Gonorrhoea: past, present and future
Evgeny A Semchenko A , Xiaofan Chen A , Caroline Thng B , Maree O’Sullivan B and Kate L Seib A CA Institute for Glycomics, Griffith University, Southport, Qld 4215, Australia
B Gold Coast Sexual Health, Southport Community Health Precinct, Southport, Qld 4215, Australia
C Tel: +61 7 555 27453, Email: k.seib@griffith.edu.au
Microbiology Australia 41(4) 205-209 https://doi.org/10.1071/MA20055
Published: 20 October 2020
Abstract
The sexually transmitted infection (STI) gonorrhoea is an ancient human disease caused by the Gram-negative bacterial pathogen Neisseria gonorrhoeae. Despite decades of research focused on preventing, diagnosing, and treating gonorrhoea, it remains a major global health concern due to its high prevalence, high rates of asymptomatic cases, the severe sequelae that can result from untreated infections, and the increasing difficulty in treating infections caused by multi-drug resistant strains of N. gonorrhoeae. It is estimated that there are more than 87 million cases of gonorrhoea worldwide each year, and the WHO, CDC and Australian National Antimicrobial Resistance (AMR) Strategy have prioritised N. gonorrhoeae as an urgent public health threat for which new therapeutics and a vaccine are needed.
Where did it all begin? The long history of gonorrhoea
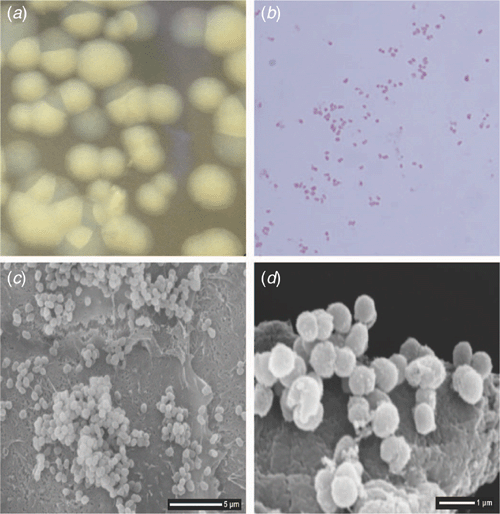
Gonorrhoea is an ancient disease of humans, with symptoms resembling gonorrhoea reportedly described in ancient Chinese and Middle Eastern records dating as far back as 3500 BC1. There is also reference to urethral discharge, believed to be gonorrhoea, in the book of Leviticus in the Old Testament of the Bible (Leviticus 15:1-3). The name gonorrhoea is credited to Greek physician Galen (AD 130-200), which means the flow of semen, derived from the Greek words ’gonos’ (semen) and ’rhoia’ (to flow)1. In the 16th century, as STIs were recognised as being more common in prostitutes, gonorrhoea became known as ‘the clap,’ likely in reference to the old Le Clapiers district of Paris where prostitutes were housed. It was not until 1879, however, that the bacteria responsible for gonorrhoea was identified and named Neisseria gonorrhoeae after Albert Neisser the German microbiologist who first isolated the bacteria (Figure 1). Over time, gonorrhoea has been described extensively in scientific literature, as well as in essays such as ‘Boswell’s Clap’ that describe James Boswell’s nineteen episodes of gonococcal urethritis between 1760–1790 based on his detailed diary accounts2, and news articles describing its antibiotic resistance status, including ‘Man has ’world’s worst’ super-gonorrhoea’ (BBC News, UK, 28 March 2018).
|
Where are we now? Current clinical aspects of gonorrhoea
The WHO estimates that more than 1 million STIs occur every day3. There are an estimated 87 million gonorrhoea infections occurring each year3 and N. gonorrhoeae has been prioritised as an urgent public health threat by the WHO4, CDC5 and Australian National AMR Strategy6. STI surveillance systems vary widely within and across WHO regions, which means that current figures likely underestimate the burden of gonorrhoea due to limitations in diagnosis and reporting. Several systems currently exist in Australia for the surveillance of N. gonorrhoeae infections, including state-level reporting via the Notification of Communicable Diseases, as well as the Australian Gonococcal Surveillance Programme (AGSP) conducted by the National Neisseria Network (NNN). These systems provide incidence, demographic, and antimicrobial resistance data to inform clinical and public health responses to continue towards gonorrhoea control. Rates of gonorrhoea have continued to increase in Australia over the last 10 years, with an 80% increase between 2013 to 20177. Rates of gonorrhoea are particularly high in gay and bisexual men (GBM), Australia’s First Peoples and younger populations (19–29 years) and there has been a recent resurgence of gonorrhoea in urban heterosexuals7. In 2019 there were 34 265 gonococcal infections notified in Australia8.
