Incorporating fungal community ecology into invasion biology: challenges and opportunities
Eleonora Egidi A and Ashley E Franks A BA Department of Physiology, Anatomy and Microbiology, La Trobe University, Kingsbury Drive, Bundoora, Vic. 3083, Australia
B Applied Environmental Microbiology Department of Physiology, La Trobe University, Bundoora, Vic. 3086, Australia. Email: a.franks@latrobe.edu.au
Microbiology Australia 39(1) 56-60 https://doi.org/10.1071/MA18015
Published: 16 February 2018
Recently, the role of the plant-associated mycobiome (i.e. the fungal community) in influencing the competitive success of invasive plant species has received increasing attention. Fungi act as primary drivers of the plant invasion process due to their ability to form both beneficial and detrimental relationships with terrestrial plant species. Here we review the role of the plant mycobiome in promoting or inhibiting plant species invasion into foreign ecosystems. Moreover, the potential to exploit these relationships for invasive plant control and restoration of native communities is discussed. Incorporating fungal community ecology into invasion and restoration biology will aid in the management and control of invasive plant species in Australia.
Alien invasive plant species represent an ever-increasing worldwide problem. The expansion of invasive species in non-native ranges can dramatically alter the structure and population dynamics of the invaded community, with the negative impact of invasive plants on ecosystem structure and function resulting in changes to native vegetation composition and productivity, nutrient cycling, soil characteristics, and even human well-being1.
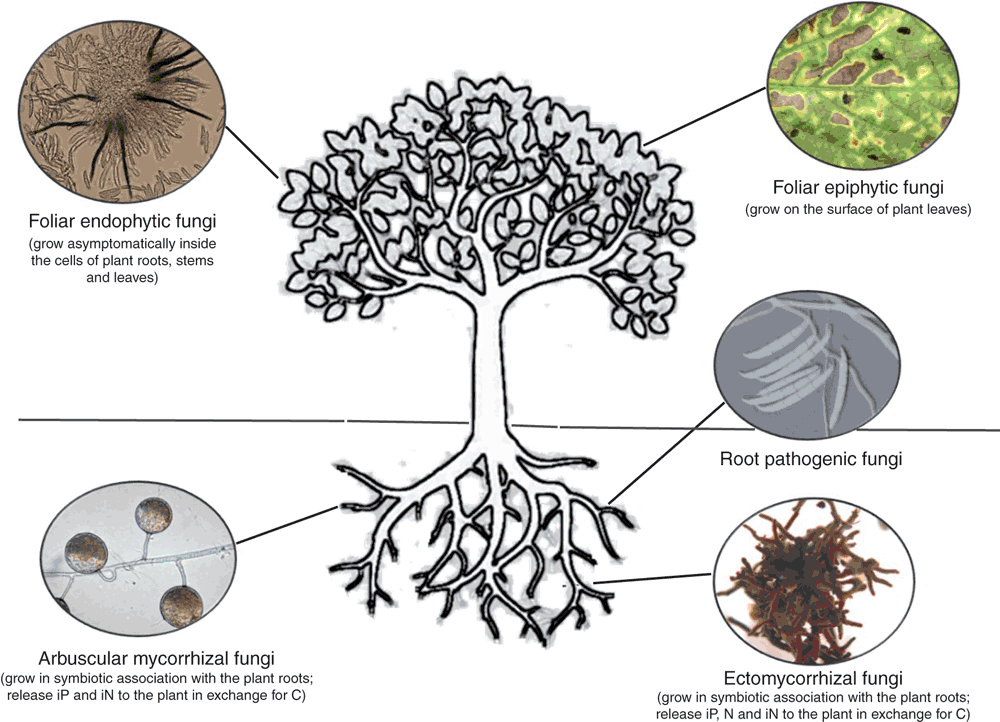
Many factors regulate exotic species naturalisation and invasion success, including the ability to rapidly access resources, allelopathy, and the modification of ecosystem processes (reviewed in Levine et al.2). However, an increasing body of evidence suggests a pivotal role for the plant-associated mycobiome (i.e. the fungal community) in influencing the competitive success of invasive species3–6. Fungi are important terrestrial ecosystem components, acting as mutualists, pathogens, decomposers, and food sources. Because of their primary role as drivers of many ecosystem functions and their ability to establish intimate relationships with terrestrial plant species (e.g. mycorrhizal fungi or leaf endophytes) (Figure 1), fungal communities can critically influence plant fitness and survival and, hence, their colonisation and invasion patterns5,7.
