Bacteriophage therapy for severe infections
Carola Venturini A B D , Aleksandra Petrovic Fabjian A and Ruby CY Lin A B CA Centre for Infectious Diseases and Microbiology, The Westmead Institute for Medical Research, Level 4, 176 Hawkesbury Road, Westmead, NSW 2145, Australia
B Sydney Medical School, Faculty of Medicine and Health, The University of Sydney, NSW 2000, Australia
C School of Medical Sciences, University of New South Wales, NSW 2052, Australia
D Tel: +61 2 8627 3415, Fax: +61 2 8627 3099, Email: carola.venturini@sydney.edu.au
Microbiology Australia 40(1) 20-23 https://doi.org/10.1071/MA19005
Published: 18 February 2019
The rise of multiple antibiotic resistance in clinically relevant bacteria has created a global crisis with increasing burden on healthcare systems. The need to optimise alternative therapies to antibiotics, particularly in high risk nosocomial settings, is therefore immediate. Bacteriophages are specialised lethal viruses of bacteria, and an underused clinical resource for the treatment of severe infections refractory to antibiotics. Both the gaps in knowledge of bacteriophage biology, particularly the details of host-pathogen dynamic interactions, and legislative hurdles related to the regulation of natural microorganisms for therapy have delayed progress in bacteriophage clinical applications. At the Westmead Institute for Medical Research (WIMR), in collaboration with Westmead Hospital (Western Sydney Local Health District, WSLHD) and the University of Sydney (USyd), we have been investigating rational design protocols for routine bacteriophage application in clinical practice and testing bacteriophage therapeutics on patients suffering from multidrug resistant (MDR) severe infections.
Bacteriophage therapy
Brief introduction
Bacteriophage (phage) therapy exploits the natural predator-prey interaction between phages and their exclusive targets, bacteria, and involves the use of purified mixes of multiple viruses (cocktails) to directly administer to patients. Only lytic phages, which replicate exponentially inside bacteria immediately after infection, are considered appropriate for therapy due to their reduced transduction potential1,2. To date most of the characterised natural phages (95%) are double-stranded DNA, tailed viruses belonging to the order Caudovirales, which are readily isolated from most environmental sources (soil, water, animal faeces, etc.)3. Their highly effective lytic activity is based on two main mechanisms: specific recognition of complementary receptors on the host cell surface, and bacterial cell lysis at the end of virion (phage progeny) replication leading to selective pathogen eradication4. Due to this unique interaction between bacterial receptors and phage anti-receptor structures, most phages have a narrow host range that can be considered advantageous for the development of targeted therapy and for the lack of collateral damage to the resident human microflora4,5, with much of the renewed commercial interest in phage applications centering around this target specificity.
The discovery of phages dates back to more than a century ago and is ascribed to both an English physician (F. Twort) and a French-Canadian microbiologist (F. d’Herelle) who independently observed and reported the lysis phenomenon caused by bacteriophage activity1,2,6. However, it was d’Herelle alone who, as early as 1919, pioneered the successful clinical application of phages to treat infections in humans2,6. Yet mixed clinical outcomes along with the discovery of broad-spectrum antibiotics in the early 1930s meant that phage therapeutic application all but ceased in Western medicine1,7. Conversely, phage research and applications continued unabated in the former USSR, particularly in two main research centres: the Eliava Institute of Bacteriophage, Microbiology, and Virology of the Georgian Academy of Sciences (Tbilisi, Georgia) and the Hirszfeld Institute of Immunology and Experimental Therapy of the Polish Academy of Sciences (Wrocław, Poland), where phages have been continuously used in preclinical and clinical treatment of common infections since the first half of the 20th century1,2,8. However, much of the accumulated experience in these countries has been anecdotal with insufficient (qualitative rather than quantitative) or inaccessible clinical records.