The gold standard for N. gonorrhoeae diagnosis remains culture due to its high specificity and the ability to perform antibiotic sensitivity tests to guide treatment. However, culture yields are highly dependent on bacterial loads, storage and transport of specimens. This impacts extragenital site sampling where culture rates could be as low as 64% in pharyngeal infections9. In addition, the intimate and invasive nature of obtaining urethral and cervical samples limit its utility for widespread screening and testing programmes. Since 2002 nucleic acid amplification tests (NAATs) have been preferred as the screening tool for N. gonorrhoeae infection. NAATs have demonstrated superior detection rates with 97–99% sensitivity, and outperform culture by up to 2-fold for rectal and 5-fold for pharyngeal gonorrhoea infections10. NAATs have also been pivotal in improving access and uptake of gonorrhoea screening programmes, with a high level of acceptability and effectiveness for detecting gonorrhoea infection11. Multiplex NAATs testing are also being developed, so that samples can be tested for up to nine different pathogens including Chlamydia trachomatis, Trichomonas vaginalis and Mycoplasma genitalium12. Concomitant infection of N. gonorrhoeae with these infections occur in up to 30% of cases. Finally, NAAT tests have the ability to deliver a result in a matter of hours. A study in the UK demonstrated that the rapid turnaround of results would reduce time to diagnosis by more than 8 days leading to potential reduction in transmission13.
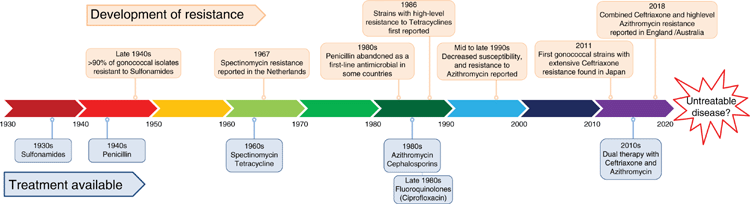
The outcome of N. gonorrhoeae infection varies by site of infection and by sex14. Symptomatic infections predominantly affect the genito-urinary tract with 90% of penile infections presenting with purulent discharge or dysuria, whilst approximately 50% of cervical infections present with changes in vaginal discharge, intermenstrual or post coital bleeding. Complications of N. gonorrhoeae can occur leading to epididymo-orchitis and pelvic inflammatory disease subsequently leading to an increased risk of infertility and ectopic pregnancy. Rarely, haematogenous spread can occur causing skin lesions, arthritis and tenosynovitis (disseminated gonococcal infection). Extragenital N. gonorrhoeae infections also occur leading to pharyngitis, proctitis and uveitis, though asymptomatic pharyngeal and rectal infections are common. Though gonorrhoea remains a curable infection, treatments have changed rapidly over time to overcome emerging drug-resistant N. gonorrhoeae (Figure 2). The future effectiveness of antibiotic treatment has been significantly compromised by the fact that N. gonorrhoeae has developed resistance to all classes of antibiotics used to treat it15. Worldwide, penicillin, ciprofloxacin and cefixime are no longer recommended first-line treatments. Instead dual antibiotic combination of ceftriaxone and azithromycin are preferred, though there is increasing concern of azithromycin resistance. In 2018, ’Super gonorrhoea’ resistant to all routine antibiotics, including the recommended dual therapy of intramuscular ceftriaxone/oral azithromycin, was reported in the UK16 and Australia17.