|
Understanding the mechanistic interactions underpinning the invasion process is predicted to be particularly important for Australia, where invasive species pose not only a threat to the biodiversity of its unique vegetation, but also an economic problem8. Following accidental and deliberate introductions after European colonisation of the continent, approximately 2700 exotic plants species are now considered established within the continent, with almost 250 having been declared harmful or being under some form of legal control measure9. In terms of economic burden, the control of alien plant species is calculated to cost approximately AU$4 billion annually for agriculture alone10. The cost associated with managing native plant communities, although difficult to estimate, is predicted to also be considerable.
Given the environmental and economic implications of invasive plant species control, the need to find effective strategies for the prevention, early detection, and eradication of invasive species now represents a priority in many economic and scientific agendas. Here we discuss the role of the plant mycobiome in promoting or inhibiting invasive plant species incursion into foreign ecosystems, and propose ways through which these relationships can be exploited for invasive plant control and native communities restoration.
Mechanisms and effects of fungi-plant interaction during invasion
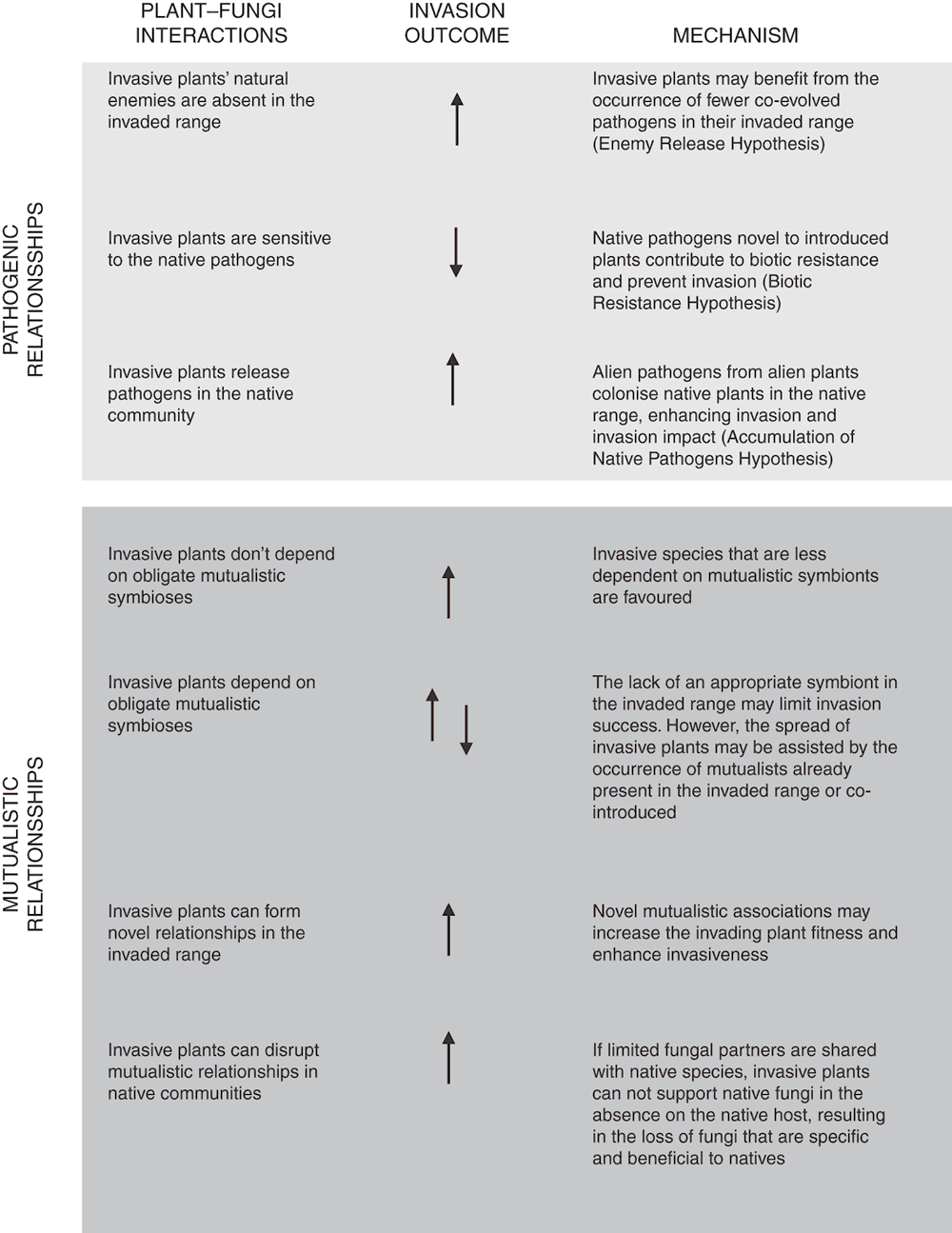
Pathogenic relationships: The interactions between invasive species and their fungal communities are complex, with invasion success often being defined by the nature of such relationships. For example, during invasive plant establishment, pathogenic fungi can form novel associations detrimental to the invasive plants11. This possibility is usually amplified if the invasive and native plants are not phylogenetically related, resulting in specific targeting of the invasive species, and thus prevention of invasion through inhibition (Figure 2). In contrast, successful invasion is often promoted by the loss of detrimental microbes, such as pathogenic fungi, particularly when they are not present in the newly colonised habitat12. Invasive species can also act as reservoirs for microorganisms that are pathogenic for the invaded community, with a consequent enhancement of the negative impact of invasion on the native vegetation, as observed during the invasion of non-native Spartina alterniflora and its fungal pathogen (Fusarium palustre) on native Chinese saltmarsh plants. This instance implies the ability of the invader population to resist or tolerate native pathogens within the invaded areas, causing an increase in pathogen loads detrimental to the native community13.
