Common pathogens found in yellowtail kingfish Seriola lalandi during aquaculture in Australia
Fran J StephensFish Health Unit
Department of Fisheries
3 Baron-Hay Court
South Perth, WA 6151, Australia
Tel: +61 8 9368 3335
Email: fran.stephens@agric.wa.gov.au
Microbiology Australia 37(3) 132-134 https://doi.org/10.1071/MA16045
Published: 11 August 2016
Yellowtail kingfish aquaculture in sea-cages is an emerging industry in Australia. Monogenean, myxozoan and bacterial pathogens sometimes cause health issues that require diagnosis or monitoring.
Yellowtail kingfish Seriola lalandi aquaculture in Australia is in its infancy with just one commercial farm in South Australia at the present time. Currently there is also interest in sea cage aquaculture of this species in Western Australia and New South Wales. The natural range of this species is from Queensland right around the south of the mainland and as far as the mid-west of Western Australia. The fish is able to spawn and grow in captivity, is very fast growing and is a firm white fleshed fish which is especially popular for sashimi.
There have been several mortality events during aquaculture of this species. The fish are generally quite robust when held in tanks on land but once they are exposed to the extra stressors associated with sea cage culture they are more likely to have periods of reduced growth and spikes of mortality. The cause of these events can be difficult to identify and are often suspected of being multifactorial in nature with various stress factors being identified and opportunistic pathogens in some but not all of the fish. Bacteria such as Vibrio harveyi, V. alginolyticus, Photobacterium damselae subspecies damselae or a P. damselae subspecies piscicida-like bacterium are sometimes isolated from the spleen and kidney of moribund fish. At times the bacteria appear to have caused a chronic hepatitis, cholangitis and pancreatitis from an ascending infection from the intestine. At other times the pseudotubercular lesions, reported in P. damselae subspecies piscicida infections overseas1, can be seen grossly in yellowtail kingfish in Australia. A deficiency in taurine due to feeding of incompletely formulated, manufactured fish pellets is one factor shown to predispose fish to ill thrift and subsequent infections resulting in mortalities. Other possible stress factors which may lead to immunosuppression in the fish could include low dissolved oxygen events, the presence of predators such as sharks, birds or seals around the cage and extremes of water temperatures outside the normal expected range for the species.
Monogenean parasites can have a severe detrimental impact on yellowtail kingfish in sea cages and continual monitoring of the fish to determine the intensity of infestation with these parasites is required. The main skin parasites of concern are the capsalid monopisthocotyleans Neobenedenia sp. (pers. comm., ID Whittington, South Australian Museum) (Figure 1) and Benedenia seriolae2. These are both tissue grazing parasites that are extremely irritating to the fish and fish become inappetant when parasite numbers are high. The blood feeding polyopisthocotylean parasite Zeuxapta seriolae2 (Figure 2) occurs on gills and can cause severe anaemia and death if infestations are not managed appropriately.
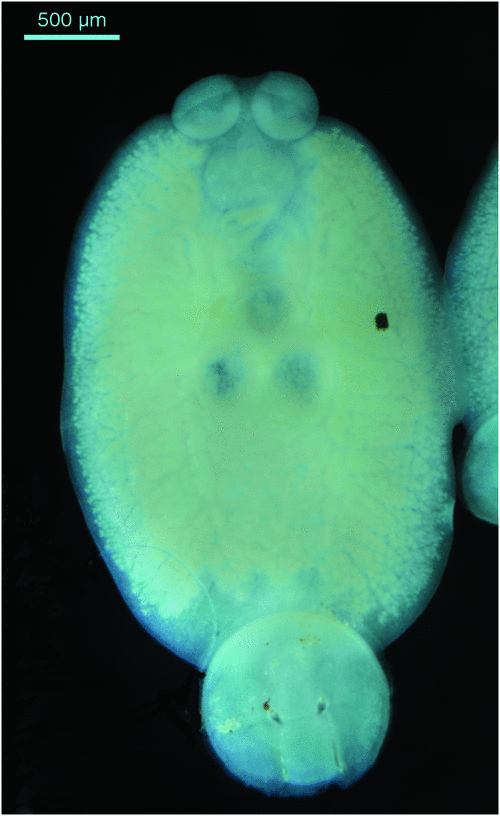
|
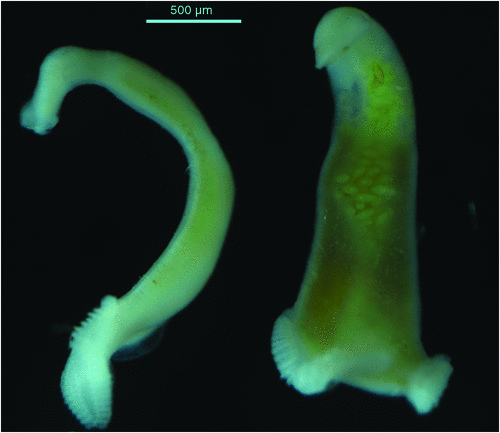
|