|
Where to next? Future therapeutic and vaccine development for gonorrhoea
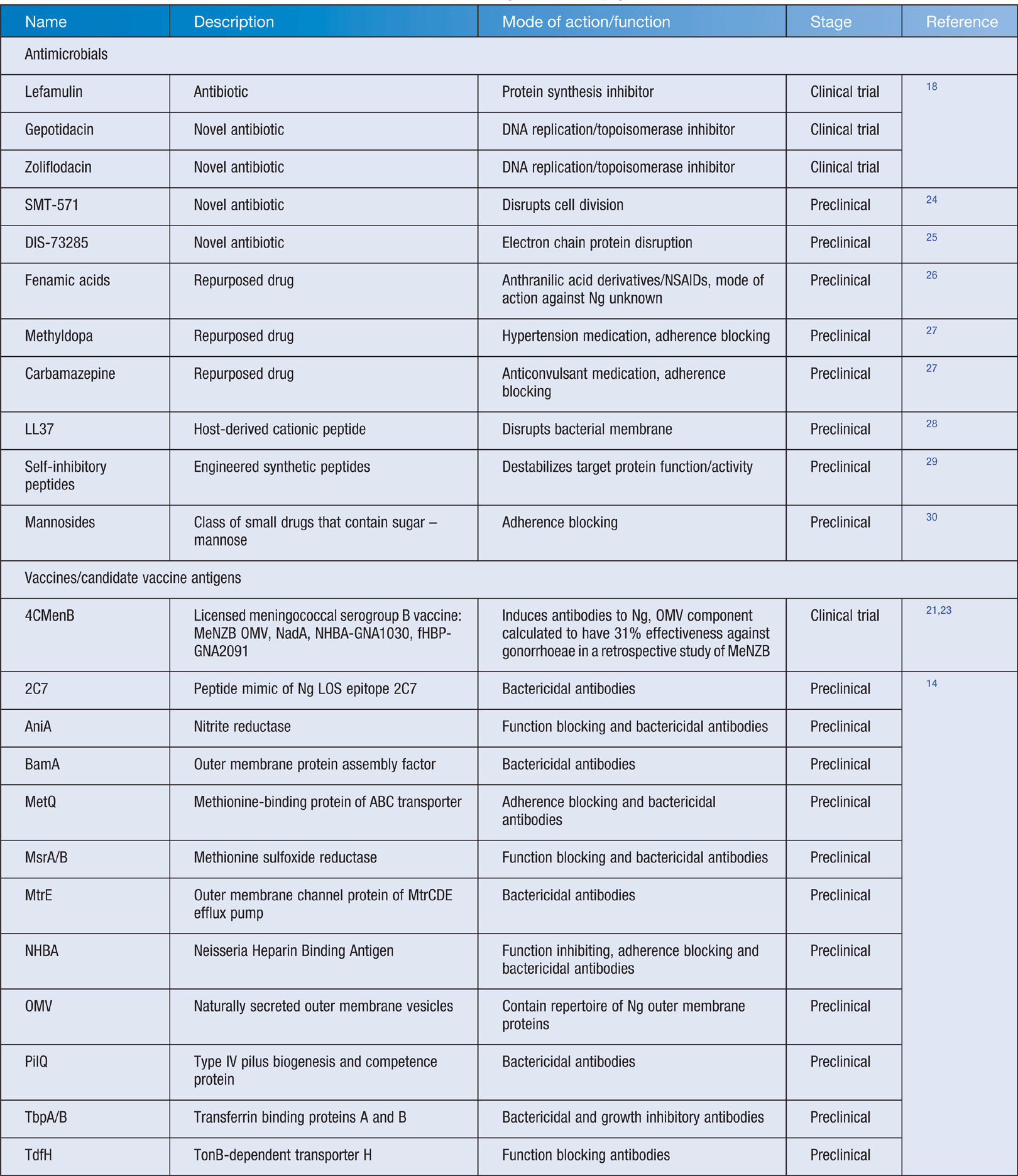
This century, scientists have made significant advances in understanding gonococcal biology, as well as its mechanisms for causing disease and evading the immune system. Most importantly, they have also discovered new approaches to prevent and treat the infection, many of which are in final stages of development. Currently there are three new antibiotics in clinical trials and there are also several other novel drugs or treatment methods in development or preclinical settings (Table 1)18. Novel diagnostics and genotyping technologies are also being developed for rapid detection of mutations to guide antibiotic therapy19.
