Algal biotechnology for pursuing omega-3 fatty acid (bioactive) production
Munish PuriA Centre for Marine Bioproducts Development (CMBD), Medical Biotechnology, Flinders Medical Science and Technology, School of Medicine, Flinders University, Bedford Park, Adelaide, SA 5042, Australia
B Bioprocessing Laboratory, Centre for Chemistry and Biotechnology, Deakin University, Waurn Ponds, Vic. 3217, Australia
C Email: Munish.puri@flinders.edu.au; Munish.puri@deakin.edu.au
Microbiology Australia 38(2) 85-88 https://doi.org/10.1071/MA17036
Published: 26 April 2017
Algae are spread in diversified ecosystems that include marine, freshwater, desert and hot springs and even snow and ice environments. Algae are classified as multicellular large sea weeds (macroalgae) or unicellular microalgae. Macroalgae are targeted for mining of natural biologically active components, which include proteins, linear peptides, cyclic peptides, and amino acids1. Recently, microalgae have been exploited for the production of high-value compounds such as lipids (omega-3 fatty acids), enzymes, polymers, toxins, antioxidants, and pigments (carotenoids)2. Thus, algal biotechnology is defined as ‘the technology developed using algae (macro or micro) to make or modify bioactive compounds, or products (nutritional supplements, fine chemicals) and renewable fuels for specific use’.
Out of all metabolites, one of the major groups of marine compounds of interest are the omega-3 fatty acids. Omega-3 fatty acids are naturally occurring important polyunsaturated fatty acids (PUFAs) that include mainly alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and docosahexaenoic acid (DHA) with EPA, DPA and DHA being the most well known in the marine world3. These PUFAs possess great relevance to the prevention of sudden death from ventricular fibrillation and are essential for brain development of infants4. Recent studies have shown that PUFAs mitigate the symptoms of diseases such as Alzheimer’s, chronic intestinal disorder, and some types of cancer5,6. The National Health and Medical Research Council (NHMRC) recommended intake of long chain omega-3 fatty acids (DHA/EPA) in order to lower the risk of chronic diseases is 610 mg/day for men and 430 mg/day for women, and it is currently estimated that Australians are getting about 160 mg of DHA+EPA per day7. The current omega-3 fatty acid products are primarily obtained from fish sources such as sardines, salmon, tuna and herring. Although the global demand is growing, fish as a source of PUFA production is a declining resource. Thus, there is current emphasis on discovering microalgal sources for the production of omega-3 fatty acids.
The algae products market is expected to reach US$44.7 billion by 2023. The target market for omega-3 fatty acids is diverse in terms of product type, from fortified food, beverages and dietary supplements, to infant food and pet food8. The carotenoid, astaxanthin, is well known for its antioxidant properties. Various reports have shown growth inhibitory effects of astaxanthin on certain types of cancers, such as colon and hepatic cancers. It can also help reduce incidence of age-related degenerative eye-disorders and reduce UV induced cell damage. These health benefits make astaxanthin a high value product, with projections suggesting that the global carotenoid market will reach $1.4 billion by 20189.
Cultivation of microalgae involves two basic processes; these are autotrophic and heterotrophic modes of growth. An open system is the simplest method of cultivation of autotrophic algae with the advantage of low production costs, and this culture system requires large surface area and shallow depth. Limited control of cultivation conditions and contamination by other microorganisms means that this type of system is restricted to microalgae that are tolerant to extreme conditions. Open pond systems are mainly suitable for the cultivation of Spirulina and Chlorella (Figure 1a, d) which is applied in places such as Japan, Thailand, California, Hawaii, Taiwan, India and China. To overcome contamination problems for less robust species, closed photo bioreactors (Figure 1c), flat panel photo and tubular bioreactors (Figure 1b, c, f) have been designed in various sizes and shapes to meet the requirement of microalgae growth and development10.
