] Yee, K.C. et al. (1988) Melioidosis, the great mimicker: a report of 10 cases from Malaysia. J. Trop. Med. Hyg. 91, 249–254.
| 3184245PubMed |
[17] Warner, J.M. et al. (2010) Melioidosis--an uncommon but also under-recognized cause of pneumonia in Papua New Guinea. P. N. G. Med. J. 53, 176–179.
| 23163189PubMed |
[18] Diefenbach-Elstob, T. et al. (2017) Fieldwork in isolated locations and under resourced settings: Practical insights from research in the remote Gogodala homelands of Papua New Guinea. SAGE Research Methods Cases.
[19] Diefenbach-Elstob, T. et al. (2018) The epidemiology of tuberculosis in the rural Balimo region of Papua New Guinea. Trop. Med. Int. Health 23, 1022–1032.
| The epidemiology of tuberculosis in the rural Balimo region of Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 29920858PubMed |
[20] Diefenbach-Elstob, T. et al. (2019) Molecular evidence of drug-resistant tuberculosis in the Balimo region of Papua New Guinea. Trop. Med. Infect. Dis. 4, 33.
| Molecular evidence of drug-resistant tuberculosis in the Balimo region of Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
[21] Stanton, A.T. and Fletcher, W. (1925) Melioidosis and its relation to glanders. J. Hyg. (Lond.) 23, 347–363.
| 20474845PubMed |
[22] Khaira, B.S. et al. (1959) Melioidosis. BMJ 1, 949–952.
| Melioidosis.Crossref | GoogleScholarGoogle Scholar | 13638596PubMed |
[23] Currie, B. (1993) Melioidosis in Papua New Guinea: is it less common than in tropical Australia? Trans. R. Soc. Trop. Med. Hyg. 87, 417.
| Melioidosis in Papua New Guinea: is it less common than in tropical Australia?Crossref | GoogleScholarGoogle Scholar | 8249067PubMed |
[24] Warner, J.M. et al. (2007) Melioidosis in a rural community of Western Province, Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 101, 809–813.
| Melioidosis in a rural community of Western Province, Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 17499321PubMed |
[25] Warner, J.M. et al. (2008) The epidemiology of melioidosis in the Balimo region of Papua New Guinea. Epidemiol. Infect. 136, 965–971.
| The epidemiology of melioidosis in the Balimo region of Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 17714600PubMed |
[26] Jenjaroen, K. et al. (2015) T-cell responses ae associated with survival in acute melioidosis patients. PLoS Negl. Trop. Dis. 9, e0004152.
| T-cell responses ae associated with survival in acute melioidosis patients.Crossref | GoogleScholarGoogle Scholar | 26495852PubMed |
[27] Koh, G.C.K.W. et al. (2013) Host responses to melioidosis and tuberculosis are both dominated by interferon-mediated signaling. PLoS One 8, e54961.
| Host responses to melioidosis and tuberculosis are both dominated by interferon-mediated signaling.Crossref | GoogleScholarGoogle Scholar |
[28] Barnes, J.L. et al. (2004) Adaptive immunity in melioidosis: a possible role for T cells in determining outcome of infection with Burkholderia pseudomallei. Clin. Immunol. 113, 22–28.
| Adaptive immunity in melioidosis: a possible role for T cells in determining outcome of infection with Burkholderia pseudomallei.Crossref | GoogleScholarGoogle Scholar | 15380526PubMed |
Biographies
Jeff Warner is an Associate Professor, Microbiology at James Cook university, Townsville. He has worked and conducted research in rural Papua New Guinea for almost 30 years and these experiences have helped shape his teaching and research interests. While he still has air in his lungs he will continue to prosecute the value of classical, descriptive microbiology because he believes these skills are still important in the rural laboratory workforce and provide valuable support to rural communities (he admits, maybe he is just old?). Jeff and Cathy Rush lead a research group of like-minded and keen students and researchers focusing infectious disease issues relevant to the tropics, including melioidosis and tuberculosis in PNG.
Cathy Rush is A/Professor of Immunology at James Cook University, Townsville. Cathy has worked in microbiology and immunology both here and overseas where she has been committed to unravelling the mysteries of the host immune response in terms of host-pathogen interactions, vaccinology and T-cell biology. Cathy has expertise in the immunobiology of tropical infectious diseases and is currently researching host susceptibility to tuberculosis and drug resistance development.




