Melioidosis in Australia
Timothy JJ InglisPathology and Laboratory Medicine, School of Medicine, and Marshall Centre, School of Biomedical Sciences, University of Western Australia; and Department of Microbiology, PathWest Laboratory Medicine, QEII Medical Centre, Nedlands, WA, Australia. Email: tim.inglis@uwa.edu.au
Microbiology Australia 42(2) 96-99 https://doi.org/10.1071/MA21027
Submitted: 15 March 2021 Accepted: 30 April 2021 Published: 20 May 2021
Journal Compilation © The Authors 2021 Open Access CC BY-NC-ND, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Melioidosis is a potentially fatal bacterial infection caused by the Gram-negative bacillus, Burkholderia pseudomallei following contact with a contaminated environmental source, normally soil or water in tropical and subtropical locations. The disease spectrum varies from rapidly progressive bacteraemic infection with or without pneumonia, to focal lesions in deep soft tissues and internal organs to superficial soft tissue infection and asymptomatic seroconversion with possible long-term dormancy. Most infections occur with a background of chronic illness such as diabetes, chronic kidney disease and alcoholic liver disease. Improvements in diagnosis, targeted antimicrobial treatment and long term follow up have improved clinical outcomes. Environmental controls following rare point source case clusters and heightened awareness of melioidosis appear to have reduced the disease burden in some parts of northern Australia. However, the impact of climate change on dispersal of environmental B. pseudomallei, and changing land use in tropical Australia is expected to change the epidemiology of melioidosis in future.
Introduction
Melioidosis has fascinated Australian microbiologists since it was first encountered as a human infection in a 32-year-old Townsville man with diabetes in 19501. Human melioidosis has a remarkable ability to cause a broad spectrum of human disease from rapidly fatal bacteraemic infection and necrotising pneumonia, through persistent localised chronic lesions to asymptomatic dormant infections that convert to more serious infection after intervals of months to years2. As a consequence, melioidosis challenges the logic of conventional clinico-pathological disease classifications and is best considered as a cluster of syndromes linked by a single bacterial aetiology, the Gram-negative, oxidase positive bacillus, Burkholderia pseudomallei and a history of environmental exposure.
Epidemiology
Melioidosis in Australia is endemic across the tropical north of Australia (Figure 1) where it occurs as a sporadic infection of people who have had exposure to contaminated soil or surface water through direct transdermal inoculation, inhalation and possibly ingestion3. Occasional point source case clusters have occurred related to contaminated water, medical solutions or hand wash products4–7, and animal case clusters have been associated with flooding of pasture land8. The majority of acute febrile melioidosis occurs as a septicaemia with or without pneumonia, peaking during the tropical wet season, and sometimes follows in the wake of tropical cyclones9, though not necessarily in all of northern Australia10. However, the potentially long symptom-free period leads to some subacute infections presenting during the dry season, or in residents of temperate Australia who previously travelled to the tropics11, including endemic locations overseas. There is a higher risk of bacteraemic infections in people with one or more of a group of co-morbidities, most notably diabetes, chronic lung disease, chronic kidney disease and alcoholic liver disease12.
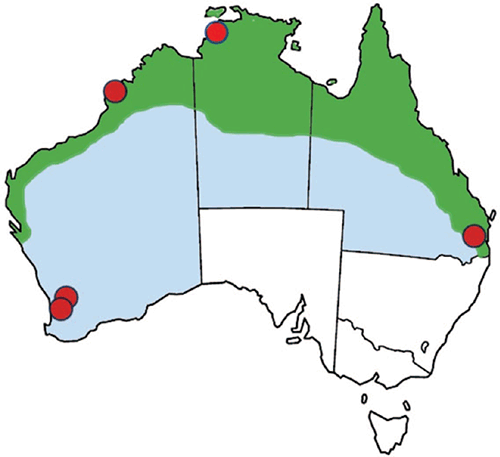
|
Diagnosis
The lack of pathognomonic clinical features, wide range of clinical presentations and potentially dormant deep soft tissue infections create difficulties for the diagnosing physician. In patients from tuberculosis-endemic settings, there is a risk of misdiagnosis of melioidosis as tuberculosis and subsequent inappropriate treatment13. Attempts have been made to standardise clinical definitions of melioidosis14. Other than in the few centres in tropical Australia that encounter enough cases to gain experience applying such classification, maintaining awareness of melioidosis is more easily said than done. The most reliable laboratory confirmation comes from isolating B. pseudomallei in blood, sputum, abscess fluid or other culture15 (Figure 2). However, confirmation of the identity of B. pseudomallei can be challenging in clinical laboratories that have not previously handled the species. Referral of a suspect isolate (Gram-negative bacillus, oxidase positive, Gentamicin and Colistin resistant) to a reference laboratory may be needed, although this will add further delays to reporting results. Laboratories in the melioidosis endemic zone will often use advanced bacterial identification methods such as specific PCR assays, MALDI-TOF mass spectrophotometry or gene sequencing to produce a definitive identification17,18. Serological assays are used as a complementary diagnostic method, particularly in the absence of a positive culture, but background antibody levels in endemic regions may confound result interpretation19. Moreover, high risk exposure activities that result in confirmed infection do not necessarily cause seroconversion20.