Fish in sea cages become infected from wild fish in the waters around the sea cage. Most of the monogenean parasites infecting yellowtail kingfish are hermaphrodites that have a direct life-cycle and tanned eggs that are resistant to chemical treatments. The eggs may have a filament that becomes entangled on structures around the cage such as the net and moorings. Individual parasites can produce large numbers of eggs daily when water temperatures are suitable. Zeuxapta seriolae is particularly fecund2. One management strategy is for nets to be regularly cleaned or changed to reduce the number of monogenean eggs present in the sea cage environment. This also removes fouling organisms that reduce water flow through the cage. Parasite infection intensity is monitored by sampling live fish from the sea cage and placing the fish in a diluted praziquantal treatment bath. The parasites detach from the fish and can be counted before the fish is returned to the cage. Chemotherapy using hydrogen peroxide or paraziquantal baths is labour intensive as the sea cage must first be surrounded by an impervious tarpaulin. Then the oxygen in the bath water must be monitored and the static water aerated to ensure the fish have access to sufficient oxygen. The treatment dose must be calculated accurately and then well dispersed through the bath water. Timing is critical in these bathing processes and must be aligned with the lifecycle of the monogenean being treated.
A number of tissue dwelling myxozoan parasites have been observed infecting yellowtail kingfish that are confined in sea cages. Of these, Unicapsula seriolae (Figure 3) has the greatest potential to impact the industry due to the parasites’ ability to cause unacceptably soft or liquefied flesh when high numbers of spores are present in the skeletal muscle3. It was first identified in wild fish in Queensland and also occurs in Western Australia4. It can infect yellowtail kingfish in sea cages that are in relatively shallow water close to the probable habitat of its intermediate host5. At the present time it does not appear to be a major impediment to the aquaculture of yellowtail kingfish. Unicapsula seriolae has not been identified as a problem in South Australia6.
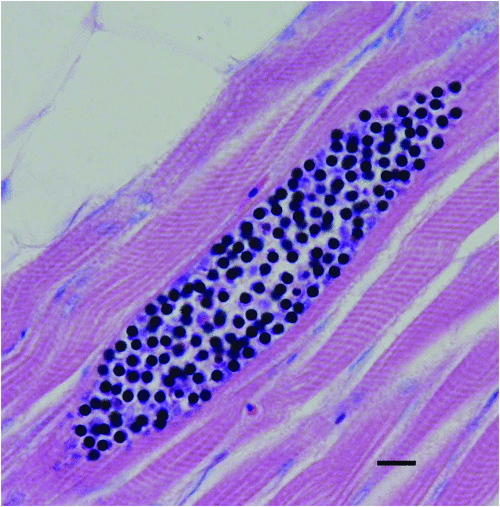
|
Several other myxozoan parasites have been seen in aquaculture operations but their presence has not been definitively linked with major morbidity or production loss in Australia. In one locality in Western Australia Kudoa neurophila was highly prevalent in the brains of yellowtail kingfish5,7. These fish had been spawned and reared in a nearby hatchery that used water pumped from the adjacent harbour where their likely invertebrate intermediate hosts (e.g. polychaete worms) were abundant. It is suspected that the fish became infected in the hatchery but the presence of infection did not present with pseudocysts in the brain until after the fish were stocked into sea cages. This parasite has not been seen in other hatcheries or yellowtail kingfish in aquaculture in Western Australia but did occur in striped trumpeter in a hatchery in Tasmania. The incoming water at that hatchery was infected with the infective stage of the parasite8.
Other potential pathogens include myxozoans in the epicardium and in the lumen of kidney tubules in some fish. The Kudoa species in the heart is similar to K. pericardialis that occurs in Japan9. Uncharacterised Apicomplexan parasites have also been seen in the intestines of fish with ill thrift. Blood flukes of the Aporocotylidae were identified as a potential risk factor for yellowtail kingfish aquaculture in Australia6 and have occurred in sea cage aquaculture in South Australia.