Symbiotic relationships: An equally important aspect of the plant-fungi relationship during the invasion process pertains to the role of symbiotic interactions, such as mycorrhizal interactions. Mutualism preservation is often vital for the invaders, and the occurrence of such beneficial interactions in the invaded habitat can be enhanced by the presence of native arbuscular mycorrhizal fungi with low endemism and low specificity in their range of associations14. Invading plants able to form novel symbiotic associations are also typically more successful. This strategy is particularly relevant for arbuscular mycorrhizal plants, which tend to establish novel associations in their exotic range, resulting in increased fitness and enhanced invasiveness15, such as in the case of the invasive North American species Ambrosia artemisiifolia. Alternatively, the co-introduction of associated symbionts in the colonised habitat may favour the invasive plants5. Co-introduction is usually due to the transport of infected plants or propagules, or from the translocation of contaminated soil. The presence of a suitable symbiotic partner, either native or alien, can significantly enhance invader fitness in the newly colonised habitat, as observed in the case of non-native willows introduced with their native fungal symbionts in Southern Australia riparian systems16.
In some instances, co-introduced symbionts may also be able to modify the native microbial community structure, including substantial loss of belowground diversity and native mutualists, with detrimental repercussions for the native host’s fitness. Such invasive processes can result in negative modifications of the functional attributes of the invaded system5. For example, the invasion of non-ectomycorrhizal communities by ectomycorrhizal plants causes both a decrease in soil carbon and a co-release of nutrients from the soil17. Plant-fungus co-invasion may also cause shifts in the total plant biomass; a change either enhanced by the increase in mutualistic fungi18 or decreased by the pathogenic invaders19,20. Both the biomass shift and the plant compositional modification can critically alter important ecosystem processes in the native community invaders19,20.