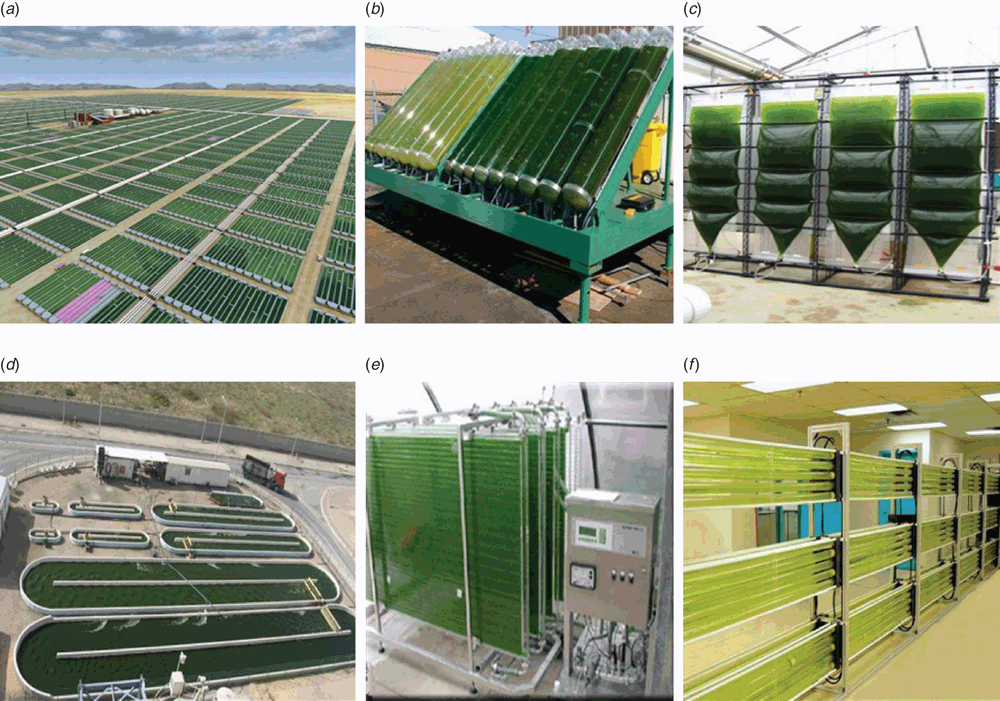
|
Heterotrophic microalgae are less abundant than autotrophic species and they require the addition of organic sources in the growth medium for energy generation and the production of various metabolites in the process. Growth is independent of light energy which allows simpler scale up processes to overcome the disadvantage of large investment in infrastructure and it also supports reproducible biomass yield in heterotrophic mode of growth. Heterotrophic microalgae such as Haemotococcus pluvialis, Chlamydomonas sp and Phaeodactylum sp, can utilise organic compounds to produce energy. They are commercially cultivated with the help of fermenters of various scales. Amongst these, thraustochytrids are microalgae-like microorganisms that can accumulate 60% of their dry weight as lipids, of which more than 25% is normally DHA. An ultrastructure study with transmission electron microscopy illustrated that the cells are heterogeneous in size, with a granular cytoplasm, containing oil micelles (Figure 2)3. The high lipid content of thraustochytrids makes them useful for the commercial production of omega-3 fatty acids.
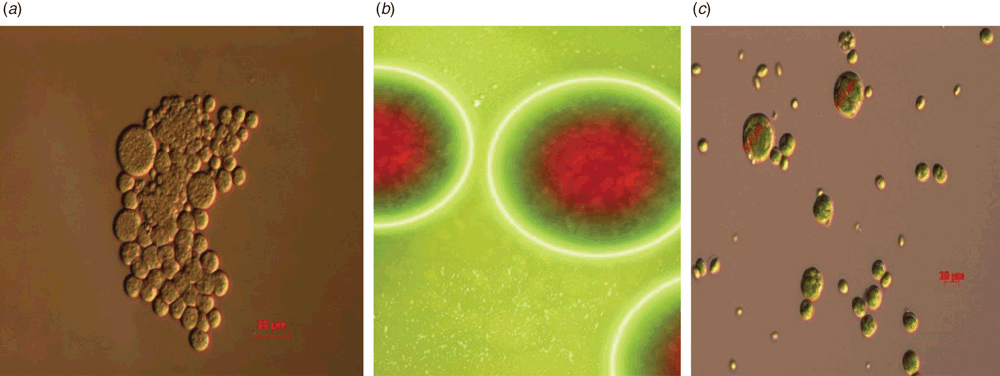
|
In this regard, we have a collection of thraustochytrids strains (strains isolated from the low-tide regions of Queenscliff and Barwon heads, Victoria, as shown in Figure 2) that produce high levels of DHA and we have shown that these microbes grow well on glucose11. An approximate three-fold improvement in carotenoid content in all isolates was achieved when glycerol was used as a carbon source in the production medium12. Based on fatty acid profiling, Australian thraustochytrids, in general, had a higher docosahexaenoic acid (DHA) content with DHA at 17–31% of total fatty acid13,14. We have evaluated the efficiency of various solvents, solvent combinations15 and cell disruption methods for lipid extraction16 to make the process cost-effective. Co-product extraction from said strains with emphasis upon astaxanthin has been optimised at lab scale17. Tween-80 has recently been investigated in a bioreactor with regard to biomass, lipase and lipid productivity in Schizochytrium sp. S3118. Another group from Tasmania has also collected Australian thraustochytrids and investigated life cycle assessment for biodiesel production19.