Regulatory framework
Phages are natural organisms, arguably the most abundant life-form on Earth9. They have evolved closely and dynamically with their bacterial host and are therefore specific and effective in selectively eliminating their target3. They have low environmental impact and have shown to have no serious side effects on bystander microorganisms2,5,7. They are self-replicating in the presence of their target, facilitating dosing regimens1,2,7, and have been successfully employed to treat even MDR infections2,7. So, why isn’t bacteriophage therapy routinely employed in the clinic yet? There are in fact a number of unresolved issues, including biology-related knowledge gaps in resistance development, transduction potential, immunogenicity, host range mechanisms, and penetrance, as well as regulatory hurdles associated with the lack of both robust scientific protocols able to withstand the scrutiny of Western regulatory agencies [e.g. Therapeutic Goods Administration (TGA, Australia), Food and Drugs Administration (FDA, US)], and of appropriate legislation for the commercialisation and use of natural organisms as therapeutics1,10. How phage therapy can be best integrated into established clinical models of drug development, pharmacokinetics and pharmacogenomics, and associated regulatory schemes remains a challenge11,12.
The use of phages and phage-based enzymes in the EU and US is currently permitted through experimental therapy only and subject to Article 37 of the Helsinki Declaration13–15. Only recently (2006) the FDA has recognised the designation of phages as ‘generally regarded as safe’, allowing for the use of phage in clinical practice and opening the road towards the implementation of bona fide clinical trials. Both the TGA and FDA define Good Manufacturing Practice (GMP)-produced phage cocktails as investigational drugs, subject to laws and regulations for this category set by each agency. In the EU, Belgium has been at forefront of progress in the regulation of phage therapy for routine clinical practice by implementation of a ‘magistral phage medicine strategy’ with magistral (Article 3, Directive 2001/83 and Article 6 quater, § 3 of the Law of 25 March 1964) phage products approved for personalised patient therapy16.
A number of phage therapy phase I and II initial (small sample sizes) trials has been conducted in recent times10,17. Although phage-based products have received FDA licensure for food safety applications, no licensed phage product prepared under GMP for infection treatment has yet reached the Western market11. The current practice for stable (prolonged shelf-life) and safe (LPS-purified GMP produced) phage cocktail preparation for therapy requires the collaboration of commercial entities and research labs2. A decade ago only a handful of companies specialised in bacteriophage products7,12. Currently bacteriophage research and development is experiencing a veritable renaissance with several new commercial enterprises established worldwide18.
Working with bacteriophage
Bacteriophages (Pyophage #051007, Eliava Institute, Georgia) were successfully trialled at Westmead Hospital more than 10 years ago under ‘compassionate use’ guidelines (TGA) on a patient suffering from a refractory Pseudomonas aeruginosa urinary tract infection19. Following this, a series of projects were aimed at both optimising the rational design of phage cocktail preparation protocols, and implementing phage therapy in critical care settings, through national and international, research and industry collaborations. A study in Adelaide showed that self-administered phage-based nasal washings (AB-SA01, AmpliPhi Biosciences Corporation) were a safe and likely effective treatment for chronic staphylococcal sinusitis20 and, in late 2018, we reported the first intravenous use of the same product for severe sepsis control in Westmead21.
Research
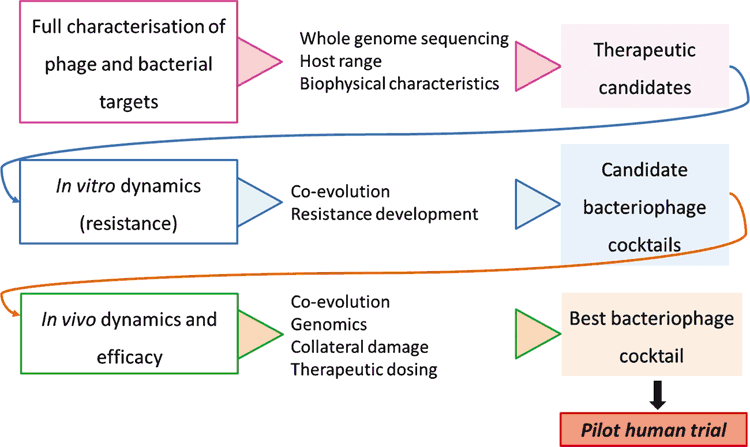
Considering the many areas of phage biology that require better understanding, a rational design approach is critical for the optimisation of phage cocktail preparations for effective and long-lasting therapy2. Therefore, in conjunction with clinical efforts, we are seeking to establish a rationalised phage cocktail preparation protocol (Fig. 1) applicable first, as proof-of-concept, to the eradication of highly virulent MDR clones (e.g. ST131 Escherichia coli and CG258 Klebsiella pneumoniae; NHMRC 1107322). Access to a large well curated collection of clinical isolates has allowed for the selection and full characterisation of exhaustive target bacterial populations, while phages were sourced both from an existing library (available through research collaboration with AmpliPhi Biosciences Corp.) and de novo isolation from environmental reservoirs.