Implications for management
A better understanding of plant-fungi interactions can have important repercussions for the mitigation of negative impacts of invasive plants and the design of better restoration strategies5. Novel microbe-mediated approaches for invasive plant control may include the inhibition of fungal symbiotic relationships that provide competitive advantages to invasive plant species (e.g. through the introduction of pathogenic microbes or inhibition of beneficial fungi)4,21. In particular, the co-introduction of native pathogens has been proposed as an effective strategy to manage alien plant species. An example of the successful application of fungi as biocontrol agents is represented by Phragmidium violaceum, used to control invasive blackberry trees in Australia22. Similarly, the rust fungus Uromyces pencanus has been proposed as a promising biocontrol agent to reduce the spread of Nassella neesiana (Chilean needle grass), a grass species invasive to the southern hemisphere23.
In addition to the invading plant management, the plant-mycobiome relationship can be harnessed to mitigate the legacies of disrupted fungal communities resulting from processes such as plant-fungi co-invasion. Effective restoration strategies may be represented by manipulations targeted to increase the fitness of native plants. Particularly, the effectiveness of using native inocula to improve the establishment, growth and diversity of plants in their native range has been demonstrated in many instances (e.g. see Maltz and Treseder24), offering an alternative approach to the native community re-establishment. In this sense, national initiatives such as the ‘Biomes of Australian Soil Environments’ (BASE) project25 may offer a useful baseline to explore the Australian microbial biodiversity and map the occurrence of suitable microbial partners in potential reintroduction ranges, thus facilitating the selection of reintroduction locations that support a fungal community similar to the native plant community of origin26.
Conclusions
In the past decade, a mounting body of evidence from ecological studies has contributed to unravelling the pivotal contribution of the plant-fungi interaction in mediating the successful plant invasion. Much of this novel insight converged to recognise that invasion dynamics cannot be understood or predicted without a thorough characterisation of the relationships occurring between invasive and native plants together with their respective microbiomes5,21. We therefore believe that approaching the plant-fungus relationships during invasion ecology studies in a mechanistic framework represents a fundamental prerequisite to better understand the processes underpinning the competitive success of invasive plant species, and thus design effective and long-lasting management and restoration strategies. Particularly, the characterisation of the microbial diversity and temporal variability, including endophytes and rhizosphere microbes, relevant to both the invasive and native plants at different growth stages, as well as the identification of their functional role and their effect on plant growth, development and tolerance21, will represent the foundation to design effective microbiome manipulation strategies and predict possible invasion outcomes. Exploring new venues to protect and restore native vegetation communities may be particularly relevant for Australia, a continent where many terrestrial systems are experiencing a rapidly increasing environmental pressure related to climate change, habitat loss and fragmentation, and growing human populations. Further research focused on elucidating the impact of linked plant-fungal invasions in the context of ecosystem-level and community assembly processes holds promise to provide a solid scientific framework to predict invasion trajectories, and thus improve the outcomes of alien plant invasion management approaches.
References
[1] Mack, R. et al. (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710.| Biotic invasions: causes, epidemiology, global consequences, and control.Crossref | GoogleScholarGoogle Scholar |
[2] Levine, J.M. et al. (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc. Biol. Sci. 270, 775–781.
| Mechanisms underlying the impacts of exotic plant invasions.Crossref | GoogleScholarGoogle Scholar |
[3] Klironomos, J.N. (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417, 67–70.
| Feedback with soil biota contributes to plant rarity and invasiveness in communities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjsFOgtbo%3D&md5=5ff933e89ff3e4f02b314c52f77a35c7CAS |
[4] Amsellem, L. et al. (2017) Importance of microorganisms to macroorganisms invasions: is the essential invisible to the eye? (The Little Prince, A. de Saint-Exupéry, 1943). In Advances in Ecological Research.