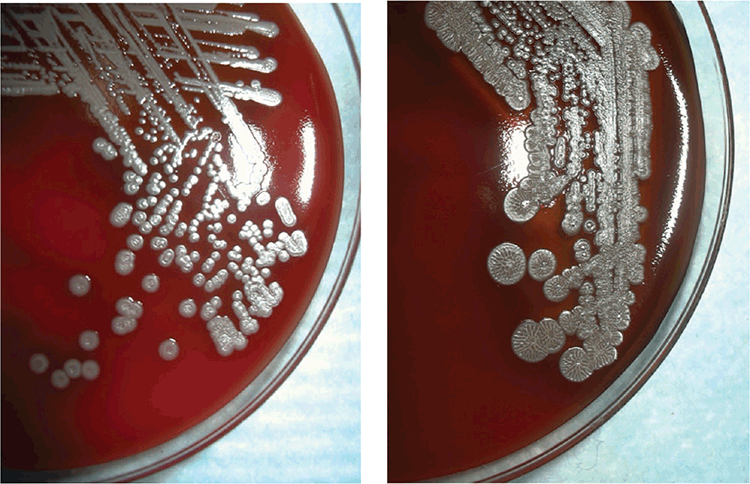
|
Treatment
Detailed treatment regimens can be found in the Therapeutic Guidelines and are updated periodically by specialists with current clinical experience21. In summary, acute bacteraemic and other severe infections are treated with an intravenous beta-lactam agent such as Ceftazidime or Meropenem in an intensive phase for between 2 and 8 weeks, then followed by an extended period of eradication with one or more oral antimicrobial agents to counter the risk of relapse in an eradication phase lasting 3–6 months. The revised guidelines vary with presence and location of focal disease. Control of co-morbid conditions such as diabetes and chronic kidney disease during this eradication phase is likely to be an important contributor to eventual success of eradication therapy, but can be confounded by poor compliance with oral treatment regimens22. The restricted range of antimicrobial agents effective against B. pseudomallei reflects its natural habitat as a soil-dwelling bacterium, where it has evolved a range of mechanisms for antibiotic inactivation, notable among these being a collection of efficient efflux pumps23. Though the success of the Darwin treatment protocol is clear from improved treatment outcomes, high levels of intrinsic antimicrobial resistance and concerns about emerging acquired resistant have prompted the application of genomics to predict antimicrobial resistance in B. pseudomallei24. Moreover, the ability of B. pseudomallei to sequestrate in cells and tissues where antimicrobial bioavailability is poor presents a challenge to guaranteeing effective intracellular antimicrobial activity.
Pathogenesis
The unusually broad range of clinical presentations of melioidosis have yet to be fully explained at a mechanistic level, but it is becoming clear that virulence of infection is predominantly a function of host risk factors25. B. pseudomallei is a facultative intracellular bacterial pathogen capable of entry into and prolonged survival within professional phagocytic cells26. Like other facultative intracellular bacteria associated with infections of public health interest, B. pseudomallei deploys a range of molecular mechanisms that likely reflect its evolutionary history as a soil-dwelling species. Indeed, its ability to invade and persist in naturally occurring soil microbiota such as free-living amoebae suggest a possible environmental origin for its cellular virulence27. However, a subset of B.pseudomallei possess a Burkholderia mallei-like sequence variation in the actin-based motility gene whose presence correlates with rapid dissemination and replication at a range of locations including the nervous system and thus have a molecular basis for neurotropism28.
