A concise overview on tick-borne human infections in Europe: a focus on Lyme borreliosis and tick-borne Rickettsia spp.
Rita Abou Abdallah A , Didier Raoult B and Pierre-Edouard Fournier A CA UMR VITROME, Aix-Marseille University, IRD, AP-HM, SSA, IHU Méditerranée-Infection, Marseille, France
B UMR MEPHI, Aix-Marseille University IRD, APHM, IHU Méditerranée-Infection, Marseille, France
C Aix-Marseille University, IRD, AP-HM, SSA, VITROME, Institut Hospitalo-Universitaire Méditerranée Infection, 19-21 Boulevard Jean Moulin 13385 Marseille Cedex 05, France. Tel: +33 (0) 4 13 73 24 01, Fax: +33 (0) 4 13 73 24 02, Email: pierre-edouard.fournier@univ-amu.fr
Microbiology Australia 39(4) 207-211 https://doi.org/10.1071/MA18065
Published: 2 November 2018
Ticks are blood-feeding external parasites of mammals. Almost all ticks belong to one of two major families, the Ixodidae or hard ticks, and the Argasidae or soft ticks. Ticks are responsible of transmitting many diseases called ‘tick-borne diseases’. Borrelia and Rickettsia spp., are the most important tick-transmitted bacterial pathogens circulating in Europe. In this review we will focus on the two tick-borne diseases caused by these bacterial pathogens, their vector, epidemiology, clinical diagnosis and symptoms.
Ticks are blood-feeding external parasites of mammals, birds and reptiles throughout the world. They belong to the order Ixodida. Almost all ticks belong to one of two major families, the Ixodidae or hard ticks, which are difficult to crush, and the Argasidae or soft ticks. The Ixodidae contain over 700 species of hard ticks with a scutum or hard shield, which is missing in the Argasidae family. The Argasidae contain about 200 species1; Table 1 shows the most important tick-borne diseases and their vectors.

|
Tick-borne diseases have been described throughout human history. A papyrus scroll dating back to the 16th century B.C. referred to what could be ‘tick fever’2. At the end of the nineteenth century, Theobald Smith and Frederick Kilbourne were the first to demonstrate that ticks were responsible for transmitting diseases. Their experiment in cattle allowed them to conclude that the absence of ticks lead to the absence of Texas fever3. In this review we will focus on Borrelia and Rickettsia spp., the most important tick-transmitted bacterial pathogens circulating in Europe.
Lyme borreliosis (LB)
Causative agent, reservoir and vector
LB is an infectious disease caused by the extracellular bacterium Borrelia burgdorferi sensu lato (sl). This microorganism was determined to be the causative agent of LB by W. Burdogorfer in the early 1980s4. It is a spirochaete belonging to the order Spirochaetales, helically shaped, motile, 20–30 µm in length and 0.2–0.3 µm in width4. Three human pathogens can be distinguished within B. burgdorferi sl: B. burgdorferi sensu stricto (ss), Borrelia afzelii and Borrelia garinii. All of them are present in Europe5.
Regarding the vector, Ixodes ticks transmit all species belonging to B. burgdorferi sl6. Eighty per cent of tick bites transmitting LB are caused by nymphal ticks. The most important reservoirs of B. burgdorferi sl in Europe are rodents, insectivores, hares and several bird species7. Birds are more likely to carry B. garinii, so this microorganism may be carried over very long distances, especially in the case of migratory sea birds8.
Epidemiology
LB is one of the most prevalent vector-borne diseases in Europe9. However, precise epidemiological data are not available for all European countries because the disease is notifiable in only a few countries10,11. The highest average incidence rates among the reporting countries were found in Belarus, Belgium, Croatia, Norway, the Russian Federation and Serbia (<5/100 000), Bulgaria, Finland, Hungary, Poland and Slovakia (<16/100 000), the Czech Republic, Estonia, and Lithuania (<36/100 000) and Slovenia (<130/100 000) (ecdc.europa.eu).
There are clear differences in LB incidence rates and clinical presentations across Europe12. Incidence rates in European countries vary from less than less than one per 100 000 inhabitants to about 350 per 100 000, with a mean annual number of notified cases in Europe exceeding 65 40013. Furthermore, the incidence rates of LB across Europe are influenced by geographical, environmental and climatic factors14,15. Studies on the future potential distribution of I. ricinus notably showed that this tick may emerge in European areas in which they are currently lacking, thus leading to an increased risk to human health16.
Clinical symptoms and diagnosis
During LB, symptoms may vary depending on the stage of the disease. Early LB may be divided into early localised (1–4 weeks after the tick-bite) and early disseminated (3–10 weeks after the tick-bite) diseases, while late disseminated LB develops months to years later. In early LB, various clinical manifestations may be identified, notably the pathognomonic erythema migrans.
