Perspectives for antivirals to limit SARS-CoV-2 infection (COVID-19)
Erik De ClercqKU Leuven, Department of Microbiology, Immunology and Transplantation, Rega Institute for Medical Research, Herestraat 49, 3000 Leuven, Belgium. Tel.: + 32 16 37 90 20; Email: erik.declercq@kuleuven.be
Microbiology Australia 42(1) 47-53 https://doi.org/10.1071/MA21013
Submitted: 5 February 2021 Accepted: 3 March 2021 Published: 12 April 2021
Journal Compilation © The Authors 2021 Open Access CC BY, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Compared with vaccines, antivirals for curbing COVID-19 (SARS-CoV-2 infection) have been developed at a much lower pace. Favipiravir has proven efficacious (in hamsters) but only at a very high dose which may not be feasible in humans. Remdesivir is the sole antiviral approved by the US FDA, but it has not been extensively evaluated for its safety. EIDD-1931 and EIDD-2801 have not been evaluated clinically. Mpro (protease) inhibitors likewise need to be subjected to clinical efficacy and safety studies. Remdesivir is a C-nucleoside and this class of compounds should be further evaluated. Polyanionic substances interfering with virus adsorption to the host cells have not been explored. They may possibly be administered by inhalation. Corticosteroids (such as dexamethasone), while virus-stimulating rather than inhibitory, may counteract the ‘cytokine storm’. Combination of (two or more of) the compounds mentioned above may offer an increased benefit through a synergistic interaction.
Introduction
Currently, vaccines are more in vogue than antivirals in attempts to curb COVID-19. Yet, for vaccines, certain requirements are imperative, which a fortiori may apply to antivirals as well. These requirements follow the rule of 5: effectiveness, safety, availability, practicality and costs.
As the success of vaccination, the use of the vaccinia virus is often cited as responsible for the global (worldwide) eradication of the variola virus (smallpox) announced by the World Health Organization (WHO) in 1980. Of all vaccinations, vaccinia is the only virus vaccine that could be credited with this unique achievement. For polio(myelitis) effective vaccines, such as those originally developed by Jonas Salk and Albert Sabin, have been described, but the total elimination of the poliovirus, although anticipated, has still not been achieved.
For influenza virus, protective vaccines still have to be adjusted on an annual basis, and for several other virus infections such as yellow fever [the only viral infection for which the discovery of the vaccine was recognised by a Nobel Prize (Max Theiler)], the disease is still prevalent, as it is transmitted by mosquitos (Aedes Aegypti) and could also originate from an animal reservoir (primates).
For hepatitis B virus (HBV), both antivirals and vaccines are available, but for other virus infections such as human immunodeficiency virus (HIV), vaccination has (so far) proven unfeasible, or unnecessary [hepatitis C virus (HCV)], as the use of direct-acting antivirals (DAAs) may be expected to eliminate the virus, or impractical (human rhinoviruses), because there are too many serotypes (>100).
SARS-CoV-2 should be situated in the Coronaviridae (genus: betacoronavirus). The first species to emerge was SARS-CoV-1 (SARS standing for ‘Severe Acute Respiratory Syndrome’) in 2003. Then followed MERS-CoV (MERS: Middle East Respiratory Syndrome) in 2012 and SARS-CoV-2 in 2019 (COVID-19). Potential antivirals against SARS were proposed in 20061 and COVID-19 in 20202.
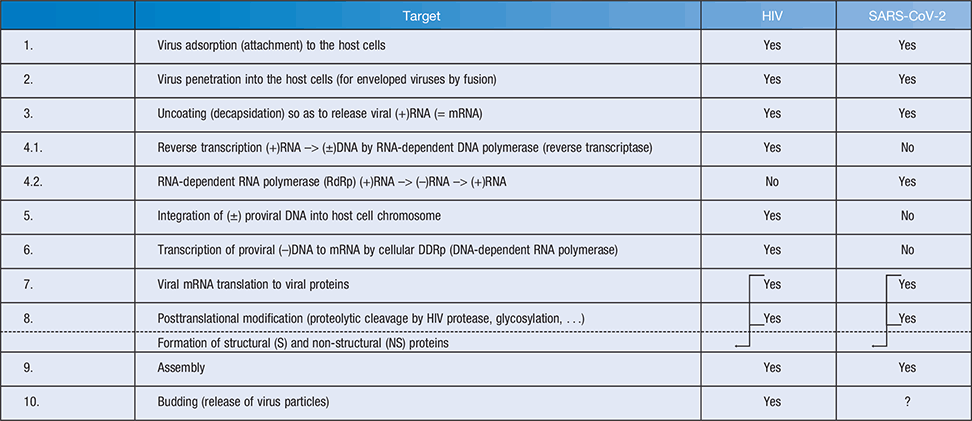
Targets for antivirals (Table 1)
Interferon
Interferon was originally discovered in 1957 by Isaacs and Lindenmann3 as a substance produced by the organism to block (‘interfere with’) the virus infection, in casu influenza. Over the past decades interferon has been formally approved for the treatment of several viral infections, including HBV and HCV, despite some undesirable side-effects that are inherently linked to exogenous administration of interferon, i.e. fever, fatigue and malaise.
|
In the treatment of COVID-19, it may act as a double-edged sword. Depending on the host’s responsiveness and the stage of the disease, interferon (whether α- or β-interferon) might in some conditions, because of its antiviral effects, favorably influence the course of the disease, but being itself a cytokine it might also worsen the infection by participating in or aggravating the cytokine storm that may accompany the SARS-CoV-2 infection.