Genomics
The explosion of microbial genomics has led to important insights into the molecular biology and immunology of melioidosis. Whole genome sequencing indicates that B. pseudomallei has one of the largest known bacterial genomes at around 6.5 Mb, arranged in two chromosomes of unequal size29. The operons associated with virulence are mainly located on the smaller of the two. Recent phylogeographic analysis indicates that the Southeast Asian clade arose from an ancient Australian clade, which may have early remnants in Papua New Guinea and the Torres Strait islands30. Non-pathogenic near neighbour species such as Burkholderia ubonensis and Burkholderia thailandensis have also been found in pristine wilderness locations during B. pseudomallei environmental survey work, raising questions about the phylogeographic origins of the wider B. pseudomallei group31. At a more pragmatic level, genotyping studies have been instrumental in confirming single points of origin for melioidosis case clusters4–7 and have shown the plausibility of occasional long-distance translocation of B. pseudomallei strains associated with human infection9.
A changing public health threat
All Australian jurisdictions in the tropics have made melioidosis a notifiable infection. Following controls applied in the aftermath of the Western Australian case cluster in 199732, bacteraemic melioidosis is now rare in WA and almost eliminated in our indigenous population. The majority of cases are in long distance travellers11 and even these have fallen recently due to pandemic travel restrictions. However, the recent Southwestern WA case cluster was a stark reminder of the greater difficulty detecting B. pseudomallei soft tissue infections6, particularly when geographic location, clinical presentation and exposure history are unexpected. We have to ask how many subacute and initially asymptomatic infections are missed. Noting the association with cyclone tracks, and the changing patterns of cyclone behaviour as a consequence of climate change9, we should be alert to the possibility of an extension of the Australian melioidosis endemic zone. The increased political instability of our region due to the effects of the COVID pandemic should also alert us to the deliberate dissemination of B. pseudomallei. This may seem far-fetched, but was under active consideration in the wake of anthrax spore/white powder events in 2001.
Conclusion
Melioidosis is a disease complex attributed to a multi-competent Gram-negative bacillus, B. pseudomallei. High rates of mortality in acute melioidosis survivors remain an unresolved problem33. The clinical and scientific experience built up in Australian centres of excellence, particularly in our tropics, has advanced diagnosis, treatment and prevention of severe and subacute disease variants. However, the natural environmental habitat of B. pseudomallei ensures that the principal reservoir of human infection cannot be eliminated. Changing patterns of land use, human encounter with environmental B. pseudomallei, and environmental influences like climate change guarantee further challenges for Australian microbiologists in years to come.
Conflicts of interest
The author declares no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] Rimington, R.A. (1962) Melioidosis in north Queensland. Med. J. Aust. 1, 50–53.| Melioidosis in north Queensland.Crossref | GoogleScholarGoogle Scholar |
[2] Gassiep, I. et al. (2020) Human melioidosis. Clin. Microbiol. Rev. 33, e00006-19.
| Human melioidosis.Crossref | GoogleScholarGoogle Scholar | 32161067PubMed |
[3] Currie, B.J. and Kaestli, M. (2016) Epidemiology: a global picture of melioidosis. Nature 529, 290–291.
| Epidemiology: a global picture of melioidosis.Crossref | GoogleScholarGoogle Scholar | 26791716PubMed |
[4] Inglis, T.J. et al. (2000) Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg. Infect. Dis. 6, 56–59.
| Burkholderia pseudomallei traced to water treatment plant in Australia.Crossref | GoogleScholarGoogle Scholar | 10653571PubMed |
[5] Currie, B.J. et al. (2001) A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am. J. Trop. Med. Hyg. 65, 177–179.
| A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates.Crossref | GoogleScholarGoogle Scholar | 11561699PubMed |
[6] Merritt, A.J. et al. (2016) Cutaneous melioidosis cluster caused by contaminated wound irrigation fluid. Emerg. Infect. Dis. 22, 1420–1427.
| Cutaneous melioidosis cluster caused by contaminated wound irrigation fluid.Crossref | GoogleScholarGoogle Scholar |
[7] Gal, D. et al. (2004) Contamination of hand wash detergent linked to occupationally acquired melioidosis. Am. J. Trop. Med. Hyg. 71, 360–362.
| Contamination of hand wash detergent linked to occupationally acquired melioidosis.Crossref | GoogleScholarGoogle Scholar | 15381819PubMed |
[8] Webb, J.R. et al. (2020) A persisting nontropical focus of Burkholderia pseudomallei with limited genome evolution over five decades. mSystems 5, e00726-20.
| A persisting nontropical focus of Burkholderia pseudomallei with limited genome evolution over five decades.Crossref | GoogleScholarGoogle Scholar | 33172968PubMed |
[9] Merritt, A.J. and Inglis, T.J.J. (2017) The role of climate in the epidemiology of melioidosis. Curr. Trop. Med. Rep. 4, 185–191.
