Role of testing for the COVID-19 response in Aboriginal, Torres Strait Islander and regional populations
David SpeersDepartment of Microbiology, Queen Elizabeth II Medical Centre, PathWest Laboratory Medicine WA; Sir Charles Gairdner Hospital, Nedlands, WA, Australia; and School of Medicine, University of Western Australia, Crawley, WA, Australia. Email: David.Speers@health.wa.gov.au
Microbiology Australia 42(1) 23-26 https://doi.org/10.1071/MA21007
Submitted: 15 February 2021 Accepted: 2 March 2021 Published: 12 April 2021
Journal Compilation © The Authors 2021 Open Access CC BY, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Aboriginal and Torres Strait Islander people may be disproportionately affected by COVID-19 and despite isolation remote communities have an increased risk of COVID-19 transmission due to overcrowding and frequent travel over long distances for family, cultural or community events. The response to an outbreak in remote settings will be logistically challenging requiring a coordinated multiagency response. The provision of timely COVID-19 diagnostic testing is central to this response.
Introduction
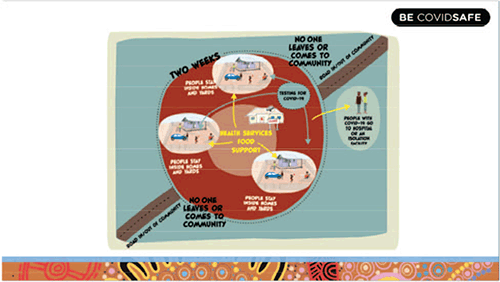
Regional populations have reduced access to timely health investigations and increased turnaround times for pathology tests, a problem compounded in Aboriginal and Torres Strait Islander people by an increased burden of disease and poorer health outcomes1. Community engagement and culturally appropriate messaging (Figure 1) are key components to combat COVID-19 and a flexible COVID-19 response model is required as there is diversity in Aboriginal culture, both between and within remote Aboriginal communities. Nationally, the Australian Health Department and the Aboriginal and Torres Strait Islander Advisory Group on COVID-19 have produced resources for Aboriginal and Torres Strait Islander people3 and country health services have established emergency operations centres to help plan and coordinate the COVID-19 responses. Aboriginal community-controlled health services are best placed to undertake culturally appropriate interactions with remote Aboriginal communities, for example, hygiene promotion to reduce the transmission risk into and within these communities.
|
Testing as part of the COVID-19 response for regional populations
Molecular testing for COVID-19
Many regional populations face delays resulting for laboratory testing compared to metropolitan areas due to longer pre-analytical transport times. COVID-19 molecular testing turnaround times for regional populations can be reduced by improved courier services for faster specimen transfer from the regions to their major centres and the central laboratory can prioritise specimens from rural/remote centres for urgent processing upon arrival into the laboratory. However, courier availability on weekends and public holidays remains problematic.
Alternatively, patient-centric molecular testing can be performed. Several manufacturers have Australian Register of Therapeutic Goods (ARTG) listed molecular detection assays for SARS-CoV-2 suitable for POCT (Abbott ID Now, MiCoBioMed Co Ltd Veri-Q PCR 316 COVID-19 Detection Kit, Roche Molecular Systems Inc. cobas® SARS-CoV-2 & Influenza A/B, Sansure Biotech Inc. Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit, Ustar Biotechnologies EasyNat Diagnostic Kit for Novel-Coronavirus (2019-nCoV) RNA). However, the good clinical performance of the Cepheid Xpert Xpress SARS-CoV-2 assay overseas2, the established regional POCT network of GeneXpert instruments from the Test, Treat and Go (TTANGO) trial for Neisseria gonorrhoeae and Chlamydia trachomatis in remote Aboriginal communities4, and the previous regional laboratory Xpert® Flu/RSV assay roll out for the 2009 influenza pandemic in several jurisdictions made this an attractive option for expansion of regional COVID-19 PCR testing around regional Australia.
Cepheid have now also produced a four-plex Xpert® Xpress SARS-CoV-2/Flu/RSV combination assay that received Emergency Use Authorization from the USA FDA in September 2020, CE-IVD marking from the European Union in November 2020 and was recently listed on the ARTG. This assay has performed well against several other commercial molecular assays5.
Currently, two regional molecular COVID-19 testing programs using the Xpert Xpress SARS-CoV-2 assay are in operation; laboratory-based testing by regional laboratory networks, and a federally funded rural and remote community POCT program initiated by the Kirby Institute in partnership with the Flinders University International Centre for Point of Care Testing6. Both programs require operator training and proficiency assessment programs, instrument service and maintenance programs with standardised control materials. The POCT program must also meet the National Pathology Accreditation Advisory Council Guidelines for Point of Care Testing7. For operator biosafety the laboratory-based testing requires handling of the open specimens in class 1 or class 2 biosafety cabinet whereas the POCT program relies on operator PPE.