|
Host range testing, matched with detailed genomic analyses of both viruses and bacteria, reveals the unique specificity of phage candidates towards the chosen targets, allowing for careful selection of optimal therapeutic cocktails. In vivo work including, but not limited to, murine models (e.g. for ST131 Escherichia coli and CG258 Klebsiella pneumoniae gut colonisation and severe bacteraemia models; NHMRC 1107322), must also be performed in order to define in vivo dynamics, resistance development potential and evolution trajectories for each bacterial population/best-specific-cocktail combination. In an effort to streamline this process towards therapy design for multiple sepsis-causing nosocomial pathogens, high-throughput susceptibility assays and host range manipulation strategies are also an essential requirement.
Clinical experience
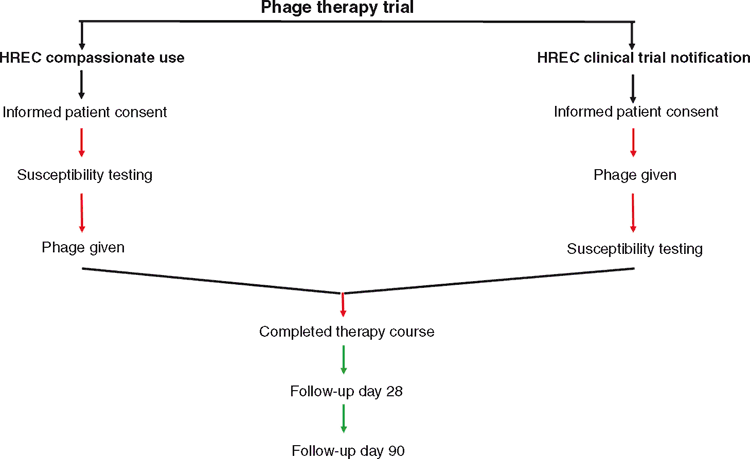
In 2018, the AB-SA01 GMP phage preparation (AmpliPhi Biosciences Corp.) was used in the treatment of severe Staphylococcus aureus infection in humans at Westmead (Sydney, NSW). The Westmead experience with intravenous administration of adjuvant bacteriophage to critically ill patients being treated for severe staphylococcal disease, including prosthetic valve endocarditis, was reported at the Infectious Diseases Society of America (IDSA) annual scientific meeting in late 201821. In Australia, S. aureus infections cause ~20–25% of lethal septic shock, and at Westmead Hospital >100 unique sterile-site isolates are identified each year. In our single site investigator-initiated study, participants were recruited under HREC (Human Research Ethics Committee, WSLHD and WIMR) approval. The phage cocktail used in this work is currently available under the US FDA’s Expanded Access regulations (http://clinicaltrials.gov)22. Critically ill patients with severe S. aureus infection were enlisted for the study under the TGA Special Access Scheme (18 May 2017 onwards) and subsequently under the TGA Clinical Trial Notification (CTN) scheme (from 6 July 2018). It is here noteworthy that the HREC allowed for CTN with ab initio bacteriophage administration after review of interim safety data from the first set of recruited patients (Fig. 2). The devised protocol prescribed treatment with phage in conjunction with standard antibiotic therapy and 90-day follow-up to define microbial kinetics as well as clinical outcomes. Treatment was reported to be associated with reduction in bacterial burden and with no adverse events21.
It is expected that the recent clinical experiences in Australia and overseas will pave the way for Phase II and III controlled randomised trials for this and other phage products at various sites. Future carefully designed controlled studies are expected to commence in 2019.