| The role of climate in the epidemiology of melioidosis.Crossref | GoogleScholarGoogle Scholar | 29188170PubMed |
[10] Stewart, J.D. et al. (2017) Melioidosis in Far North Queensland is not correlated with severe weather events. Med. J. Aust. 207, 394.
| Melioidosis in Far North Queensland is not correlated with severe weather events.Crossref | GoogleScholarGoogle Scholar | 29092705PubMed |
[11] Dan, M. (2015) Melioidosis in travelers: review of the literature. J. Travel Med. 22, 410–414.
| Melioidosis in travelers: review of the literature.Crossref | GoogleScholarGoogle Scholar | 26503093PubMed |
[12] Currie, B.J. et al. (2004) Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop. Med. Int. Health 9, 1167–1174.
| Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia.Crossref | GoogleScholarGoogle Scholar | 15548312PubMed |
[13] Garg, R. et al. (2020) Melioidosis in suspected recurrent tuberculosis: a disease in disguise. J. Infect. Dev. Ctries. 14, 312–316.
| Melioidosis in suspected recurrent tuberculosis: a disease in disguise.Crossref | GoogleScholarGoogle Scholar | 32235093PubMed |
[14] Cheng, A.C. (2010) Melioidosis: advances in diagnosis and treatment. Curr. Opin. Infect. Dis. 23, 554–559.
| Melioidosis: advances in diagnosis and treatment.Crossref | GoogleScholarGoogle Scholar | 20847695PubMed |
[15] Fairley, L. et al. (2021) Systematic review and meta-analysis of diagnostic tests for diagnosis of melioidosis. Acta Trop. 214, 105784.
| Systematic review and meta-analysis of diagnostic tests for diagnosis of melioidosis.Crossref | GoogleScholarGoogle Scholar | 33296681PubMed |
[16] Inglis, T.J. et al. (2015) Volatile-sulfur-compound profile distinguishes Burkholderia pseudomallei from Burkholderia thailandensis. J. Clin. Microbiol. 53, 1009–1011.
| Volatile-sulfur-compound profile distinguishes Burkholderia pseudomallei from Burkholderia thailandensis.Crossref | GoogleScholarGoogle Scholar | 25568444PubMed |
[17] Gassiep, I. et al. (2021) Diagnosis of melioidosis: the role of molecular techniques. Future Microbiol. 16, 271–288.
| Diagnosis of melioidosis: the role of molecular techniques.Crossref | GoogleScholarGoogle Scholar | 33595347PubMed |
[18] Inglis, T.J. et al. (2012) Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis for rapid confirmation of Burkholderia pseudomallei in septicemic melioidosis. Am. J. Trop. Med. Hyg. 86, 1039–1042.
| Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis for rapid confirmation of Burkholderia pseudomallei in septicemic melioidosis.Crossref | GoogleScholarGoogle Scholar | 22665614PubMed |
[19] Chaichana, P. et al. (2018) Antibodies in melioidosis: the role of the indirect hemagglutination assay in evaluating patients and exposed populations. Am. J. Trop. Med. Hyg. 99, 1378–1385.
| Antibodies in melioidosis: the role of the indirect hemagglutination assay in evaluating patients and exposed populations.Crossref | GoogleScholarGoogle Scholar | 30298810PubMed |
[20] Grivas, R. et al. (2015) A prospective study of melioidosis after environmental exposure of healthy participants to Burkholderia pseudomallei during a muddy endurance challenge. Am. J. Trop. Med. Hyg. 92, 773–775.
| A prospective study of melioidosis after environmental exposure of healthy participants to Burkholderia pseudomallei during a muddy endurance challenge.Crossref | GoogleScholarGoogle Scholar | 25624406PubMed |
[21] Sullivan, R.P. et al. (2020) 2020 Review and revision of the 2015 Darwin melioidosis treatment guideline; paradigm drift not shift. PLoS Negl. Trop. Dis. 14, e0008659.
| 2020 Review and revision of the 2015 Darwin melioidosis treatment guideline; paradigm drift not shift.Crossref | GoogleScholarGoogle Scholar | 32986699PubMed |
[22] Stewart, J.D. et al. (2017) The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management. PLoS Negl. Trop. Dis. 11, e0005411.
| The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management.Crossref | GoogleScholarGoogle Scholar | 28264029PubMed |
[23] Krishnamoorthy, G. et al. (2019) Efflux pumps of Burkholderia thailandensis control the permeability barrier of the outer membrane. Antimicrob. Agents Chemother. 63, e00956-19.