Erythema migrans (EM) is the most specific and frequent finding in patients with LB. European studies showed that it is present in 40 to 77% of LB patients17. Primary erythema migrans is a round or oval, expanding erythematous skin lesion that develops at the site of the infecting tick-bite18. Three to 30 days after the tick bite the skin lesion becomes apparent (most commonly 7–14 days). It is not associated with significant pruritis19. If the skin lesion disappears within a few days, it is not considered as an EM. Other secondary ring-shaped lesions may develop in certain cases and are named multiple EM. Less common LB symptoms are cited in Table 2.
|
The diagnosis of LB is difficult due to the unspecific nature of the majority of clinical symptoms, and makes laboratory support crucial. The culture of Borrelia spp. is time consuming and labor intensive, and is thus not used for the routine diagnosis20. In addition, in Europe, the microbiological diagnosis of LB must consider the heterogeneity of the agents depending on countries.
Serology is usually the routine method used to support clinical diagnosis19,20. However, serology suffers limitations. In particular, the antibody response in early LB may be weak or absent, especially in EM and early LNB21. Western blotting is used currently as a confirmatory assay in the serodiagnosis of LB, but is usually only employed following a positive screening assay. In addition, many biomolecular tests were developed in order to supplement western blot such as sensitive and specific Lyme Multiplex PCR-dot blot assay (LM-PCR assay) and nested polymerase chain reaction (nPCR)21 applicable to blood and urine samples. However, PCR-based methods are not standardised.
Rickettsioses
Causative agents and vectors
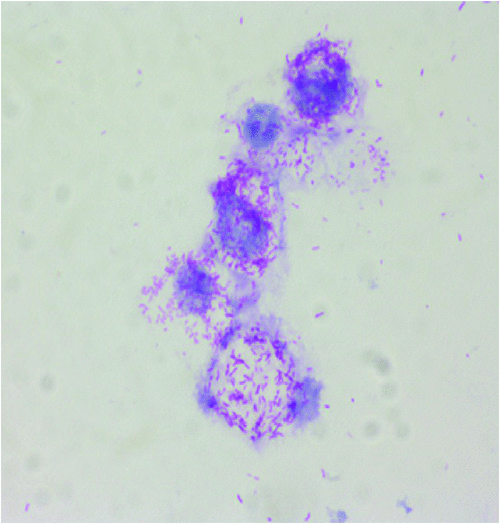
Rickettsioses are worldwide zoonosis caused by obligate intracellular bacteria from the genera Rickettsia and Orientia. These bacteria belong to the alpha-Proteobacteria (Figure 1) and are transmitted by arthropods, mainly ticks, but also fleas, lice and mites22. These zoonoses are among the oldest known vector-borne diseases. In Europe, only Rickettsia spp. are etiological agents of rickettsioses23. Tick-borne rickettsioses (TBR) are the main rickettsial infections in Europe and will be developed in the next section.
|
Epidemiology
The prevalence of tick-borne rickettsioses depends on several parameters and most of them are directly related to the tick vectors: (i) the abundance of the tick itself, which is influenced by many factors, including climatic and ecological conditions; (ii) their affinity for humans; and (iii) the prevalence of rickettsia-infected ticks. In Europe, several Rickettsia species are responsible for tick-borne rickettsioses24. Rickettsia conorii, the main etiological agent of Mediterranean spotted fever (MSF), is the most important in terms of numbers of cases24. Its distribution is restricted to the Mediterranean area where it occurs in Spring and Summer. Other pathogenic Rickettsia species in Europe include R. aeschlimannii, R. helvetica, R. hoogstraalii, R. massiliae, R. monacensis, R. raoultii, R. sibirica mongolitimonae, and R. slovaca (Table 1, Figure 1).
Clinical symptoms and diagnosis
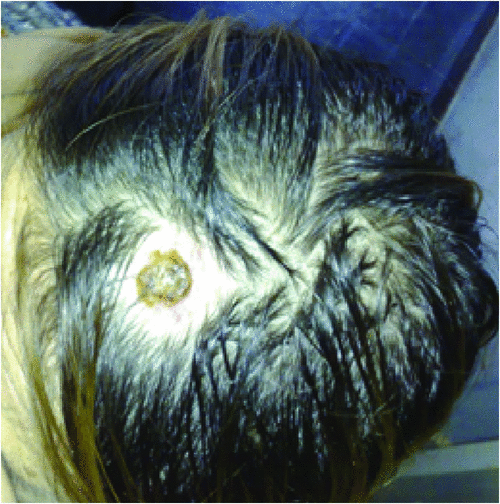
Typically, the clinical symptoms of tick-borne rickettsioses develop 6 to10 days after a tick-bite. Human rickettsioses are characterised by various combinations of symptoms, the most common being a triad consisting of a generalised maculopapular rash, an eschar at the inoculation site (Figure 2) and flu-like symptoms including high grade fever, myalgia, malaise, headache and nausea25. However, depending on species and the underlying patient’s status, these major clinical signs vary greatly.
|
The diagnosis of rickettsial infections usually relies on epidemio-clinical data, and may be confirmed by laboratory testing6. In addition, the arthropods collected at the bite site or eschar may be useful for the diagnosis.
