Salmonella in Australia: understanding and controlling infection
Joshua PM NewsonDepartment of Microbiology and Immunology
The University of Melbourne at the Peter Doherty Institute for Infection and Immunity
Vic. 3000, Australia
Tel: +61 3 8344 0825
Email: joshua.newson@unimelb.edu.au
Microbiology Australia 38(3) 112-115 https://doi.org/10.1071/MA17044
Published: 9 August 2017
The bacterium Salmonella causes a spectrum of foodborne diseases ranging from acute gastroenteritis to systemic infections, and represents a significant burden of disease globally. In Australia, Salmonella is frequently associated with outbreaks and is a leading cause of foodborne illness, which results in a significant medical and economic burden. Salmonella infection involves colonisation of the small intestine, where the bacteria invades host cells and establishes an intracellular infection. To survive within host cells, Salmonella employs type-three secretion systems to deliver bacterial effector proteins into the cytoplasm of host cells. These bacterial effectors seek out and modify specific host proteins, disrupting host processes such as cell signalling, intracellular trafficking, and programmed cell death. This strategy of impairing host cells allows Salmonella to establish a replicative niche within the cell, where they can replicate to high numbers before escaping to infect neighbouring cells, or be transmitted to new hosts. While the importance of effector protein translocation to infection is well established, our understanding of many effector proteins remains incomplete. Many Salmonella effectors have unknown function and unknown roles during infection. A greater understanding of how Salmonella manipulates host cells during infection will lead to improved strategies to prevent, control, and eliminate disease. Further, studying effector proteins can be a useful means for exploring host cell biology and elucidating the details of host cell signalling.
Salmonella infections in Australia
Australia experiences an unusually high number of reported Salmonella infections, comprising a significant baseline of individually acquired infections, combined with a string of well-publicised foodborne outbreaks1. Typically, outbreaks are associated with bacterial contamination of livestock and animal products in farms and factories, which are then carried on through to consumers. Several high-profile outbreaks have been linked to contamination of animal products: the outbreak at the Langham hotel in Melbourne in 2015 was linked to raw egg mayonnaise2, while chicken and pork products were linked to an outbreak at a Sydney bakery in 20163. Interestingly, several recent outbreaks have been linked to contaminated fruits and vegetables: Salmonella Anatum in bagged salads4, Salmonella Saintpaul on beansprouts5, and Salmonella Hvittingfoss on rockmelons6. These outbreaks translate to a significant economic impact to the affected industries, in addition to the medical burden caused to the affected individuals7,8.
Australia enjoys a high standard of public health surveillance, and Salmonella infections are notifiable and publicly reported through the National Notifiable Diseases Surveillance System. These reports indicate more than 17 000 cases of Salmonella infection were reported in 20169, and already more than 10 000 reported cases in 2017 at the time of writing10. These numbers are derived from a symptomatic person presenting to a healthcare professional, and so the true number of infections is likely to be significantly higher11. Salmonella therefore represents a significant public health concern.
Salmonella causes a range of disease states
At first glance, the phylogeny of Salmonella appears relatively simple, with the genus comprising only two species. S. enterica is the species most frequently associated with disease states in humans and other animals, while S. bongori lacks several important virulence factors and consequently rarely causes disease in humans12. Within S. enterica however lies an astonishing variety of serologically distinct serovars, some of which have undergone selective pressures resulting in host adaptation and acquisition of additional virulence factors. Serovars within S. enterica can cause drastically different disease states: S. enterica serovars Typhimurium and Enteritidis are frequently the causative agents of gastroenteritis outbreaks, while S. enterica serovar Typhi causes typhoid fever, a systemic infection with broadly different clinical outcomes13. Even within serovars, variation and selection can result in strikingly different strains. For example, the globally disseminated S. enterica Typhimurium strain ST19 causes self-limiting gastroenteritis, while the genetically similar S. enterica Typhimurium strain ST313 is causative of bloodstream infections resulting in significant mortality rates in Sub-Saharan Africa14.
In Australia, the burden of disease arises from a variety of Salmonella serovars. Individually acquired infections and outbreak-associated infections are most typically caused by serovar S. Typhimurium15, which is a generalist strain capable of infecting different animals. Some serovars are specialised to infect certain livestock animals: S. Gallinarum and S. Pullorum are adapted to chickens, while S. Choleraesuis is associated with pigs. Occasionally, outbreaks are associated with rarer serovars, such as S. Anatum and S. Hvittingfoss. Nearly all cases of S. Typhi infection in Australia occur in travellers that have contracted the bacteria while overseas1.
Ultimately, the diversity of Salmonella serovars and spectrum of diseases that it causes demonstrate a bacterium that is adept at persisting through the food supply chain, from contamination of farms and factories to restaurants and household kitchens, despite stringent food safety standards and public health surveillance.