The major limitations to these programs have been the limited Cepheid cartridge supply and the low capacity of the GeneXpert instruments (up to four specimens concurrently) making them unsuitable for an urgent testing response to a large outbreak. There is a national Virtual Working Group for Cepheid SARS-CoV-2 test cartridges allocation to ensure equitable distribution of supply around the country and test approval systems have been introduced to ensure the most urgent cases are tested as rapidly as possible without outstripping supply. For large outbreaks, a system using a palm-sized thermal cycler (Figure 2) linked to a small liquid handling device, an extraction system and a laptop computer can be deployed by air with a trained operator to the regional laboratory. The system requires a small bench footprint and access to the laboratory’s biosafety cabinet. In Western Australia, such a system with a rapid throughput of up to 150 specimens per day has been validated and successfully trialled in a regional laboratory during an exercise.

|
Another limitation of all molecular assays is the potential for false positive reactions and the social disruption this can cause in isolated communities. In most parts of the world where SARS-CoV-2 is prevalent it is difficult to distinguish between weak true positives due to residual low-level SARS-CoV-2 RNA following infection and false positives. In Madagascar the Xpert Xpress SARS-CoV-2 assay was assessed against the Detection Kit for 2019 Novel Coronavirus (2019-nCoV) RNA Da An Gene assay and found to be 100% sensitive, but only 80% (16 of 20) specific. The apparent false positives were due to high crossing threshold (>40 cycles) N2 gene results with no amplification of the E gene8. A similar problem with the Xpert Xpress SARS-CoV-2 assay has been found in a small number of cases without COVID-19 exposure risk in Western Australia. Investigation of these cases has shown this false reactivity to be due to cross-reacting DNA in the throat and nasal specimens (unpublished results). This illustrates that performance of diagnostic tests developed and validated overseas may not always show similar performance characteristics when used in remote regions of Australia. If a weak Xpert Xpress SARS-CoV-2 assay N2 gene only result is found without supporting epidemiological information confirmatory testing on a second PCR platform is recommended.
Antigen tests
COVID-19 antigen testing of respiratory tract samples provides results within minutes, is relatively cheap and easy to use with little operator training required, and is suitable for POCT. However, COVID-19 antigen testing is less sensitive than molecular testing and therefore is best placed for use in specific settings where the pre-test probability is high, such as an outbreak in a high risk setting, or where community transmission is established. One such scenario could be an outbreak in an isolated community where antigen testing would provide immediate results for public health action and relieve pressure on the limited availability of local molecular testing. COVID-19 cases are likely to be antigen positive in the first few days of their illness when they are most infectious. Performing antigen testing daily to capture this period of their illness can help negate the reduced sensitivity compared to molecular tests. The need to reflex to molecular testing to confirm antigen positives is dependent on the prevalence of COVID-19 in the population being tested.
Wastewater testing
Testing of wastewater for SARS-CoV-2 RNA fragments has been introduced by several jurisdictions as an additional surveillance tool to clinical testing. This is most often performed on collections from wastewater treatment plants servicing large urban centres, but is now being expanded to regional centres. Targeted testing of communities and mine sites with increased movement of people across borders has the potential for an early detection system for SARS-CoV-2 incursion into a regional community. However, there are logistical challenges to regular wastewater collection from multiple remote locations, so it is unlikely to be available to all regional communities.
Whole genome sequencing
SARS-CoV-2 genomics can be used for cluster analysis to inform and assess the impact of public health interventions and for tracking of variant lineages of concern, as has been recently reported from the United Kingdom (B.1.1.7) and South Africa (B.1.351). SARS-CoV-2 genomics applied to regional cases could help identify transmission chains and sources of introduction into communities with this information used to guide control measures. Also, since most published sequences are from large population centres in overseas countries it is important that strains circulating in more remote regions around the world, including Australia, are identified. Each jurisdiction in Australia has access to SARS-CoV-2 genomics to facilitate the sequencing of all suitable PCR-positive specimens to help build the picture of SARS-CoV-2 transmission in the unique Australian environment.