| Efflux pumps of Burkholderia thailandensis control the permeability barrier of the outer membrane.Crossref | GoogleScholarGoogle Scholar | 31383661PubMed |
[24] Madden, D.E. et al. (2021) Taking the next-gen step: comprehensive antimicrobial resistance detection from Burkholderia pseudomallei. EBioMedicine 63, 103152.
| Taking the next-gen step: comprehensive antimicrobial resistance detection from Burkholderia pseudomallei.Crossref | GoogleScholarGoogle Scholar | 33285499PubMed |
[25] Webb, J.R. et al. (2019) Burkholderia pseudomallei lipopolysaccharide genotype does not correlate with severity or outcome in melioidosis: host risk factors remain the critical determinant. Open Forum Infect. Dis. 6, ofz091.
| Burkholderia pseudomallei lipopolysaccharide genotype does not correlate with severity or outcome in melioidosis: host risk factors remain the critical determinant.Crossref | GoogleScholarGoogle Scholar | 30949536PubMed |
[26] Whiteley, L. et al. (2017) Entry, intracellular survival, and multinucleated-giant-cell-forming activity of Burkholderia pseudomallei in human primary phagocytic and nonphagocytic cells. Infect. Immun. 85, e00468-17.
| Entry, intracellular survival, and multinucleated-giant-cell-forming activity of Burkholderia pseudomallei in human primary phagocytic and nonphagocytic cells.Crossref | GoogleScholarGoogle Scholar | 28760929PubMed |
[27] Inglis, T.J. et al. (2000) Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape. Infect. Immun. 68, 1681–1686.
| Interaction between Burkholderia pseudomallei and Acanthamoeba species results in coiling phagocytosis, endamebic bacterial survival, and escape.Crossref | GoogleScholarGoogle Scholar | 10678988PubMed |
[28] Morris, J.L. et al. (2017) Increased neurotropic threat from Burkholderia pseudomallei strains with a B. mallei-like variation in the bimA motility gene, Australia. Emerg. Infect. Dis. 23, 740–749.
| Increased neurotropic threat from Burkholderia pseudomallei strains with a B. mallei-like variation in the bimA motility gene, Australia.Crossref | GoogleScholarGoogle Scholar |
[29] Songsivilai, S. and Dharakul, T. (2000) Multiple replicons constitute the 6.5-megabase genome of Burkholderia pseudomallei. Acta Trop. 74, 169–179.
| Multiple replicons constitute the 6.5-megabase genome of Burkholderia pseudomallei.Crossref | GoogleScholarGoogle Scholar | 10674646PubMed |
[30] Baker, A.L. et al. (2018) Burkholderia pseudomallei distribution in Australasia is linked to paleogeographic and anthropogenic history. PLoS One 13, e0206845.
| Burkholderia pseudomallei distribution in Australasia is linked to paleogeographic and anthropogenic history.Crossref | GoogleScholarGoogle Scholar | 30395628PubMed |
[31] Levy, A. et al. (2008) Expanded range of Burkholderia species in Australia. Am. J. Trop. Med. Hyg. 78, 599–604.
| Expanded range of Burkholderia species in Australia.Crossref | GoogleScholarGoogle Scholar | 18385355PubMed |
[32] Inglis, T.J. et al. (2011) The aftermath of the Western Australian melioidosis outbreak. Am. J. Trop. Med. Hyg. 84, 851–857.
| The aftermath of the Western Australian melioidosis outbreak.Crossref | GoogleScholarGoogle Scholar | 21633018PubMed |
[33] Hanson, J. and Smith, S. (2019) High rates of premature and potentially preventable death among patients surviving melioidosis in tropical Australia. Am. J. Trop. Med. Hyg. 101, 328–331.
| High rates of premature and potentially preventable death among patients surviving melioidosis in tropical Australia.Crossref | GoogleScholarGoogle Scholar | 31264566PubMed |
Biography
Dr Inglis is Head of Pathology and Laboratory Medicine at the University of Western Australia, and appointed as a Medical Microbiologist at PathWest Laboratory Medicine WA. He qualified in Medicine at the University of Southampton, and followed this with a Doctor of Medicine thesis on ventilator associated pneumonia. After three years in Singapore where he first encountered melioidosis, he moved to Western Australia to take up his position with the state pathology service. Shortly afterwards he investigated the Kimberley melioidosis outbreak and its aftermath, completing a PhD on the environmental biology of Burkholderia pseudomallei. Since then he has expanded on laboratory biopreparedness, regional pathology capability building and the laboratory response to emerging infectious diseases, including the current pandemic.