|
It is widely considered that vaccination will be the best long-term solution to gonorrhoea. However, gonococcal vaccine development is challenging. N. gonorrhoeae infection does not protect against subsequent infection, therefore there are no correlates of protection from natural immunity to guide vaccine development14. Four gonococcal vaccine candidates have been tested in human clinical trials (all pre-2000) but none provided any protection against N. gonorrhoeae infection14. However, several new vaccine antigens are currently in preclinical development (Table 1)14,20. Considerable funding from the US National Institute of Health (NIH) was recently allocated for creation of large collaborative research groups, aiming to deliver a gonococcal vaccine into clinical trials within 5 years.
The feasibility of a gonococcal vaccine was supported by recent findings from a retrospective study that showed decreased N. gonorrhoeae rates following vaccination with an outer membrane vesicle (OMV)-based vaccine (MeNZB) licenced to protect against the closely related bacteria Neisseria meningitidis21. MeNZB was estimated to have a vaccine effectiveness of 31% against N. gonorrhoeae21. Mathematical modelling has indicated that a gonococcal vaccine with 30% efficacy would be expected to halve gonorrhoea prevalence within 20 years22. The MeNZB vaccine was succeeded by a multicomponent meningococcal serogroup B vaccine – 4CMenB (tradename Bexsero), that in addition to the MeNZB OMVs, contains additional recombinant antigens. 4CMenB has been shown to induce cross-reactive antibodies to N. gonorrhoeae in humans23, and is now in clinical trials to investigate its efficacy against gonorrhoea. One of these studies is underway in a population at high risk of contracting N. gonorrhoeae in Australia (MenGO; ANZCTR Identifier: 12619001478101). Two additional efficacy studies will commence shortly in Australia (GoGoVax; ClinicalTrials.gov Identifier: NCT04415424) and United States (NCT04350138), with estimated completions dates in 2023.
Conclusions
The discovery of antibiotics ushered a new age in medicine, allowing treatment of many bacterial infections that plagued mankind, including millennia old gonorrhoea. However, the gonococcus was able to acquire resistance to new antibiotics as quickly as they were developed and we have reached the point where strains resistant to ‘last line of defence’ antibiotics have emerged, prompting urgent action. Currently there are several new antibiotics and vaccine antigens being investigated at all stages of the clinical development pipeline, which will hopefully deliver new treatments and a cure for the clap.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Kollar, L. and Shmaefsky, B.R. (2005) History of gonorrhea and sexually transmitted diseases. In Deadly Diseases and Epidemics: Gonorrhea. (Alcamo, I.E., ed), Chelsea House.[2] Ober, W.B. (1970) Boswell’s clap. JAMA 212, 91–95.
| Boswell’s clap.Crossref | GoogleScholarGoogle Scholar | 4907557PubMed |
[3] Rowley, J. et al. (2019) Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull. World Health Organ. 97, 548–562P.
| Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016.Crossref | GoogleScholarGoogle Scholar | 31384073PubMed |
[4] WHO (2017) Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/
[5] CDC Antibiotic Resistance Threats in the United States (2013 ) http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
[6] Department of Health (2015) Responding to the threat of antimicrobial resistance: Australia’s First National Antimicrobial Resistance Strategy 2015–2019. Department of Health, Australian Government, Canberra.
[7] Kirby Institute (2018) HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2018. The Kirby Institute, UNSW Sydney.
[8] Lahra, M.M. et al. (2020) Australian Gonococcal Surveillance Programme Annual Report, 2019. Commun. Dis. Intell. 44., .