Constraints in omega-3 fatty acid production
Industrial production of omega-3 fatty acids usually requires two prerequisites, low cost and high productivity. High productivity can be achieved only with high cell density and high DHA content. Fermentation in submerged level is of two types, one stage fermentation and fed-batch fermentation or two stage fermentation; the difference in the process lies with the supply of carbon source. The carbon source needs to be supplied in fed batch fermentation as it decreases the negative effect of substrate inhibition. High cell density can be achieved through fed-batch fermentation.
Future perspectives
Biotechnological solution: Innovation both at the laboratory and Industry level should be aimed at devising upstream and downstream strategies for cost-effective polyunsaturated and co-product production. New marine microorganisms from varied habitats need to be continuously to be explored in terms of the isolation of new algal strains that have the capacity to accumulate high concentration of omega-3 fatty acid naturally and other valuable products. Novel marine sources possessing environmental stress should be explored for the isolation of new algal strains.
Various inexpensive carbon sources, such as whey from the dairy industry, processed fruit pulp from the food processing industry (an important resource for recovering nutrients), bread and bakery waste,20 crude glycerol,14 oil cake, lignocellulose resource21 and molasses can be tested for growing novel microalgal strains22. Such cost-effective carbon sources may lead to higher or comparative yield to glucose and glycerol, thus decreasing upstream processing costs. Currently, the cost of fermentation of DHA and EPA is significantly higher than obtaining these fatty acids from fish oils. Thus, controlled fermentation methods are required for cost-effective production of omega-3 fatty acids.
Downstream processing: Rupturing of biomass cell walls prior to solvent contact can improve lipid extraction yields. In addition, process optimisation for co-product production during algal fermentation can improve the overall economics of large-scale algal fermentation.
This write-up presents the potential of microalgae to provide the growing population with a secure, sustainable alternative source of health promoting omega-3 fatty acids for use in food and feeds.
Acknowledgements
This write-up entails information about the research work with reference to Omega-3 fermentation carried at the Bioprocessing laboratory at Centre for Chemistry and Biotechnology, Deakin University. The author acknowledges contributions from all his PhD graduates, i.e. Dr Adarsha Gupta, Dr Reinu E Abraham, Dr Tamilselvi Thyagrajan, Dr Avinesh Byreddy and Dr Dilip Singh for pursuing research working in the area. Encouragement and assistance received from Professor Colin J Barrow (an authority on omega-3 biotechnology) Director, Centre for Chemistry and Biotechnology, to pursue this area is appreciated.
References
[1] Harnedy, P.A. and Fitzgerald, R. (2011) Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 47, 218–232.| Bioactive proteins, peptides, and amino acids from macroalgae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntFCktbw%3D&md5=0e46ce49116ca1a6f9f3ff08f9b486e1CAS |
[2] Markou, G. and Nerantzis, E. (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol. Adv. 31, 1532–1542.
| Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVagt77I&md5=9778aae927e82f70124587f365b8191bCAS |
[3] Gupta, A. et al. (2012) Omega-3 biotechnology: Thrasutochytrids as a novel source of omega-3 oils. Biotechnol. Adv. 30, 1733–1745.
| Omega-3 biotechnology: Thrasutochytrids as a novel source of omega-3 oils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XktlCis7Y%3D&md5=43c3191bb3daceb1d8e7c8e3c2d9e028CAS |
[4] Connor, W.E. (2000) Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 71, 171S–175S.
| 1:CAS:528:DC%2BD3cXlsFGrtg%3D%3D&md5=92ec29b52b0dc4fdb66e1f9b03d15f75CAS |
[5] Cockbain, A.J. et al. (2012) Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer Gut 61, 135–149.
| Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancerCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitFWnurk%3D&md5=27981547bf1d88ce22103fd64607e7d0CAS |
[6] Finco, A. M. et al. (2016) Technological trends and market perspectives for production of microbial oils rich in omega-3. Crit. Rev. Biotechnol. , 1–16.
| Technological trends and market perspectives for production of microbial oils rich in omega-3.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhtlSktb0%3D&md5=3c282954f24e7993893e7b2676eaa31aCAS |
[7] Herron, M. (2014) Is omega-3 too good to be true? Available at: https://www.choice.com.au/health-and-body/medicines-and-supplements/vitamins-and-supplements/articles/omega-3-supplements (accessed 3 March 2017).
[8] Credence Research (2016) Algae products market by application. Available at: http://www.credenceresearch.com/report/algae-products-market (accessed 3 March 2017).
[9] Global Market Insights (2016) Available at: https://www.gminsights.com/industry-analysis/astaxanthin-market (accessed 3 March 2017).
[10] Thayagrajan, T. (2015) Fermentation of omega-3 and carotenoid producing marine microorganisms. PhD thesis, Deakin University.