Ribavirin
Ribavirin (Virazole) is the first nucleoside analogue ever described as a broad-spectrum antiviral agent4. It was reported to be effective in the treatment of Lassa fever (a hemorrhagic fever (HF) arena virus infection)5. In combination with (pegylated) interferon-α, ribavirin has been used for almost a full decade (2000-2010) in the treatment of hepatitis C, where it has now been replaced by the more effective DAAs.
Although traditionally regarded as an antiviral agent, its principal mode of action, the inhibition of the biosynthesis of GTP, consequently to the inhibition of the IMP dehydrogenase6, is also compatible with an immunosuppressive effect7. As an antiviral agent, ribavirin may not have sufficient potency to block SARS-CoV-2 replication, but as an immunosuppressant it could exhibit the desirable utility to curtail the cytokine storm accompanying the SARS-CoV-2 infection.
(Hydroxy)chloroquine
(Hydroxy)chloroquine has been claimed, without any indication of its mode of action, to inhibit SARS-CoV-2 replication in vitro (hydroxychloroquine being more potent than chloroquine)8–10. However, Hoffmann et al. definitely ruled out that chloroquine could inhibit the replication of SARS-CoV-2 in human lung (Calu-3) cells11. Also, Kaptein et al.12 found that hydroxychloroquine was totally inactive against SARS-CoV-2 infections in hamsters. Therefore, there is no compelling reason to further advocate its use in the treatment of COVID-19.
Polyanionic substances
For more than half of a century, polyanionic compounds have been envisaged as potential antiviral agents: i.e. double-stranded RNAs such as poly(I).poly(C) as inducers of interferon and sulfated polysaccharides such as dextran sulfate as inhibitors of HIV adsorption. The structurally related polysulfonate suramin, originally identified as a potent (murine) reverse transcriptase (RT) inhibitor13 was the first compound ever shown to block HIV infection, both in vitro14 and in vivo15.
Sulfated polysaccharides encompass an extremely wide family of compounds, including natural (marine) substances such as carrageenans derived from seaweeds16. These compounds may be expected to block virus adsorption of all enveloped viruses, including coronaviruses. It is surprising that no data have ever been reported on the activity of any sulfated (or anionic) polymers against SARS-CoV-2.
Polyanionic substances may not be suitable for systemic administration, as they may not easily reach their target cells upon such administration, but they could be amenable to topical administration, i.e. inhalation, which may be well suited in the therapy of SARS-CoV-2 infection.
Protease inhibitors
The combination of Lopinavir with Ritonavir (the latter to increase the plasma half-life of the former through inhibition of cytochrome P450) has been formally approved as a specific HIV protease inhibitor duo for the treatment of HIV infections, and so are 8 other HIV protease inhibitors (i.e. saquinavir, indinavir, nelfinavir, amprenavir, fosamprenavir, atazanavir, tipranavir and darunavir). It is surprising that Lopinavir and Ritonavir (and later on some other HIV protease inhibitors) were advocated for the treatment of SARS-CoV-2 infections.
This application has been generated by the fact that Lopinavir and Ritonavir were reported to show in vitro inhibitory activity against SARS-CoV-217–19. It is reassuring therefore that in hospitalised adult patients with severe COVID-19 Lopinavir (+ Ritonavir) treatment did not offer additional benefit beyond standard care20.
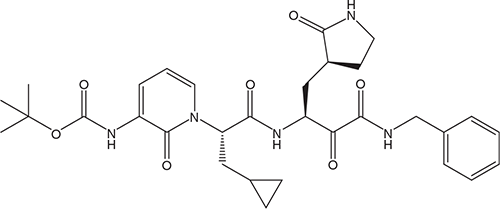
The HIV protease is fundamentally different from the coronavirus main protease Mpro or 3CLpro, which since 2003 has been considered as an attractive target for the design of anti-SARS drugs21. A representative compound, the α-ketoamide 13b (Figure 1) proved highly effective in inhibiting SARS-CoV-2 replication in human Calu-3 lung cells22. This compound might be suitable for administration by inhalation in the treatment of COVID-19.
|
In general, α-ketoamides display low-micromolar EC50 values against a broad range of corona- and enteroviruses23. What remains to be ascertained is whether any of the α-ketoamides, and if so, which one, has practical utility in the therapy (or prophylaxis) of COVID-19 by any route, i.e. inhalative, oral, or parental. This will require in-depth exploration of the clinical candidate α-ketoamides for their safety, clinical efficacy and pharmacokinetic and -dynamic profile.