[9] Fairley, C.K. et al. (2011) Is it time to move to nucleic acid amplification tests screening for pharyngeal and rectal gonorrhoea in men who have sex with men to improve gonorrhoea control? Sex. Health 8, 9–11.
| Is it time to move to nucleic acid amplification tests screening for pharyngeal and rectal gonorrhoea in men who have sex with men to improve gonorrhoea control?Crossref | GoogleScholarGoogle Scholar | 21371376PubMed |
[10] Cornelisse, V.J. et al. (2017) Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex. Transm. Dis. 44, 114–117.
| Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing.Crossref | GoogleScholarGoogle Scholar | 27984552PubMed |
[11] Barbee, L.A. et al. (2016) Implementation and operational research: effectiveness and patient acceptability of a sexually transmitted infection self-testing program in an HIV care setting. J. Acquir. Immune Defic. Syndr. 72, e26–e31.
| Implementation and operational research: effectiveness and patient acceptability of a sexually transmitted infection self-testing program in an HIV care setting.Crossref | GoogleScholarGoogle Scholar | 26959189PubMed |
[12] Kriesel, J.D. et al. (2016) Multiplex PCR testing for nine different sexually transmitted infections. Int. J. STD AIDS 27, 1275–1282.
| Multiplex PCR testing for nine different sexually transmitted infections.Crossref | GoogleScholarGoogle Scholar | 26538551PubMed |
[13] Whitlock, G.G. et al. (2018) Rapid testing and treatment for sexually transmitted infections improve patient care and yield public health benefits. Int. J. STD AIDS 29, 474–482.
| 29059032PubMed |
[14] Edwards, J.L. et al. (2016) Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development. Crit. Rev. Microbiol. 42, 928–941.
| Is gonococcal disease preventable? The importance of understanding immunity and pathogenesis in vaccine development.Crossref | GoogleScholarGoogle Scholar | 26805040PubMed |
[15] Unemo, M. and Shafer, W.M. (2014) Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin. Microbiol. Rev. 27, 587–613.
| Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future.Crossref | GoogleScholarGoogle Scholar | 24982323PubMed |
[16] Eyre, D.W. et al. (2018) Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill. 23, 1800323.
| Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018.Crossref | GoogleScholarGoogle Scholar | 29991383PubMed |
[17] Whiley, D.M. et al. (2018) Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect. Dis. 18, 717–718.
| Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin.Crossref | GoogleScholarGoogle Scholar | 29976521PubMed |
[18] Suay-García, B. and Pérez-Gracia, M.T. (2018) Future prospects for Neisseria gonorrhoeae treatment. Antibiotics (Basel) 7, 49.
[19] Pond, M.J. et al. (2016) Accurate detection of Neisseria gonorrhoeae ciprofloxacin susceptibility directly from genital and extragenital clinical samples: towards genotype-guided antimicrobial therapy. J. Antimicrob. Chemother. 71, 897–902.
| Accurate detection of Neisseria gonorrhoeae ciprofloxacin susceptibility directly from genital and extragenital clinical samples: towards genotype-guided antimicrobial therapy.Crossref | GoogleScholarGoogle Scholar | 26817487PubMed |
[20] Gottlieb, S.L. et al. (2020) Gonococcal vaccines: public health value and preferred product characteristics; report of a WHO global stakeholder consultation, January 2019. Vaccine 38, 4362–4373.
| Gonococcal vaccines: public health value and preferred product characteristics; report of a WHO global stakeholder consultation, January 2019.Crossref | GoogleScholarGoogle Scholar | 32359875PubMed |
[21] Petousis-Harris, H. et al. (2017) Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 390, 1603–1610.
| Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study.Crossref | GoogleScholarGoogle Scholar | 28705462PubMed |
[22] Craig, A.P. et al. (2015) The potential impact of vaccination on the prevalence of gonorrhea. Vaccine 33, 4520–4525.
| The potential impact of vaccination on the prevalence of gonorrhea.Crossref | GoogleScholarGoogle Scholar | 26192351PubMed |
[23] Semchenko, E.A. et al. (2019) The serogroup B meningococcal vaccine Bexsero elicits antibodies to Neisseria gonorrhoeae. Clin. Infect. Dis. 69, 1101–1111.
| The serogroup B meningococcal vaccine Bexsero elicits antibodies to Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 30551148PubMed |
[24] Jacobsson, S. et al. (2019) In vitro activity of the novel oral antimicrobial SMT-571, with a new mechanism of action, against MDR and XDR Neisseria gonorrhoeae: future treatment option for gonorrhoea? J. Antimicrob. Chemother. 74, 1591–1594.