[11] Gupta, A. et al. (2013) Exploring potential use of Australian thraustochytrids for the bioconversion of glycerol to lipids and carotenoids. Biochem. Eng J. 78, 11–17.
| Exploring potential use of Australian thraustochytrids for the bioconversion of glycerol to lipids and carotenoids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXovVCntbc%3D&md5=02b4168b0067cca9b471a0f1e3ba9dedCAS |
[12] Gupta, A. et al. (2013)a Pine pollen baiting facilitates the isolation of marine thraustochytrids with potential in omega-3 and biodiesel production. J. Ind. Microbiol. Biotechnol. 40, 1231–1240.
| Pine pollen baiting facilitates the isolation of marine thraustochytrids with potential in omega-3 and biodiesel production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtl2qtrrJ&md5=fd9e1062da260c0f4d1e37a71d597542CAS |
[13] Gupta, A. et al. (2016) Exploring Australian and Indian thraustochytrids for omega-3 fatty acids, enzymes and biodiesel production. Biotechnol. J. 11, 345–355.
| Exploring Australian and Indian thraustochytrids for omega-3 fatty acids, enzymes and biodiesel production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvFKhu7rJ&md5=f84b200e706364ab38bb8955cb8565d9CAS |
[14] Lee Chang, K.J. et al. (2015) Australian thraustochytrids: potential production of dietary long-chain omega-3 oils using crude glycerol. J. Funct. Foods 19, 810–820.
| Australian thraustochytrids: potential production of dietary long-chain omega-3 oils using crude glycerol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitFyntrY%3D&md5=e8eb43a1baf05fd06e928e17ba9ef1f6CAS |
[15] Byreddy, A.R. et al. (2015) Comparison of cell disruption methods for improving lipid extraction from thraustochytrid strains. Mar. Drugs 13, 5111–5127.
| Comparison of cell disruption methods for improving lipid extraction from thraustochytrid strains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XksVCjur0%3D&md5=1d2114b7203f73dd8efdf40e1e35bb2fCAS |
[16] Byreddy, A.R. et al. (2016) Bead milling for lipid recovery from thraustochytrid cells and selective hydrolysis of Schizochytrium DT3 oil using lipase. Bioresour. Technol. 200, 464–469.
| Bead milling for lipid recovery from thraustochytrid cells and selective hydrolysis of Schizochytrium DT3 oil using lipase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhs1Kqtb%2FN&md5=07c5a9d405a01ee167e021d14bc58945CAS |
[17] Singh, D. et al. (2015) Understanding response surface optimisation to the modelling of astaxanthin extraction from a novel strain of Thraustochytrium sp.S7. Algal Res. 11, 113–120.
| Understanding response surface optimisation to the modelling of astaxanthin extraction from a novel strain of Thraustochytrium sp.S7.Crossref | GoogleScholarGoogle Scholar |
[18] Byreddy, A.B. et al. (2017) Tween-80 influences the production of intracellular lipase by Schizochytrium S31 in a stirred tank reactor. Process Biochem. 53, 30–35.
| Tween-80 influences the production of intracellular lipase by Schizochytrium S31 in a stirred tank reactor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XitVOltrbP&md5=d4d39e3b19b9d8c3390fa1b28feff4ddCAS |
[19] Lee Chang, K.J. et al. (2015) Life cycle assessment: heterotrophic cultivation of thraustochytrids for biodiesel production. J. Appl. Phycol. 27, 639–647.
| Life cycle assessment: heterotrophic cultivation of thraustochytrids for biodiesel production.Crossref | GoogleScholarGoogle Scholar |
[20] Thyagarajan, T. et al. (2014) Evaluation of bread crumbs as a potential carbon source for the growth of thruasctochytrid species for oil and omega-3 production. Nutrients 6, 2104–2114.
| Evaluation of bread crumbs as a potential carbon source for the growth of thruasctochytrid species for oil and omega-3 production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1Gjsr3K&md5=65cee5b44432ddaf5fb0cf5bd25dcca4CAS |
[21] Abraham, R.E. et al. (2014) Suitability of magnetic nanoparticles immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol. Biofuels 7, 90.
| Suitability of magnetic nanoparticles immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass.Crossref | GoogleScholarGoogle Scholar |
[22] Pleissner, D. et al. (2013) Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour. Technol. 137, 139–146.
| Food waste as nutrient source in heterotrophic microalgae cultivation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmvV2kt7Y%3D&md5=aeac6e7bfd4acd465e204d8e416487d9CAS |
Biography
Dr Munish Puri is an Associate Professor of Bioprocessing and serves as Deputy Director, Centre for Marine Bioproducts Development (CMBD), Medical Biotechnology, School of Medicine at Flinders University. He leads the microalgal biotechnology program. He has recently moved to Flinders after establishing a research program in industrial biotechnology and bioprocessing at Deakin University, Australia. Professor Puri’s research interests include microbial screening for bioactives production, extracting nutraceuticals for human health and food biotechnology applications. His group employs nanobiotechnology approaches for enhancing enzyme stability with respect to food processing and biofuel applications.