[5] Dickie, I.A. et al. (2017) The emerging science of linked plant-fungal invasions. New Phytol. 215, 1314–1332.
| The emerging science of linked plant-fungal invasions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXht1Ois7zI&md5=84048effbcea1d55b27b558f898e5613CAS |
[6] Pringle, A. et al. (2009) Mycorrhizal symbioses and plant invasions. Annu. Rev. Ecol. Evol. Syst. 40, 699–715.
| Mycorrhizal symbioses and plant invasions.Crossref | GoogleScholarGoogle Scholar |
[7] Peay, K.G. et al. (2016) Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 14, 434–447.
| Dimensions of biodiversity in the Earth mycobiome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xps12ntLw%3D&md5=60896fd76dcf8d7d9334e21ea4617ec5CAS |
[8] Hoffmann, B. et al. (2016) The economic cost of managing invasive species in Australia. NeoBiota 31, 1–18.
| The economic cost of managing invasive species in Australia.Crossref | GoogleScholarGoogle Scholar |
[9] Hoyle, F.C. and Murphy, D. V. (2007) The Australian Weeds Strategy – a national strategy for weed management in Australia. Australian Government Department of the Environment and Water Resources, Canberra, ACT. (Natural Resource Management Ministerial Council)
[10] Sinden, J. et al. (2004) The economic impact of weeds in Australia. Tech. Ser. 8.
[11] Stricker, K.B. et al. (2016) Emergence and accumulation of novel pathogens suppress an invasive species. Ecol. Lett. 19, 469–477.
| Emergence and accumulation of novel pathogens suppress an invasive species.Crossref | GoogleScholarGoogle Scholar |
[12] Rúa, M.A. et al. (2011) The role of viruses in biological invasions: friend or foe? Curr. Opin. Virol. 1, 68–72.
| The role of viruses in biological invasions: friend or foe?Crossref | GoogleScholarGoogle Scholar |
[13] Lee, K.A. and Klasing, K.C. (2004) A role for immunology in invasion biology. Trends Ecol. Evol. 19, 523–529.
| A role for immunology in invasion biology.Crossref | GoogleScholarGoogle Scholar |
[14] Grove, S. et al. (2017) Mycorrhizae, invasions, and the temporal dynamics of mutualism disruption. J. Ecol. 105, 1496–1508.
| Mycorrhizae, invasions, and the temporal dynamics of mutualism disruption.Crossref | GoogleScholarGoogle Scholar |
[15] Nuñez, M.A. and Dickie, I.A. (2014) Invasive belowground mutualists of woody plants. Biol. Invasions 16, 645–661.
| Invasive belowground mutualists of woody plants.Crossref | GoogleScholarGoogle Scholar |
[16] McInerney, P.J. and Rees, G.N. (2017) Co-invasion hypothesis explains microbial community structure changes in upland streams affected by riparian invader. Freshw. Sci. 36, 297–306.
| Co-invasion hypothesis explains microbial community structure changes in upland streams affected by riparian invader.Crossref | GoogleScholarGoogle Scholar |
[17] Dickie, I.A. et al. (2014) Conflicting values: ecosystem services and invasive tree management. Biol. Invasions 16, 705–719.
| Conflicting values: ecosystem services and invasive tree management.Crossref | GoogleScholarGoogle Scholar |
[18] Dickie, I. et al. (2011) Podocarp roots, mycorrhizas, and nodules. Smithson. Contrib. Bot. 95, 175–187.
| Podocarp roots, mycorrhizas, and nodules.Crossref | GoogleScholarGoogle Scholar |
[19] Cobb, R.C. and Rizzo, D.M. (2016) Litter chemistry, community shift, and non-additive effects drive litter decomposition changes following invasion by a generalist pathogen. Ecosystems 19, 1478–1490.