Salmonella subverts host cells to achieve infection
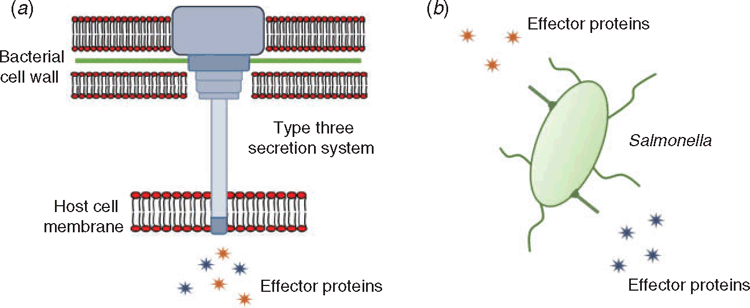
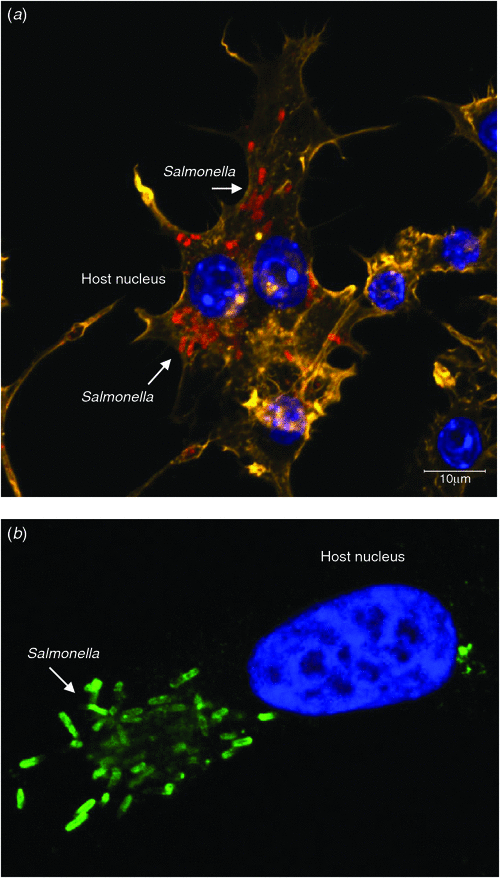
Infection by Salmonella spp. follows ingestion of live bacteria, which survive passage through the stomach and attach to the gastrointestinal epithelium. The infection strategy of Salmonella involves the invasion of host cells (either by localised disruption of the membrane of non-phagocytic epithelial cells, or by endocytic uptake into professional phagocytes) followed by the establishment of an intracellular replicative niche16–18 (Figure 1). These activities are heavily mediated by the use of type-three secretion systems (T3SS), which function as molecular syringes to penetrate host cells and deliver bacterial effector proteins into the host cytoplasm (Figure 2). These effector proteins typically recognise specific host proteins, and disrupt the normal function of these proteins often by catalysing post-translational modifications19,20.
|
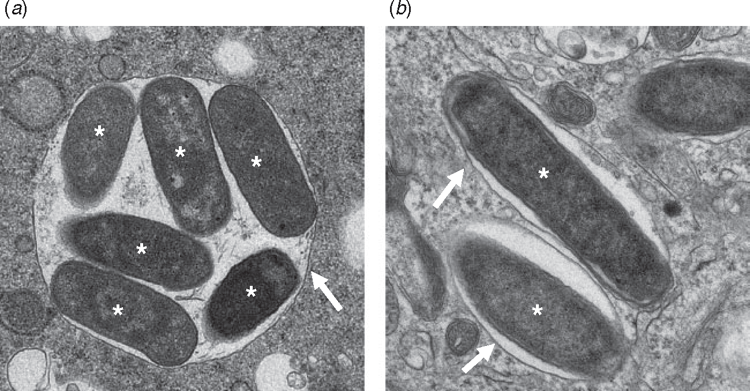
Salmonella employs two distinct type-three secretion systems at different stages of infection. These secretion systems translocate distinct cohorts of effector proteins into the host cell to achieve different goals for the bacterium. At early stages of infection, Salmonella uses the SPI-1 secretion system (encoded on the genomic region termed Salmonella pathogenicity island 1) to achieve uptake into non-phagocytic epithelial cells. Effector proteins translocated by this secretion system manipulate the host actin cytoskeleton to induce membrane ruffling, which enables bacterial uptake21. Shortly after invasion, the SPI-1 secretion system is downregulated and the bacterium is taken up into a phagocytic vacuole. Sensing the acidification of the vacuole, Salmonella then deploys the SPI-2 secretion system, which delivers another set of effectors into the host cytoplasm. Broadly, these effectors are responsible for remodelling the vacuole into a replication-permissive space (Figure 3), translocating and extending the vacuole along host microtubules, and interfering with the secretion of inflammatory cytokines22,23. Together, these activities enable Salmonella to survive within both epithelia and phagocytic cells, to replicate to high numbers, and to disseminate to adjacent cells and on to new hosts.