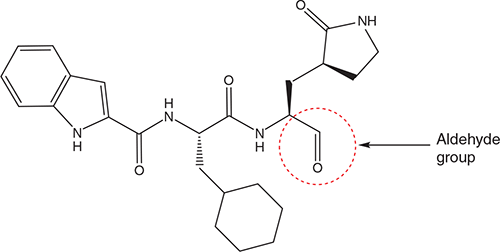
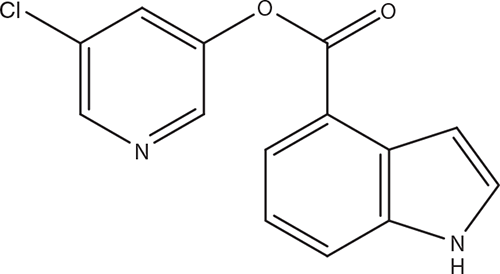
The SARS-CoV-2 main protease Mpro (3CLpro) can also be targeted by a series of compounds carrying an aldehyde group that could be covalently bound to cysteine 145 of Mpro. This has been shown for compounds 11a and 11b24. Compound 11a (Figure 2) also exhibited low cytotoxicity and inhibited SARS-CoV-2 replication in Vero E6 cells at an EC50 of 0.53 µM. The authors concluded that compound 11a would be a good candidate for further study.
|
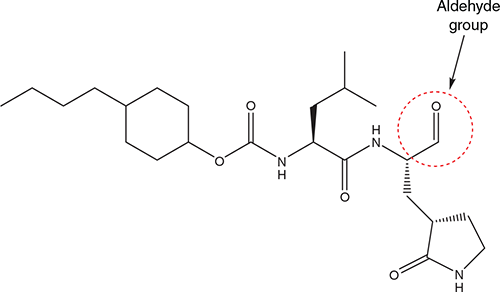
Rathnayake et al.25 described several 3C-like protease inhibitors also containing an aldehyde group, with one of the lead compounds being 6e (Figure 3), which blocked SARS-CoV-2 replication in primary human airway epithelial cells [EC50 : 0.17 µM in a FRET (fluorescence resonance energy transfer) assay]; it increased survival from 0 to 100% in a murine model of MERS-CoV infection.
|
Hattori et al.26 reported that an indole chloropyridinyl ester, GRL-0920 (Figure 4), could completely block SARS-CoV-2 infection in Vero E6 cells (whether or not overexpressing TMPRSS2 (EC50 ≃ 9 µM) as the result of covalent bonding with Cys 145 and His 41. Whether GRL-0920 or any other indole chloropyridinyl ester has any therapeutic or prophylactic usefulness against COVID-19 in humans remains to be determined.
|
RdRp (RNA-dependent RNA polymerase) inhibitors
Besides Mpro, the SARS-CoV-2 RdRp can also be considered as an attractive target in the design of specific SARS-CoV-2 inhibitors. The structure of this enzyme (nsp12 polymerase bound to nsp7 and nsp8 cofactors) was published in 201927. Shortly thereafter, Chien and co-workers, including R.N. Kirchdoerfer, reported that the SARS-CoV-2 RdRp could be inhibited by the 5′-triphosphate forms of sofosbuvir, AZT (3′-azido,2′,3′-dideoxy-thymidine, azidothymidine) and FdT (3′-fluoro-2′,3′-dideoxythymidine, alovudine) and the 5′-diphosphate metabolite of tenofovir, which following their incorporation into the RdRp product, would act as a chain terminator of the latter28.
That sofosbuvir, azidothymidine and alovudine would be able to do so was again repeated in a paper published by Ju et al.29. Then, emtricitabine and tenofovir, the active compounds present in Truvada® and Descovy® used as PrEP (Pre-Exposure Prophylaxis) to prevent HIV infections were singled out as chain terminators for SARS-CoV-2 RdRp30. This inspired some authors to undertake some molecular docking studies31. This made me, in turn, to caution that antiviral drug development should be based on rigorous testing for antiviral activity that could not be substituted by molecular docking32.
In the meantime, the number of nucleoside analogues claimed to act against SARS-CoV-2 RdRp according to the same modus operandi (incorporation followed by chain termination) has increased to another 11 molecules, including carbovir triphosphate, stavudine triphosphate, ganciclovir triphosphate, entecavir triphosphate and cidofovir diphosphate33. As this list of compounds encompasses a number of antiviral drugs formally approved for the treatment of HIV, HBV and herpesvirus infections, it is highly questionable, if not unlikely, that any of these compounds would have any utility for the treatment of COVID-19.