Serology
COVID-19 serology is most often used for the diagnosis of historical cases, or as an aid for the release from isolation of cases with persistent PCR positivity. Serology also has a role in assessing the true prevalence of COVID-19 after a community outbreak, especially if there was limited access to PCR testing. The Australian COVID-19 Vaccination Policy9 includes Aboriginal and Torres Strait Islander people, as one of the priority population groups. The COVID-19 vaccine Roadmap10 involves a three-phase roll-out strategy with Aboriginal and Torres Strait Islander people vaccinated in Phase 1b (over 55 years) and Phase 2a (18–54 years). With the introduction of the COVID-19 vaccines it is important to assess vaccine responsiveness in Aboriginal and Torres Strait Islander people. Serology may have a role in this but the availability of neutralising antibody tests, which correlates better with immunity, is limited. There may also be a role for the interferon-gamma release assays, such as the QuantiFERON®SARS-CoV-2 and the EUROIMMUN Interferon-gamma ELISA for the measurement of the T-cell responses to COVID-19 vaccines.
Conclusion
Thankfully remote communities have so far been relatively spared from COVID-19, but could be disproportionately affected. Factors that contribute to their vulnerability include limited access to health services and telecommunications, and inadequate infrastructure, including unsuitable housing which can lead to overcrowding. The response to an outbreak in a remote community will be logistically challenging with locally available accurate testing a key component of this response.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Yashadhana, A. et al. (2020) Indigenous Australians at increased risk of COVID-19 due to existing health and socioeconomic inequities. Lancet Regional Health – Western Pacific 1, 100007.| Indigenous Australians at increased risk of COVID-19 due to existing health and socioeconomic inequities.Crossref | GoogleScholarGoogle Scholar |
[2] Loeffelholz, M.J. et al. (2020) Multicenter evaluation of the Cepheid Xpert Xpress SARS-CoV-2 test. J. Clin. Microbiol 58, e00926-20.
| Multicenter evaluation of the Cepheid Xpert Xpress SARS-CoV-2 test.Crossref | GoogleScholarGoogle Scholar | 32843530PubMed |
[3] Australian Government, Department of Health (2021) Coronavirus (COVID-19) advice for Aboriginal and Torres Strait Islander peoples and remote communities. https://www.health.gov.au/news/health-alerts/novel-coronavirus-2019-ncov-health-alert/advice-for-people-at-risk-of-coronavirus-covid-19/coronavirus-covid-19-advice-for-aboriginal-and-torres-strait-islander-peoples-and-remote-communities
[4] Guy, R.J. et al. (2013) A randomised trial of point-of-care tests for chlamydia and gonorrhoea infections in remote Aboriginal communities: Test, Treat ANd GO- the ‘TTANGO’ trial protocol. BMC Infect. Dis. 13, 485.
| A randomised trial of point-of-care tests for chlamydia and gonorrhoea infections in remote Aboriginal communities: Test, Treat ANd GO- the ‘TTANGO’ trial protocol.Crossref | GoogleScholarGoogle Scholar | 24138699PubMed |
[5] Mostafa, H.H. et al. (2020) Multicenter evaluation of the Cepheid Xpert® Xpress SARS-CoV-2/Flu/RSV test. J. Clin. Microbiol. 59, e02955-20.
| Multicenter evaluation of the Cepheid Xpert® Xpress SARS-CoV-2/Flu/RSV test.Crossref | GoogleScholarGoogle Scholar |
[6] Ministers Department of Health (2020) World first rapid COVID-19 testing to protect Aboriginal and Torres Strait Islander communities. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/world-first-rapid-covid-19-testing-to-protect-aboriginal-and-torres-strait-islander-communities
[7] National Pathology Accreditation Advisory Council (NPAAC) (2015) Guidelines for Point of Care Testing (PoCT) First Edition. https://www1.health.gov.au/internet/main/publishing.nsf/Content/health-npaac-poctguid
[8] Rakotosamimanana, N. et al. (2020) GeneXpert for the diagnosis of COVID-19 in LMICs. Lancet Glob. Health 8, e1457–e1458.
| GeneXpert for the diagnosis of COVID-19 in LMICs.Crossref | GoogleScholarGoogle Scholar | 33091372PubMed |
[9] Australian Government (2020) Australian COVID-19 vaccination policy. https://www.health.gov.au/sites/default/files/documents/2020/12/australian-covid-19-vaccination-policy.pdf
[10] Australian Government (2020) Australia’s epidemiology and COVID-19 vaccine roadmap. https://www.health.gov.au/sites/default/files/documents/2021/01/australia-s-epidemiology-and-covid-19-vaccine-roadmap-australia-s-covid-19-vaccine-national-rollout-strategy.pdf
Biography
Dr David Speers is a fellow of the Royal Australasian College of Physicians, the Royal College of Pathologists of Australasia, and the Australasian College of Tropical Medicine. He is Chair of Microbiology at PathWest, Western Australia’s public health laboratory, which has implemented diagnostic SARS-CoV-2 PCR testing, serology, and genomics. He is a member of the Public Health Laboratory Network of Australia and has been involved in several state and national COVID-19 working groups.