Serology is the most commonly used and available method worldwide due to its quick turnaround time, need for minimal sample preparation and to the serological cross-reactions observed among Rickettsia species that enable limiting the number of tested antigens6. Molecular testing is also well adapted for the diagnosis of tick-borne rickettsioses26. Molecular techniques overcome the drawback of seroconversion time, needed with serological testing27. PCR (either real-time or conventional) can be performed on whole blood, buffy coat or eschar material (crust, swabs, or biopsies)6,28.
Rickettsial culture is also time consuming and should be performed in a BSL3 laboratory29. It is thus reserved to highly specialised laboratories. Matrix-assisted laser desorption/ionisation-time of flight Mass Spectrometry (MALDI-TOF MS) is a promising technique enabling both tick speciation and determining infection with Rickettsiaceae30.
Conclusion
In Europe, the number of vector-borne disease is increasing in some regions. Ticks are notably expanding their range with climate changes. In addition, increased human travel and animal transport result in the epidemiology of tick-borne disease to be in a continuous dynamic change, thus leading to the emergence and/or spread of numerous tick-borne pathogens in Europe. Preventive measures that minimise tick-bite risk are one of the best ways to avoid contracting these diseases. Standardised diagnostic tools are crucial for treating and combating vector-borne diseases, especially when clinical symptoms are not specific. Finally, an increased interest should be given to tick-borne disease to avoid the small bite causing a big problem.
References
[1] Guglielmone, A.A. et al. (2010) The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa 2528, 1–28.[2] Krantz, G.W. and Walter, D.E. (eds) (2009) A Manual of Acarology. Third edition. Texas Tech University Press, Lubbock, Texas.
[3] Assadian, O. and Stanek, G. (2002) Theobald Smith-the discoverer of ticks as vectors of disease. Wien. Klin. Wochenschr. 114, 479–481.
[4] Burgdorfer, W. et al. (1982) Lyme disease-a tick-borne spirochetosis? Science 216, 1317–1319.
| Lyme disease-a tick-borne spirochetosis?Crossref | GoogleScholarGoogle Scholar |
[5] Baranton, G. et al. (1992) Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and Group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42, 378–383.
[6] Brouqui, P. et al. (2004) Guidelines for the diagnosis of tick‐borne bacterial diseases in Europe. Clin. Microbiol. Infect. 10, 1108–1132.
| Guidelines for the diagnosis of tick‐borne bacterial diseases in Europe.Crossref | GoogleScholarGoogle Scholar |
[7] Gern, L. (2008) Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis: life in the wilds. Parasite 15, 244–247.
| Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis: life in the wilds.Crossref | GoogleScholarGoogle Scholar |
[8] Olsen, B. et al. (1995) Transhemispheric exchange of Lyme disease spirochetes by seabirds. J. Clin. Microbiol. 33, 3270–3274.
[9] Stanek, G. et al. (2012) Lyme borreliosis. Lancet 379, 461–473.
| Lyme borreliosis.Crossref | GoogleScholarGoogle Scholar |
[10] Vorou, R.M. et al. (2007) Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol. Infect. 135, 1231–1247.
[11] Hubálek, Z. (2009) Epidemiology of Lyme borreliosis. Lyme Borreliosis 37, 31–50.
| Epidemiology of Lyme borreliosis.Crossref | GoogleScholarGoogle Scholar |
[12] van Dam, A.P. et al. (1993) Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17, 708–717.
| Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis.Crossref | GoogleScholarGoogle Scholar |
[13] Rizzoli, A. et al. (2011) Lyme borreliosis in Europe. Euro Surveill. 16, 19906.
[14] Lindgren, E. and Jaenson, T.G.Y. (2006) Lyme borreliosis in Europe: influences of climate and climate change, epidemiology, ecology and adaptation measures. World Health Organization Regional Office for Europe, Copenhagen, Denmark.