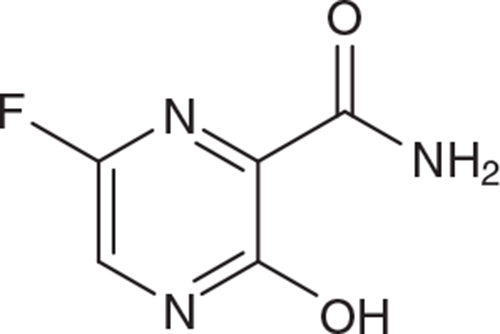
Favipiravir
Favipiravir (T-705; Figure 5) is a pyrazine analogue that has been formally approved in Japan and South Korea for the treatment of influenza virus infections. It has been demonstrated to be active against a broad range of (+)RNA and (−)RNA viruses, including hemorrhagic fever RVA viruses34. In hamsters, it was reported to have potent antiviral activity against SARS-CoV-2 infection, but only at high doses (1 g/kg twice per day)12. It is doubtful that such high doses would be safely attainable in humans. Clinical trials with favipiravir are currently ongoing in several countries35.
|
In vitro activity with favipiravir against SARS-CoV-2 was not observed in the experiments carried out by Professor Masanori Baba (personal communication, 2020). To be antivirally active, favipiravir has to fulfil two requirements. As a pyrazine analogue it must be first converted by phosphoribosyl transferase, which, like hypoxanthine/guanine phosphoribosyl transferase (4GPT) converts favipiravir directly to favipiravir ribosyl 5′-monophosphate, should then be further phosphorylated to the 5′-diphosphate and 5′-triphosphate before the latter could act as inhibitor of the SARS-CoV-2 RdRp. Whether the latter (following removal of its diphosphate moiety) could eventually act as an inhibitor, or possibly as a non-obligate chain terminator of the viral RNA synthesis, has not been experimentally shown. Such possibility is not excluded, since favipiravir contains a carboxamide (-CO-NH2) moiety that mimics the C6-N1 part of guanine. which would be apt to base pairing (through hydrogen bond formation) with cytosine (cf. Watson-Crick base pairing).
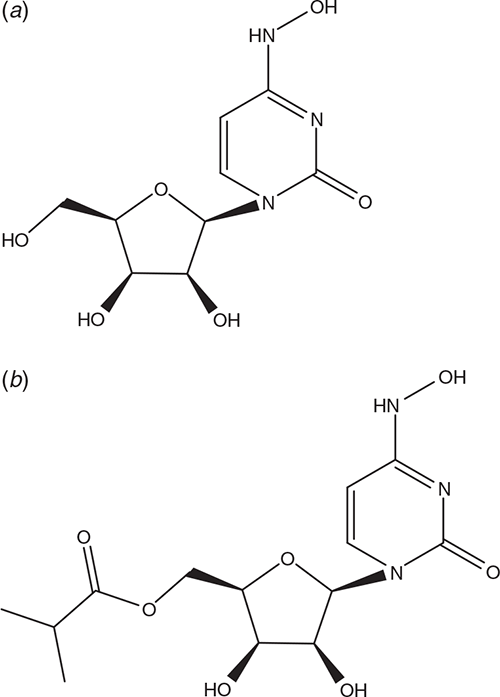
NHC (N4-hydroxycytidine, EIDD-1931)
β-D-N4-Hydroxycytidine (NHC, EIDD-1931; Figure 6a) has been shown to be active against various unrelated RNA viruses including influenza, respiratory syncytial virus (RSV), Ebolavirus (EBOV), alpha- and coronaviruses36–40.
|
An orally bioavailable prodrug of EIDD-1931, namely its 5′-isopropyl ester EIDD-2801 (Figure 6b) has proved to be inhibitory to SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice41.
From a mechanistic viewpoint, NHC, if converted to its 5′-triphosphate, might be expected to interfere with the SARS-CoV-2 RdRp, and to act, if incorporated into the viral RNA, as a possible non-obligate chain terminator due to base pairing, through hydrogen bonding, with guanine. This mechanistic viewpoint, the possible clinical efficacy of NHC (EIDD-1931) and its prodrug EIDD-2801, and all the required safety and pharmacokinetic assessments remain to be carried out.
Remdesivir
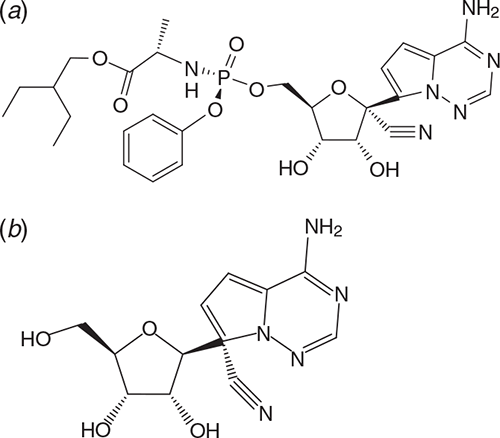
Remdesivir (Veklury®; Figure 7a) is currently the only compound that has been formally approved by the US FDA for the treatment of COVID-19 at a dose of 200 mg on day 1 followed by once daily doses of 100 mg for a total duration of 5 days, all by the intravenous route. If the patient does not show clinical improvement, treatment may be extended to 10 days. The parent of Remdesivir is GS-441524 (Figure 7b), which is the predominant metabolite reaching the lungs. GS-441524 was claimed to be superior to Remdesivir for COVID-19 treatment42.
|
GS-441524 was originally synthesised by Choung U Kim and his colleagues at Gilead Sciences in 201243. The purpose was to find activity against HCV, but activity was detected against a wide variety of RNA viruses with RdRp as the target enzyme.