Discovering the function of Salmonella effector proteins
It is well established that Salmonella relies on effector protein translocation to achieve infection – mutant strains lacking type-three secretion systems do not invade epithelia effectively, and are deficient in intracellular persistence24. However, the contribution of individual effector proteins is less well defined. Some effector proteins are relatively well described, others are poorly understood, and many have no known function and no known role during infection. Our research aims to characterise the poorly understood effector proteins of the SPI-2 secretion system.
Classical methods for identifying interactions between a given protein and its potential binding partner rely on the strength of the interaction between the two. Approaches such as yeast two-hybrid screening and immunoprecipitation have found success in identifying the host substrates of many Salmonella effector proteins. However, these approaches are unsuitable if the binding affinity between two proteins is weak or transient. For instance, effector proteins that are predicted to be enzymes are not likely to have strong or stable binding affinity for their host substrates.
Our research focuses on the enzymatic activity of a family of Salmonella effector proteins. These effectors are known substrates of the SPI-2 secretion system, and are predicted to be glycosyltransferases. Therefore, we have screened the proteome of infected host cells to detect changes in glycosylation activity. This approach combines custom immunoprecipitation techniques with highly sensitive mass spectrometry to detect glycosylated peptides during infection. Our approaches are not limited by the binding affinity of an effector for its substrate, and also allow for interrogating the activity of effector proteins without overexpression or ectopic expression. Preliminary results suggest Salmonella engages in a blockade of apoptotic cell death signalling, a finding that expands our understanding of how bacteria survive within host cells.
Directions and applications
Antibiotic treatment is generally ineffective at reducing the impact of Salmonella infection, and vaccines that are effective and economically viable are still in development25. The successful control and prevention of Salmonella infection requires a more robust understanding of the interaction between the pathogen and the host cell. Given that type-three secretion mutants are significantly attenuated during infection, it stands to reason that interfering with effector translocation represents a viable opportunity for developing novel anti-infective therapeutics. As we move towards a more complete understanding of the Salmonella–host interaction, it is likely that new opportunities will arise to antagonise the intracellular lifestyle of the bacteria. Ultimately, effective control and prevention of Salmonella infection will require a combination of human and animal vaccination, public health surveillance and food safety compliance, and effective therapeutic options.
Acknowledgements
The author gratefully acknowledges contributions to the project by Dr Nichollas Scott, Dr Cristina Giogha, Dr Tania Wong Fok Lung, Dr Jaclyn Pearson and Professor Elizabeth Hartland.
References
[1] OzFoodNet Working Group (2015) Monitoring the incidence and causes of diseases potentially transmitted by food in Australia: annual report of the OzFoodNet network, 2011. Commun. Dis. Intell. 39, 236–264.[2] Younger, E. (2015) Melbourne’s Langham Hotel salmonella outbreak blamed on raw egg mayonnaise. ABC News, Melbourne, Vic. http://www.abc.net.au/news/2015-08-25/raw-egg-mayonnaise-blamed-for-langham-hotel-salmonella-outbreak/6722062 (accessed 8 July 2017).
[3] Meddows, D. and Rapana, J. (2016) Dozens of customers ‘poisoned’ after eating at Box Village Bakery at Sylvania. Daily Telegraph, Sydney NSW. http://www.dailytelegraph.com.au/news/dozens-of-customers-poisoned-after-eating-at-sylvania-bakery/news-story/d0b6b20dc9c03f3cd47a1e3f48fac39e (accessed 8 July 2017).
[4] Spooner, R. (2016) Salmonella salad cases across Australia continue to rise. The Age, Melbourne, Vic. http://www.theage.com.au/victoria/salmonella-salad-cases-across-australia-continue-to-rise-20160217-gmwr4t.html (accessed 8 July 2017).
[5] Stokes, K. (2016) Contaminated bean sprout mystery solved after source traced to South Australian factory. The Advertiser, Adelaide, SA. http://www.adelaidenow.com.au/news/south-australia/contaminated-bean-sprout-mystery-solved-after-source-traced-to-south-australian-factory/news-story/9d77e218fa6333179ffdaf020dbc1dc4 (accessed 8 July 2017).
[6] Aubusson, K. and Levy, M. (2016) ’Red Dirt’ rockmelons recalled as Salmonella outbreak affects 80 people. The Sydney Morning Herald, Sydney, NSW. http://www.smh.com.au/business/consumer-affairs/rockmelon-linked-to-spike-in-salmonella-cases-in-australia-20160802-gqjnaa.html (accessed 8 July 2017).