| In vitro activity of the novel oral antimicrobial SMT-571, with a new mechanism of action, against MDR and XDR Neisseria gonorrhoeae: future treatment option for gonorrhoea?Crossref | GoogleScholarGoogle Scholar | 30778550PubMed |
[25] Jacobsson, S. et al. (2020) High in vitro activity of DIS-73285, a novel antimicrobial with a new mechanism of action, against MDR and XDR Neisseria gonorrhoeae. J. Antimicrob. Chemother. , .
| High in vitro activity of DIS-73285, a novel antimicrobial with a new mechanism of action, against MDR and XDR Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 32929456PubMed |
[26] Seong, Y.J. et al. (2020) Repurposing fenamic acid drugs to combat multidrug-resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 64, e02206-19.
| Repurposing fenamic acid drugs to combat multidrug-resistant Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 32393483PubMed |
[27] Poole, J. et al. (2020) Repurposed Drugs That Block the gonococcus-complement receptor 3 interaction can prevent and cure gonococcal infection of primary human cervical epithelial cells. MBio 11, e03046-19.
| Repurposed Drugs That Block the gonococcus-complement receptor 3 interaction can prevent and cure gonococcal infection of primary human cervical epithelial cells.Crossref | GoogleScholarGoogle Scholar | 32127453PubMed |
[28] John, C.M. et al. (2019) Cationic cell-penetrating peptide is bactericidal against Neisseria gonorrhoeae. J. Antimicrob. Chemother. 74, 3245–3251.
| Cationic cell-penetrating peptide is bactericidal against Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 31424547PubMed |
[29] Zhan, J. et al. (2019) Self-derived structure-disrupting peptides targeting methionine aminopeptidase in pathogenic bacteria: a new strategy to generate antimicrobial peptides. FASEB J. 33, 2095–2104.
| Self-derived structure-disrupting peptides targeting methionine aminopeptidase in pathogenic bacteria: a new strategy to generate antimicrobial peptides.Crossref | GoogleScholarGoogle Scholar | 30260702PubMed |
[30] Semchenko, E.A. et al. (2019) Glycointeractome of Neisseria gonorrhoeae: identification of host glycans targeted by the gonococcus to facilitate adherence to cervical and urethral epithelial cells. MBio 10, e01339-19.
| Glycointeractome of Neisseria gonorrhoeae: identification of host glycans targeted by the gonococcus to facilitate adherence to cervical and urethral epithelial cells.Crossref | GoogleScholarGoogle Scholar | 31289181PubMed |
Biographies
Evgeny Semchenko is a Postdoctoral Researcher at the Institute for Glycomics at Griffith University. He is a microbiologist and an expert in the field of pathogenic Neisseria, working on investigating interactions between bacteria and host glycans, identifying and characterising bacterial glycan-binding proteins and applying this knowledge to the development of new vaccines and treatment options.
Xiaofan Chen is a student at the Institute for Glycomics at Griffith University. She just finished her honours degree and continues to focus on pathogenic Neisseria during her PhD, investigating new ways to treat gonorrhoea.
Caroline Thng is a Sexual Health and HIV physician at Gold Coast Sexual Health. She has worked as a specialist in both the UK and Australia, and is an expert in complex STI and HIV management. She has delivered clinical trials, observational cohort studies, social research, and epidemiological research both locally and in collaborative projects. She is the co-PI in the first Australian trial using Meningococcal B vaccine as a potential prevention strategy against gonorrhoea infection.
Maree O’Sullivan is the Clinical Director at the Gold Coast Sexual Health Service. She has clinical trials experience over 20 years, including multinational drug trials predominately in the HIV and vaccine sectors. She has also undertaken self -initiated, and collaborative, qualitative and quantitative clinical research in HIV and Chlamydia.
Kate Seib is a Research Leader and the Associate Director (Research) at the Institute for Glycomics at Griffith University. Her expertise is in the field of molecular microbiology, with a focus on understanding virulence mechanisms and characterising vaccine antigens of human mucosal pathogens (e.g. Neisseria gonorrhoeae, Neisseria meningitidis, Moraxella catarrhalis, Haemophilus influenzae).