That Remdesivir was active against SARS-CoV-2 replication in vitro was first reported by Wang on 4 February 20208, but the validity of these findings could be questioned since several other compounds of questionable activity such as chloroquine and penciclovir were also reported to be active in this study.
That Remdesivir should be effective against SARS-CoV-2 could be predicted from its activity against both epidemic and zoonotic coronaviruses44 and various human endemic and zoonotic deltacoronaviruses with a highly divergent RNA-dependent RNA polymerase45.
The target for the activity of Remdesivir against SARS-CoV-2 is its RdRp46,47. Incorporation of the Remdesivir 5′-triphosphate (as its 5′-monophosphate) at position i caused termination of RNA synthesis at position i+348,49.
Remdesivir has proven to be efficacious in the treatment of both MERS CoV and SARS-CoV-2 infection in rhesus macaques50,51. To achieve a clinical benefit, treatment should be initiated early during the viral infection.
The clinical studies with Remdesivir in humans have yielded mixed results. On 10 April 2020, Grein et al.52 reported that the compassionate use of Remdesivir for patients with severe COVID-19 afforded clinical improvement in 36 of 53 patients (68%). On 29 April 2020, Wang et al.53 reported that in a randomised, double-blind, placebo-controlled, multicentre trial Remdesivir was not associated with statistically significant clinical benefits. On 22 May 2020, Beigel et al.54 reported that Remdesivir was superior to placebo in shortening the time to recovery in adults hospitalised with COVID-19. On 27 May 2020, Goldmann et al.55 reported that in patients with severe COVID-19 not requiring mechanical ventilation, there was no significant difference between a 5-day course and a 10-day course of Remdesivir. On 21 August 2020, Spinner et al.56 reported that among patients with moderate COVID-19 those randomised to a 10-day course of Remdesivir did not have a statistically significant difference in clinical status compared with standard care at 11 days after initiation of treatment; patients randomised to a 5-day course of Remdesivir had a statistical difference in clinical status compared with standard care, but the difference was of uncertain clinical importance.
In Europe, the formal marketing authorisation of Remdesivir by the European Medicines Agency (EMA) is still pending. The Manufacturer, Gilead Sciences, has set a price of US$2340 for a 5-day treatment course in late June 2020. The question now is whether Remdesivir for treatment of COVID-19 in Europe will provide value for money57?
Although approved in the US, and its approval pending in the EU, a number of issues of Remdesivir still remain to be resolved, i.e. oral bioavailability, safety, optimal dosage, optimal route of administration, pharmacodynamics and -therapeutics, risk for resistance development, structure-activity (SAR) relationship, etc.
C-nucleosides
The fact that Remdesivir, the only antiviral drug that has been formally approved by the US FDA for the treatment of COVID-19, is a C-nucleoside, makes it the first C-nucleoside in history to be ever approved as a C-nucleoside. In contrast with the N-nucleosides to which all nucleoside analogues belong that are currently on the antiviral (or antitumor) drug market, C-nucleosides are not susceptible to phosphorolytic cleavage which with the help of inorganic phosphate (Pi) cleaves the N-glycosidic linkage between the heterocyclic ring (e.g. purine or pyrimidine) and the sugar (e.g. ribose or deoxyribose) moiety. The phosporolytic cleavage is normally carried out by a purine or pyrimidine phosphorylase responsible for a perfectly reversible reaction of the general type Nucleoside + Pi 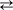 Purine/Pyrimidine + (Deoxy)ribose – MP, which occurs in the degradation (catabolism) as well as biosynthesis (salvage) of nucleotides.
Purine/Pyrimidine + (Deoxy)ribose – MP, which occurs in the degradation (catabolism) as well as biosynthesis (salvage) of nucleotides.
The potential therapeutic application of Remdesivir could have been foreseen, when in 2016 I wrote that C-nucleosides should be revisited as antiviral agents58. A case in point is provided by pyrazofurin (alias pyrazomycin), which in the 1970s we found to be antivirally far superior to ribavirin59. There is no experimental evidence that except for GS-441524 and its phosphoramidate (Remdesivir), any other C-nucleoside analogues or their phosphoramidates would be effective against COVID-19, but this possibility has not been thoroughly explored.
Conclusion
The paucity of antivirals to treat SARS-CoV-2 infections currently available contrasts with the abundance of virus vaccines which have been developed to prevent the infection. Also, we do not know how exactly SARS-CoV-2 could develop resistance to these antivirals and/or how many virus variants exist today or may emerge in the future.
Foremost among the most likely antiviral drug candidates are those targeted at the main viral protease (Mpro) and the viral RNA-dependent RNA polymerase (RdRp), although virus attachment (adsorption) to the host cells may also be envisaged as a potential target.
The situation with COVID-19 may, to some extent, remind the early AIDS days, with a major difference between COVID-19 and AIDS that with AIDS there was never a prospect for effective vaccination, whereas for COVID-19, we have too many either present or waiting.
For the treatment of COVID-19, there are only few antivirals at hand or forthcoming, but what we learned from HIV infection, and tuberculosis as well, is that drug combinations could be envisioned so as to obtain synergism, diminish the individual dosages and reduce the risk for drug resistance development.