[15] van den Wijngaard, C.C. et al. (2017) Surveillance perspective on Lyme borreliosis across the European Union and European Economic Area. Euro Surveill. 22, 30569.
| Surveillance perspective on Lyme borreliosis across the European Union and European Economic Area.Crossref | GoogleScholarGoogle Scholar |
[16] Alkishe, A.A. et al. (2017) Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS One 12, e0189092.
| Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus.Crossref | GoogleScholarGoogle Scholar |
[17] Boyé, T. (2007) [What kind of clinical, epidemiological, and biological data is essential for the diagnosis of Lyme borreliosis? Dermatological and ophtalmological courses of Lyme borreliosis.] Med. Mal. Inf. 37, S175–S188.
[18] Flisiak, R. and Prokopowicz, D. (1996) [Epidemiologic and clinical characteristics of Lyme borreliosis in northeastern Poland]. Pol Tyg Lek 51, 326–8–330.
[19] Wormser, G.P. et al. (2006) The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 43, 1089–1134.
| The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America.Crossref | GoogleScholarGoogle Scholar |
[20] Wilske, B. et al. (2007) Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol. Med. Microbiol. 49, 13–21.
| Microbiological and serological diagnosis of Lyme borreliosis.Crossref | GoogleScholarGoogle Scholar |
[21] Shah, J.S. et al. (2018) Development of a sensitive PCR-dot blot assay to supplement serological tests for diagnosing Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. 37, 701–709.
| Development of a sensitive PCR-dot blot assay to supplement serological tests for diagnosing Lyme disease.Crossref | GoogleScholarGoogle Scholar |
[22] Parola, P. et al. (2013) Update on tick-borne rickettsioses around the world: a geographic approach. Clin. Microbiol. Rev. 26, 657–702.
| Update on tick-borne rickettsioses around the world: a geographic approach.Crossref | GoogleScholarGoogle Scholar |
[23] Didier, R. (2010) Introduction to Rickettsioses, Ehrlichioses, and Anaplasmosis. In Principles and Practice of Infectious Diseases, 7th edition (Mandell, G.L. et al., eds). pp. 2495–2498. Elsevier, London.
[24] Portillo, A. et al. (2015) Rickettsioses in Europe. Microbes Infect. 17, 834–838.
| Rickettsioses in Europe.Crossref | GoogleScholarGoogle Scholar |
[25] Raoult, D. and Roux, V. (1997) Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10, 694–719.
| Rickettsioses as paradigms of new or emerging infectious diseases.Crossref | GoogleScholarGoogle Scholar |
[26] La Scola, B. (1997) Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J. Clin. Microbiol. 35, 2715–2727.
[27] Portillo, A. et al. (2017) Guidelines for the detection of Rickettsia spp. Vector Borne Zoonotic Dis. 17, 23–32.
| Guidelines for the detection of Rickettsia spp.Crossref | GoogleScholarGoogle Scholar |
[28] Mouffok, N. et al. (2011) Diagnosis of rickettsioses from eschar swab samples, Algeria. Emerg. Infect. Dis. 17, 1968–1969.
| Diagnosis of rickettsioses from eschar swab samples, Algeria.Crossref | GoogleScholarGoogle Scholar |
[29] Angelakis, E. et al. (2012) Comparison of real-time quantitative PCR and culture for the diagnosis of emerging rickettsioses. PLoS Negl. Trop. Dis. 6, e1540.
| Comparison of real-time quantitative PCR and culture for the diagnosis of emerging rickettsioses.Crossref | GoogleScholarGoogle Scholar |
[30] Yssouf, A. et al. (2015) Identification of tick species and disseminate pathogen using hemolymph by MALDI-TOF MS. Ticks Tick Borne Dis. 6, 579–586.
| Identification of tick species and disseminate pathogen using hemolymph by MALDI-TOF MS.Crossref | GoogleScholarGoogle Scholar |
Biographies
Rita Abou Abdallah is an MS, PharmD holder in clinical pharmacy and pharmaco-Epidemiology. She is also a PhD holder in microbiology and infectious diseases. Her research activities are focused on genomic analysis of bacterial human pathogens and mainly the study of the relationship between genomic and clinical features. She works in the IHU (Institut Hospitalo-Universitaire, Méditerranée-lnfection, Marseille, France).
Didier Raoult is Professor of Microbiology at Aix-Marseille University. He created in Marseille the sole medical institute dedicated to infectious diseases, which hosts 75 hospital beds, a large diagnostic laboratory and unique research platforms. The institute is currently the most productive and the largest of its kind in Europe. Pr Raoult is the most productive and most cited European microbiologist in the past 10 years.
Pierre-Edouard Fournier is MD, PhD, professor in medical microbiology at the Mediterranee Infection institute in Marseille, France. He is the director of the French reference center for rickettsioses, Q fever and bartonelloses. His research activities focus on the use of genomic sequences for the description of new human-associated bacteria and the development of new diagnostic assays.