| Litter chemistry, community shift, and non-additive effects drive litter decomposition changes following invasion by a generalist pathogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtFOku7zM&md5=ab01dc0fc55032ad42bb5db6f8b8e283CAS |
[20] Preston, D.L. et al. (2016) Disease ecology meets ecosystem science. Ecosystems 19, 737–748.
| Disease ecology meets ecosystem science.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XjtVOjtrw%3D&md5=f893815bd024fb64817f6edfec90bce8CAS |
[21] Kowalski, K.P. et al. (2015) Advancing the science of microbial symbiosis to support invasive species management: a case study on Phragmites in the Great Lakes. Front. Microbiol. 6, 95.
| Advancing the science of microbial symbiosis to support invasive species management: a case study on Phragmites in the Great Lakes.Crossref | GoogleScholarGoogle Scholar |
[22] Morin, L. and Evans, K. (2012) Rubus fruticosus L. aggregate-European blackberry. In Biological control of weeds in Australia. pp. 499–509.
[23] Anderson, F.E. et al. (2017) Plant/pathogen interactions observed during host range testing of the rust fungus Uromyces pencanus, a classical biological control agent for Chilean needle grass (Nassella neesiana) in Australia and New Zealand. Biocontrol Sci. Technol. 27, 1096–1117.
| Plant/pathogen interactions observed during host range testing of the rust fungus Uromyces pencanus, a classical biological control agent for Chilean needle grass (Nassella neesiana) in Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar |
[24] Maltz, M.R. and Treseder, K.K. (2015) Sources of inocula influence mycorrhizal colonization of plants in restoration projects: a meta-analysis. Restor. Ecol. 23, 625–634.
| Sources of inocula influence mycorrhizal colonization of plants in restoration projects: a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[25] Bissett, A. et al. (2016) Introducing BASE: the Biomes of Australian Soil Environments soil microbial diversity database. Gigascience 5, 21.
| Introducing BASE: the Biomes of Australian Soil Environments soil microbial diversity database.Crossref | GoogleScholarGoogle Scholar |
[26] Rigg, J.L. et al. (2017) Conservation by translocation: establishment of Wollemi pine and associated microbial communities in novel environments. Plant Soil 411, 209–225.
| Conservation by translocation: establishment of Wollemi pine and associated microbial communities in novel environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xhtlylu7nK&md5=a3141b8ea646fbf2305006825895287cCAS |
Biographies
Dr Eleonora Egidi is mycologist interested in different aspects of fungal research, including fungal ecology, biology and phylogeny. Eleonora has an international background and has been involved in collaborative projects for the identification of new fungal barcoding regions, study of fungal communities from extreme environments, and use of fungi in phytoremediation. She is currently a postdoc in the Applied and Environmental Microbiology Laboratory at La Trobe University, where she is investigating the biodiversity of Australian fungi. Her scientific interest focusses on the role of fungi as drivers of plant diversity in Australian natural ecosystems, as well as understanding how these communities respond to environmental changes.
Associate Professor Ashley E Franks is head of the Applied and Environmental Microbiology Laboratory at La Trobe University. He conducted his doctorate research as part of the Centre of Marine Biofouling and Bioinnovation at the University of New South Wales by investigating antifungal compounds produced by marine bacteria in biofilms. During his PhD he spent 4 months at the University of Exeter in the UK on an Adrian Lee Fellowship to develop dual bacterial/yeast biofilm systems. On graduating he moved to the Biomerit Research Centre in Cork, Ireland to work on bacterial plant interactions as a Government of Ireland Fellow in Science Technology and Engineering. This research looked at how to use bacteria to help plant growth. He then took a position as a Senior Scientist and Research Professor within the Geobacter Project at the University of Massachusetts Amherst in the USA where he worked on microbes that make electricity. He currently serves as the Chair of the Awards Committee for the International Society of Microbial Electrochemical Technology and was previously the Secretary of Synthetic Biology Australasia.