Conflicts of interest
The author declares no conflicts of interest.
Addendum
Since this article was written (January–February 2021) and peer-reviewed, new potential antivirals for the treatment of COVID-19 were reported, such as: (1) AT-511, announced as the ‘free base form’ of AT-52760, but basically the same compound as described by Berliba et al.61; (2) PF-07304814 and PF-00835231 (SARS-CoV-2 3CL protease inhibitors)62; and (3) ALG-0971163 that would directly inhibit the SARS-CoV-2 3CL protease inhibitor and K77764 that would indirectly do so (via inhibition of cathepsin L).
Acknowledgements
This research did not receive any specific funding.
References
[1] De Clercq, E. (2006) Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev. Anti Infect. Ther. 4, 291–302.| Potential antivirals and antiviral strategies against SARS coronavirus infections.Crossref | GoogleScholarGoogle Scholar | 16597209PubMed |
[2] Li, G. and De Clercq, E. (2020) Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug Discov. 19, 149–150.
| Therapeutic options for the 2019 novel coronavirus (2019-nCoV).Crossref | GoogleScholarGoogle Scholar | 32127666PubMed |
[3] Isaacs, A. and Lindenmann, J. (1957) Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147, 258–267.
| Virus interference. I. The interferon.Crossref | GoogleScholarGoogle Scholar | 13465720PubMed |
[4] Sidwell, R.W. et al. (1972) Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 177, 705–706.
| Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide.Crossref | GoogleScholarGoogle Scholar | 4340949PubMed |
[5] McCormick, J.B. et al. (1986) Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 314, 20–26.
| Lassa fever. Effective therapy with ribavirin.Crossref | GoogleScholarGoogle Scholar | 3940312PubMed |
[6] Streeter, D.G. et al. (1973) Mechanism of action of 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc. Natl. Acad. Sci. USA 70, 1174–1178.
| Mechanism of action of 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent.Crossref | GoogleScholarGoogle Scholar | 4197928PubMed |
[7] Potter, C.W. et al. (1976) Antiviral, immunosuppressive and antitumour effects of ribavirin. Nature 259, 496–497.
| Antiviral, immunosuppressive and antitumour effects of ribavirin.Crossref | GoogleScholarGoogle Scholar | 1256547PubMed |
[8] Wang, M. et al. (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30, 269–271.
| Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro.Crossref | GoogleScholarGoogle Scholar | 32020029PubMed |
[9] Liu, J. et al. (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6, 16.
| Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro.Crossref | GoogleScholarGoogle Scholar | 33731711PubMed |
[10] Yao, X. et al. (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 71, 732–739.
| In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).Crossref | GoogleScholarGoogle Scholar | 32150618PubMed |
[11] Hoffmann, M. et al. (2020) Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature 585, 588–590.
| Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2.Crossref | GoogleScholarGoogle Scholar | 32698190PubMed |
[12] Kaptein, S.J.F. et al. (2020) Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc. Natl. Acad. Sci. USA 117, 26955–26965.
| Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity.Crossref | GoogleScholarGoogle Scholar |
[13] De Clercq, E. (1979) Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses. Cancer Lett. 8, 9–22.
| Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses.Crossref | GoogleScholarGoogle Scholar |
[14] Mitsuya, H. et al. (1984) Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III. Science 226, 172–174.
| Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III.Crossref | GoogleScholarGoogle Scholar | 6091268PubMed |
[15] Broder, S. et al. (1985) Effects of suramin on HTLV-III/LAV infection presenting as Kaposi’s sarcoma or AIDS-related complex: clinical pharmacology and suppression of virus replication in vivo. Lancet 326, 627–630.
| Effects of suramin on HTLV-III/LAV infection presenting as Kaposi’s sarcoma or AIDS-related complex: clinical pharmacology and suppression of virus replication in vivo.Crossref | GoogleScholarGoogle Scholar |
[16] Witvrouw, M. and De Clercq, E. (1997) Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 29, 497–511.
| Sulfated polysaccharides extracted from sea algae as potential antiviral drugs.Crossref | GoogleScholarGoogle Scholar | 9352294PubMed |
[17] Chu, C.M. et al. (2004) Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 59, 252–256.
| Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings.Crossref | GoogleScholarGoogle Scholar | 14985565PubMed |
[18] Chen, F. et al. (2004) In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 31, 69–75.
| In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds.Crossref | GoogleScholarGoogle Scholar | 15288617PubMed |
[19] Wu, C.Y. et al. (2004) Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. USA 101, 10012–10017.
| Small molecules targeting severe acute respiratory syndrome human coronavirus.Crossref | GoogleScholarGoogle Scholar | 15226499PubMed |
[20] Cao, B. et al. (2020) A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 382, 1787–1799.
| A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19.Crossref | GoogleScholarGoogle Scholar | 32187464PubMed |
[21] Anand, K. et al. (2003) Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 300, 1763–1767.
| Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs.Crossref | GoogleScholarGoogle Scholar | 12746549PubMed |
[22] Zhang, L. et al. (2020) Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368, 409–412.
| Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors.Crossref | GoogleScholarGoogle Scholar | 32198291PubMed |
[23] Zhang, L. et al. (2020) α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J. Med. Chem. 63, 4562–4578.
| α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment.Crossref | GoogleScholarGoogle Scholar | 32045235PubMed |
[24] Dai, W. et al. (2020) Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 368, 1331–1335.
| Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease.Crossref | GoogleScholarGoogle Scholar | 32321856PubMed |
[25] Rathnayake, A.D. et al. (2020) 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV-infected mice. Sci. Transl. Med. 12, eabc5332.
| 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV-infected mice.Crossref | GoogleScholarGoogle Scholar | 32747425PubMed |
[26] Hattori, S.I. et al. (2020) GRL-0920, an indole chloropyridinyl ester, completely blocks SARS-CoV-2 Infection. MBio 11, e01833-20.
| GRL-0920, an indole chloropyridinyl ester, completely blocks SARS-CoV-2 Infection.Crossref | GoogleScholarGoogle Scholar | 32820005PubMed |
[27] Kirchdoerfer, R.N. and Ward, A.B. (2019) Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 10, 2342.
| Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors.Crossref | GoogleScholarGoogle Scholar | 31138817PubMed |
[28] Chien, M. et al. (2020) Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J. Proteome Res. 19, 4690–4697.
| Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19.Crossref | GoogleScholarGoogle Scholar | 32692185PubMed |
[29] Ju, J. et al. (2020) Nucleotide analogues as inhibitors of SARS-CoV polymerase. Pharmacol. Res. Perspect. 8, e00674.
| Nucleotide analogues as inhibitors of SARS-CoV polymerase.Crossref | GoogleScholarGoogle Scholar | 33124786PubMed |
[30] Jockusch S.et al2020 Triphosphates of the two components in DESCOVY and TRUVADA are inhibitors of the SARS-CoV-2 polymerase.bioRxiv2020.04.03.022939
[31] Elfiky, A.A. (2020) Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 253, 117592.
| Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study.Crossref | GoogleScholarGoogle Scholar | 32222463PubMed |
[32] De Clercq, E. (2020) Letter to the editor. Life Sci. 252, 117714.
| Letter to the editor.Crossref | GoogleScholarGoogle Scholar | 32334012PubMed |
[33] Jockusch, S. et al. (2020) A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antiviral Res. 180, 104857.
| A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19.Crossref | GoogleScholarGoogle Scholar | 32562705PubMed |
[34] De Clercq, E. (2019) New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem. Asian J. 14, 3962–3968.
| New nucleoside analogues for the treatment of hemorrhagic fever virus infections.Crossref | GoogleScholarGoogle Scholar | 31389664PubMed |
[35] Du, Y.X. and Chen, X.P. (2020) Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Ther. 108, 242–247.
| Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection.Crossref | GoogleScholarGoogle Scholar | 32246834PubMed |
[36] Reynard, O. et al. (2015) Identification of a new ribonucleoside inhibitor of Ebola virus replication. Viruses 7, 6233–6240.
| Identification of a new ribonucleoside inhibitor of Ebola virus replication.Crossref | GoogleScholarGoogle Scholar | 26633464PubMed |
[37] Urakova, N. et al. (2018) β-d-N4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J. Virol. 92, e01965-17.
| β-d-N4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome.Crossref | GoogleScholarGoogle Scholar | 29899097PubMed |
[38] Yoon, J.J. et al. (2018) Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob. Agents Chemother. 62, e00766-18.
| Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses.Crossref | GoogleScholarGoogle Scholar | 29891600PubMed |
[39] Agostini, M.L. et al. (2019) Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J. Virol. 93, e01348-19.
| Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance.Crossref | GoogleScholarGoogle Scholar | 31578288PubMed |
[40] Toots, M. et al. (2019) Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 11, eaax5866.
| Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia.Crossref | GoogleScholarGoogle Scholar | 31645453PubMed |
[41] Sheahan, T.P. et al. (2020) An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 12, eabb5883.
| An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice.Crossref | GoogleScholarGoogle Scholar | 32253226PubMed |
[42] Yan, V.C. and Muller, F.L. (2020) Advantages of the parent nucleoside GS-441524 over remdesivir for Covid-19 treatment. ACS Med. Chem. Lett. 11, 1361–1366.
| Advantages of the parent nucleoside GS-441524 over remdesivir for Covid-19 treatment.Crossref | GoogleScholarGoogle Scholar | 32665809PubMed |
[43] Cho, A. et al. (2012) Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med. Chem. Lett. 22, 2705–2707.
| Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides.Crossref | GoogleScholarGoogle Scholar | 22446091PubMed |
[44] Sheahan, T.P. et al. (2017) Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 9, eaal3653.
| Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses.Crossref | GoogleScholarGoogle Scholar | 28659436PubMed |
[45] Brown, A.J. et al. (2019) Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 169, 104541.
| Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase.Crossref | GoogleScholarGoogle Scholar | 31233808PubMed |
[46] Gao, Y. et al. (2020) Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 368, 779–782.