Finally, at Westmead we are working toward the development of bacteriophage bio-banking and linked patient sample collections as a state-wide resource available for all pathogen researchers in NSW, with the aim of implementing sustainable national and international networks. We are active supporters of the newly established ASM Bacteriophage Biology and Therapeutics Special Interest Group (SIG) (https://bacteriophagesig.blogspot.com/), aiming to promote bacteriophage research in Australia and to connect phage researchers and any others who have an interest in this field.
Conflicts of interest
The authors declare no conflicts of interest. CV, APF and RCYL are not employees of AmpliPhi BioSciences and do not own any shares.
Acknowledgements
The work performed at Westmead was funded by the NHMRC (J Iredell and C Venturini GNT1107322, research component), and AmpliPhi Biosciences partially contributed to the funding for the clinical trial. We acknowledge Prof J Iredell as the principal lead in all the bacteriophage studies at Westmead, as well as the teams at AmpliPhi Biosciences Corp. and WSLHD for their collaboration and support.
References
[1] Kutter, E. and Sulakvelidze, A. (2005) Bacteriophage therapy in humans. In Bacteriophages: biology and applications (Kutter, E. and Sulakvelidze, A., eds). pp. 372–427. CRC Press.[2] Chan, B.K. et al. (2013) Phage cocktails and the future of phage therapy. Future Microbiol. 8, 769–783.
| Phage cocktails and the future of phage therapy.Crossref | GoogleScholarGoogle Scholar | 23701332PubMed |
[3] Fokine, A. and Rossmann, M.G. (2014) Molecular architecture of tailed double-stranded DNA phages. Bacteriophage 4, e28281.
| Molecular architecture of tailed double-stranded DNA phages.Crossref | GoogleScholarGoogle Scholar | 24616838PubMed |
[4] Nobrega, F.L. et al. (2018) Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 16, 760–773.
| Targeting mechanisms of tailed bacteriophages.Crossref | GoogleScholarGoogle Scholar | 30104690PubMed |
[5] Sulakvelidze, A. et al. (2001) Bacteriophage therapy. Antimicrob. Agents Chemother. 45, 649–659.
| Bacteriophage therapy.Crossref | GoogleScholarGoogle Scholar | 11181338PubMed |
[6] Summers, W.C. (2012) The strange history of phage therapy. Bacteriophage 2, 130–133.
| The strange history of phage therapy.Crossref | GoogleScholarGoogle Scholar | 23050223PubMed |
[7] Pirnay, J.-P. et al. (2012) Introducing yesterday’s phage therapy in today’s medicine. Future Virol. 7, 379–390.
| Introducing yesterday’s phage therapy in today’s medicine.Crossref | GoogleScholarGoogle Scholar |
[8] Lin, D.M. et al. (2017) Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 8, 162–173.
| Phage therapy: an alternative to antibiotics in the age of multi-drug resistance.Crossref | GoogleScholarGoogle Scholar | 28828194PubMed |
[9] Kutter, E. and Sulakvelidze, A. eds (2005) Bacteriophages: biology and applications. CRC Press.
[10] Furfaro, L.L. et al. (2018) Bacteriophage therapy: clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 8, 376–382.
| Bacteriophage therapy: clinical trials and regulatory hurdles.Crossref | GoogleScholarGoogle Scholar | 30406049PubMed |
[11] Henein, A. (2013) What are the limitations on the wider therapeutic use of phage? Bacteriophage 3, e24872.
| What are the limitations on the wider therapeutic use of phage?Crossref | GoogleScholarGoogle Scholar | 24228220PubMed |
[12] Abedon, S.T. et al. (2017) Editorial: phage therapy: past, present and future. Front. Microbiol. 8, 981–987.
| Editorial: phage therapy: past, present and future.Crossref | GoogleScholarGoogle Scholar | 28663740PubMed |
[13] World Medical Association (2013) World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194.
| World medical association declaration of Helsinki: ethical principles for medical research involving human subjects.Crossref | GoogleScholarGoogle Scholar | 24141714PubMed |
[14] Debarbieux, L. et al. (2016) A bacteriophage journey at the European Medicines Agency. FEMS Microbiol. Lett. 363, fnv225.