[7] Abelson, P. et al. (2006) The annual cost of foodborne illness in Australia. Canberra: Australian Government Department of Health and Ageing.
[8] Kirk, M. et al. (2014) Foodborne illness, Australia, circa 2000 and circa 2010. Emerg. Infect. Dis. 20, 1857–1864.
| Foodborne illness, Australia, circa 2000 and circa 2010.Crossref | GoogleScholarGoogle Scholar |
[9] Communicable Diseases Network Australia (2016) National Notifiable Diseases Surveillance System – CDNA fortnightly summary notes 2016. Australian Government Department of Health. http://www.health.gov.au/internet/main/publishing.nsf/Content/nndss-fortnightly-summary-notes-2016
[10] Communicable Diseases Network Australia (2017) National Notifiable Diseases Surveillance System – CDNA fortnightly report. Australian Government Department of Health. http://www.health.gov.au/internet/main/publishing.nsf/Content/cdnareport.htm
[11] Hall, G. et al. (2008) Estimating community incidence of Salmonella, Campylobacter, and Shiga toxin-producing Escherichia coli infections, Australia. Emerg. Infect. Dis. 14, 1601–1609.
| Estimating community incidence of Salmonella, Campylobacter, and Shiga toxin-producing Escherichia coli infections, Australia.Crossref | GoogleScholarGoogle Scholar |
[12] Fookes, M. et al. (2011) Salmonella bongori provides insights into the evolution of the Salmonellae. PLoS Pathog. 7, e1002191.
| Salmonella bongori provides insights into the evolution of the Salmonellae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFOitrzN&md5=2aee81c2e6d8a65709800ae771d9b3bcCAS |
[13] Coburn, B. et al. (2007) Salmonella, the host and disease: a brief review. Immunol. Cell Biol. 85, 112–118.
| Salmonella, the host and disease: a brief review.Crossref | GoogleScholarGoogle Scholar |
[14] Feasey, N.A. et al. (2012) Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379, 2489–2499.
| Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa.Crossref | GoogleScholarGoogle Scholar |
[15] Ford, L. et al. (2016) Increasing incidence of Salmonella in Australia, 2000–2013. PLoS One 11, e0163989.
| Increasing incidence of Salmonella in Australia, 2000–2013.Crossref | GoogleScholarGoogle Scholar |
[16] Vazquez-Torres, A. et al. (1999) Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401, 804–808.
| Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXntFaqs78%3D&md5=e9b2f9cbd0e245556fe5793bdc6634d3CAS |
[17] Wallis, T.S. and Galyov, E.E. (2000) Molecular basis of Salmonella-induced enteritis. Mol. Microbiol. 36, 997–1005.
| Molecular basis of Salmonella-induced enteritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktF2luro%3D&md5=cbb243cfe99db920c60a3bfad90cda01CAS |
[18] Rescigno, M. et al. (2001) Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2, 361–367.
| Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXis1aktbo%3D&md5=4660b702dbffaac16e0b3830bf829922CAS |
[19] McGhie, E.J. et al. (2009) Salmonella takes control: effector-driven manipulation of the host. Curr. Opin. Microbiol. 12, 117–124.
| Salmonella takes control: effector-driven manipulation of the host.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhvFGksro%3D&md5=318b55e8e90dac1c776e27d4e7555d5eCAS |
[20] Figueira, R. and Holden, D.W. (2012) Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 158, 1147–1161.
| Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptFems70%3D&md5=4c2f84c1eed28f610a9ce2684326b900CAS |
[21] Zhou, D. and Galan, J. (2001) Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 3, 1293–1298.
| Salmonella entry into host cells: the work in concert of type III secreted effector proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisFOg&md5=aac057cd166d2a9886bb1e0e38467653CAS |
[22] Waterman, S.R. and Holden, D.W. (2003) Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell. Microbiol. 5, 501–511.
| Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmtVykt7k%3D&md5=35550f689b184d1c991162a851d5c37fCAS |
[23] Ramos-Morales, F. (2012) Impact of Salmonella enterica type III secretion system effectors on the eukaryotic host cell. Biochem. Biophys. Res. Commun. 449, 419–424.
[24] Galán, J.E. (2001) Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17, 53–86.
| Salmonella interactions with host cells: type III secretion at work.Crossref | GoogleScholarGoogle Scholar |
[25] Chen, H.M. et al. (2013) Nontyphoid Salmonella infection: microbiology, clinical features, and antimicrobial therapy. Pediatr. Neonatol. 54, 147–152.
| Nontyphoid Salmonella infection: microbiology, clinical features, and antimicrobial therapy.Crossref | GoogleScholarGoogle Scholar |
Biography
Joshua Newson is a PhD student at the Doherty Institute, The University of Melbourne. His research involves identifying mechanisms by which Salmonella survive within infected immune cells, and elucidating the function of translocated effector proteins.