In summary the Monogenea have been the most troublesome pathogens of yellowtail kingfish culture in Australia. Their management requires regular monitoring of the intensity of infestation together with time consuming and logistically demanding therapeutic bathing at strategic times. Other pathogens cause sporadic problems that require management on a case by case basis.
References
[1] Avci, H. et al. (2013) Comparative histopathological and immunohistochemical evaluations in juvenile sea bass (Dicentrrarhus labrax) and gilthead sea bream (Sparus aurata) naturally infected with Photobacterium damselae subsp. piscicida. Revue Méd. Vét. 164, 72–79.[2] Tubbs, L.A. et al. (2005) Effects of temperature on fecundity in vitro, egg hatching and reproductive development of Benedenia seriolae and Zeuxapta seriolae (Monogenea) parasitic on yellowtail kingfish Seriola lalandi. Int. J. Parasitol. 35, 315–327.
| Effects of temperature on fecundity in vitro, egg hatching and reproductive development of Benedenia seriolae and Zeuxapta seriolae (Monogenea) parasitic on yellowtail kingfish Seriola lalandi.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2M%2FptFynuw%3D%3D&md5=41ff0941d8716c3aa3e76220bb890ec8CAS | 15722083PubMed |
[3] Lester, R.J.G. (1982) Unicapsula seriolae n. sp. (Myxosporea, Multivalvulida) from Australian Yellowtail Kingfish Seriola lalandi. Protozool 29, 584–587.
| Unicapsula seriolae n. sp. (Myxosporea, Multivalvulida) from Australian Yellowtail Kingfish Seriola lalandi.Crossref | GoogleScholarGoogle Scholar |
[4] Miller, T.L. and Adlard, R.D. (2013) Unicapsula species (Myxosporea: Trilosporidae) of Australian marine fishes including the description of Unicapsula andersenae n. sp. in five teleost families off Queensland. Parasitol. Res. 112, 2945–2957.
| Unicapsula species (Myxosporea: Trilosporidae) of Australian marine fishes including the description of Unicapsula andersenae n. sp. in five teleost families off Queensland.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sjmvVyhtQ%3D%3D&md5=a44c7e0a24aa6ece76587d16cce7ccc5CAS | 23812600PubMed |
[5] Stephens, F.J. and Savage, A. (2010) Two mortality events in sea-caged yellowtail kingfish Seriola lalandi Valenciennes, 1833 (Nannopercidae) from Western Australia. Aust. Vet. J. 88, 414–416.
| Two mortality events in sea-caged yellowtail kingfish Seriola lalandi Valenciennes, 1833 (Nannopercidae) from Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cfjs1Oqsw%3D%3D&md5=bb92431d5b904c11bc61604b21c9e76cCAS | 20854300PubMed |
[6] Hutson, K.S. et al. (2007) Risk assessment for metazoan parasites of yellowtail kingfish Seriola lalandi (Perciformes: Carangidae) in South Australian sea-cage aquaculture. Aquaculture 271, 85–99.
| Risk assessment for metazoan parasites of yellowtail kingfish Seriola lalandi (Perciformes: Carangidae) in South Australian sea-cage aquaculture.Crossref | GoogleScholarGoogle Scholar |
[7] Burger, M.A.A. and Adlard, R.D. (2010) Phenotypic variation in a significant spore character in Kudoa (Myxosporea: Multivalvulida) species infecting brain tissue. Parasitology 137, 1759–1772.
| Phenotypic variation in a significant spore character in Kudoa (Myxosporea: Multivalvulida) species infecting brain tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Gjs7%2FP&md5=838ba0c3a8a911711ed7e8d23ad0ffb9CAS |
[8] Grossel, G. (2005) Kudoa neurophila in striped trumpeter: identification, diagnostic development and histopathology. PhD thesis, University or Tasmania. pp. 137.
[9] Egusa, S. (1983) Disease problems in Japanese yellowtail, Seriola quinqueradiata, culture: a review. Rapp. P.-V. Reun.- Cons. Int. Explor. Mer 182, 10–18.
Biography
Fran Stephens is a Fish Pathologist at the Fish Health Unit of the Department of Fisheries in Western Australia. She has an interest in diseases of aquatic animals and the management of aquaculture production units.