| A bacteriophage journey at the European Medicines Agency.Crossref | GoogleScholarGoogle Scholar | 26656541PubMed |
[15] Maciejewska, B. et al. (2018) Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: an ambitious and also a realistic application? Appl. Microbiol. Biotechnol. 102, 2563–2581.
| Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: an ambitious and also a realistic application?Crossref | GoogleScholarGoogle Scholar | 29442169PubMed |
[16] Pirnay, J.P. et al. (2018) The magistral phage. Viruses 10, 64.
| The magistral phage.Crossref | GoogleScholarGoogle Scholar |
[17] Jault, P. et al. (2019) Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 19, 35–45.
| Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial.Crossref | GoogleScholarGoogle Scholar | 30292481PubMed |
[18] Abedon, S.T. Phage companies. http://www.companies.phage.org/ (accessed 28 December 2018).
[19] Khawaldeh, A. et al. (2011) Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J. Med. Microbiol. 60, 1697–1700.
| Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection.Crossref | GoogleScholarGoogle Scholar | 21737541PubMed |
[20] Drilling, A.J. et al. (2017) Long-term safety of topical bacteriophage application to the frontal sinus region. Front. Cell. Infect. Microbiol. 7, 49–56.
| Long-term safety of topical bacteriophage application to the frontal sinus region.Crossref | GoogleScholarGoogle Scholar | 28286740PubMed |
[21] Aslam, S. et al. (2018) 1642. Safety and efficacy of bacteriophage therapy: analysis of clinical case series data. Open Forum Infect. Dis. 5, S47.
| 1642. Safety and efficacy of bacteriophage therapy: analysis of clinical case series data.Crossref | GoogleScholarGoogle Scholar |
[22] Speck, P.G. and Wormald, P.J. ( ) Is phage therapy suitable for treating chronic sinusitis Staphylococcus aureus infection? Future Microbiol. 13, 605–608.
| Is phage therapy suitable for treating chronic sinusitis Staphylococcus aureus infection?Crossref | GoogleScholarGoogle Scholar |
Biographies
Dr Carola Venturini is a research microbiologist whose work has primarily focused on the role of mobile genetic elements in the evolution of infectious bacteria. Her work has a multidisciplinary approach that combines traditional microbiology molecular methods with bioinformatics (genomics). Since 2013, Carola has been part of Prof Jon Iredell’s Bacterial Pathogenesis research group at The Westmead Institute for Medical Research (Sydney, NSW) leading as project manager applied research investigating the ecology of the gut microbiome related to mechanisms of antibiotic resistance in the Enterobacteriacae and exploring the use of bacteriophage in combating infective multidrug resistant bacterial clones of clinical relevance. Carola is the NSW-representative executive committee member of the ASM Bacteriophage Biology SIG.
Dr Aleksandra Petrovic Fabijan obtained her Bachelor, Master and PhD in Biology at University of Novi Sad (Serbia). During her Master Thesis she studied antibiotic resistance of E. coli isolated from digestive tracts of wild birds. She completed her PhD in Microbiology in 2016 investigating Bordetella bronchiseptica specific bacteriophages and their antimicrobial potential. During her PhD studies she also investigated alternative antimicrobial agents against multi-resistant Acinetobacter baumannii and Helicobacter pylori strains. Aleksandra recently joined Iredell’s team as a Phage Biologist and her work is focused on bacteria-bacteriophage interaction and host response in septic patients receiving adjuvant bacteriophage therapy.
A/Prof Ruby Lin joined the Iredell lab at The Westmead Institute for Medical Research at the end of October 2017 as project manager, after a short stint in industry. She is the scientific lead for an investigator-led clinical trial involving treatment of severe staphylococcal infections using bacteriophage therapy. Her research focus has been microRNA driven dysfunctions in eukaryotic disease model systems including mouse/rat models and humans. She was named NHMRC Peter Doherty fellow (2005–8) and UNSW Global postdoctoral fellow (2009–14). She is a conjoint Associate Professor at the UNSW. During her presidency at Australasian Genomic Technologies Association (AGTA), a prominent society in genomics in Australia and NZ with members from industry and academia, she implemented gender equality at its annual meetings. She is heavily involved in promoting gender balance and women in STEM through various professional networks.