| Structure of the RNA-dependent RNA polymerase from COVID-19 virus.Crossref | GoogleScholarGoogle Scholar | 32277040PubMed |
[47] Gordon, C.J. et al. (2020) Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 295, 6785–6797.
| Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency.Crossref | GoogleScholarGoogle Scholar | 32284326PubMed |
[48] Agostini, M.L. et al. (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 9, e00221-18.
| Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease.Crossref | GoogleScholarGoogle Scholar | 29511076PubMed |
[49] Shannon, A. et al. (2020) Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites. Antiviral Res. 178, 104793.
| Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites.Crossref | GoogleScholarGoogle Scholar | 32283108PubMed |
[50] de Wit, E. et al. (2020) Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 117, 6771–6776.
| Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection.Crossref | GoogleScholarGoogle Scholar | 32054787PubMed |
[51] Williamson, B.N. et al. (2020) Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585, 273–276.
| Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2.Crossref | GoogleScholarGoogle Scholar | 32516797PubMed |
[52] Grein, J. et al. (2020) Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 382, 2327–2336.
| Compassionate use of remdesivir for patients with severe Covid-19.Crossref | GoogleScholarGoogle Scholar | 32275812PubMed |
[53] Wang, Y. et al. (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395, 1569–1578.
| Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial.Crossref | GoogleScholarGoogle Scholar | 32423584PubMed |
[54] Beigel, J.H. et al. (2020) Remdesivir for the treatment of Covid-19 – final report. N. Engl. J. Med. 383, 1813–1826.
| Remdesivir for the treatment of Covid-19 – final report.Crossref | GoogleScholarGoogle Scholar | 32445440PubMed |
[55] Goldman, J.D. et al. (2020) Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 383, 1827–1837.
| Remdesivir for 5 or 10 days in patients with severe Covid-19.Crossref | GoogleScholarGoogle Scholar | 32459919PubMed |
[56] Spinner, C.D. et al. (2020) Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 324, 1048–1057.
| Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial.Crossref | GoogleScholarGoogle Scholar | 32821939PubMed |
[57] Dal-Ré, R. et al. (2021) Remdesivir for COVID-19 in Europe: will it provide value for money? Lancet Respir. Med. 9, 127–128.
| Remdesivir for COVID-19 in Europe: will it provide value for money?Crossref | GoogleScholarGoogle Scholar | 33341157PubMed |
[58] De Clercq, E. (2016) C-nucleosides to be revisited. J. Med. Chem. 59, 2301–2311.
| C-nucleosides to be revisited.Crossref | GoogleScholarGoogle Scholar | 26513594PubMed |
[59] Descamps, J. and De Clercq, E. (1978) Broad-spectrum antiviral activity of pyrazofurin (pyrazomycin). In Current Chemotherapy Proceedings of the 10th International Congress of Chemotherapy (Siegenthaler, W., Lüthy, R., eds), 18–23 September 1977, Zürich, Switzerland. p. 354. Washington, DC: American Society for Microbiology.
[60] Good S.S.et al2020 AT-527 is a potent in vitro replication inhibitor of SARS-CoV-2, the virus responsible for the COVID-19 pandemic.bioRxiv2020.08.11.242834.
[61] Berliba, E. et al. (2019) Safety, pharmacokinetics and antiviral activity of AT-527, a novel purine nucleotide prodrug, in HCV-infected subjects with and without cirrhosis. Antimicrob. Agents Chemother. 63, e01201-19.
| Safety, pharmacokinetics and antiviral activity of AT-527, a novel purine nucleotide prodrug, in HCV-infected subjects with and without cirrhosis.Crossref | GoogleScholarGoogle Scholar |
[62] Hoffman, R.L. et al. (2020) Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J. Med. Chem. 63, 12725–12747.
| Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19.Crossref | GoogleScholarGoogle Scholar | 33054210PubMed |
[63] Vandyck K.et al2021 ALG-097111, a potent and selective SARS-CoV-2 3-chymotrypsin-like cysteine protease inhibitor exhibits in vivo efficacy in a Syrian Hamster model.bioRxiv2021.02.14.431129.
[64] Mellott D.et al2020 A cysteine protease inhibitor blocks SARS-CoV-2 infection of human and monkey cells.bioRxiv2020.10.23.347534
Biography
Erik De Clercq, MD, PhD, is Emeritus Professor of KU Leuven (Belgium) and has, since 2007 (until present), been teaching the course of ‘Chemistry at the Service of Medicine’ at the Faculty of Sciences at the University of South Bohemia (České Budějovice, Czech Republic) in a joint program with Keppler University (Linz, Austria). He received in 2010 jointly with Dr Anthony S. Fauci the Dr Paul Janssen Award for Biomedical Research. He has (co)discovered a number of antiviral drugs currently used in the treatment of HSV (valaciclovir), VZV (brivudin), CMV (cidofovir), HBV [adefovir dipivoxil, tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF)], and HIV infections (TDF, TAF). The combination of TDF with emtricitabine (Truvada®) has been approved worldwide for the pre-exposure prophylaxis (PrEP) of HIV infections.


