Animal models of human cytomegalovirus congenital infection
Helen FarrellA School of Chemistry and Molecular Biology
University of Queensland
St Lucia, Qld 4067, Australia
B Centre for Children’s Health Research
University of Queensland
South Brisbane, Qld 4101, Australia
Email: h.farrell1@uq.edu.au
Microbiology Australia 36(4) 196-199 https://doi.org/10.1071/MA15068
Published: 20 October 2015
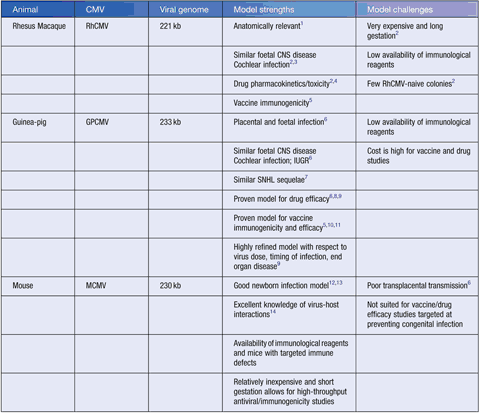
Human cytomegalovirus (HCMV) infection is highly species-specific, which means that it is unable to productively infect laboratory animals. Despite this caveat, studies of animal CMV counterparts in their natural hosts have revealed significant correlations with observed neuropathological effects of congenital HCMV infection and have improved our understanding of host responses to vaccination. The biological relatedness between human and animal CMVs has been confirmed by phylogenetic analyses; the conservation of ‘core’ genes that are essential for virus replication as well as genes that contribute similar mechanisms for virus persistence in their respective host species. The common animal models of HCMV congenital infection include Rhesus CMV (RhCMV), guinea-pig CMV (GPCMV) and mouse CMV (MCMV). Whilst animal models of CMV do not fully recapitulate HCMV infection, they each offer specific advantages in understanding HCMV congenital/perinatal infection (summarised in Table 1).
|
Transplacental transmission and neonatal infections
The placentae of the guinea-pig and the rhesus macaque are structurally similar to the human placenta1. Experimental infections with RhCMV and GPCMV result in foetal infection, with clinical manifestations that include CNS involvement and (for GPCMV) sensorineural hearing loss (SNHL)2,6,7. Systemic maternal infection causes syndromes deleterious for the developing foetus, (e.g. intrauterine growth restriction) with the incidence of foetal morbidity and mortality being highest when transmission occurs during early gestation2,3. These key pathological features are similar to congenital HCMV infection15.
Despite poor transplacental transmission of MCMV in the laboratory setting, direct injection into the foetus or the newborn pup has been shown to mimic HCMV-induced congenital disease12,13. Similar to RhCMV, the susceptibility of neuronal stem cells to MCMV infection is maturation stage-dependent, with a rapid resistance to infection of the brain developing after birth16. MCMV also infects the auditory nerve spiral ganglion and cochlea of newborn pups with measurable cytopathic effects and neuronal loss, and thus offers an amenable model for studying viral and host factors that contribute to SNHL17.
Evaluation of antiviral therapies to ameliorate effects of congenital infection
Current antiviral therapies for HCMV target the viral replicative machinery (e.g. ganciclovir, valganciclovir, foscarnet and cidifivor)18,19. However, due to their toxicity, none are licenced for use during pregnancy and only ganciclovir/valganciclovir (that target the HCMV-encoded UL97 kinase) are administered to symptomatic HCMV-infected newborns at high risk of SNHL18,20,21. Despite similarities in the pharmacokinetics between humans and rhesus macaques, and similar sensitivities to HCMV antivirals, assessments of drug efficacies have not been performed due to the expense of this model2,4. By comparison, GPCMV model has proved a highly valuable model to evaluate the action of hexadecyloxypropyl-cidofovir (brincidofovir or CMX001) in the reduction of foetal morbidity, virus load and the manifestation of SNHL8. Although GPCMV is resistant to ganciclovir, the generation of GPCMV/HCMV UL97 chimaeric viruses will enable future antiviral testing of ganciclovir in vivo9. Poor placental transmission by MCMV precludes the evaluation of antivirals on foetal infection. Nevertheless, the newborn infection model offers excellent prospects for rapid screening of novel drugs on CNS infection and disease, including SNHL. Maribavir, an inhibitor of the UL97-kinase, has become a promising alternative to ganciclovir due to its reduced toxicity: its efficacy in ameliorating SNHL in newborns awaits testing in animal model systems19.
Vaccination studies
Because maternal immunity reduces the severity of congenital CMV disease, the development of a vaccine is a high priority22. The GPCMV model has been used extensively for evaluating vaccine efficacy, by virtue of the ability to quantify the maternal immune responses, virus loads, as well as developmental sequelae10. Several GPCMV vaccines (live attenuated, subunit and DNA) administered before conception, have been evaluated5,11. Whilst sterilising immunity to any vaccination program has not been demonstrated, the model has informed HCMV vaccination strategies with respect to choice of the immunogen and adjuvant as well as identifying diagnostic correlates of foetal protection, such as the magnitude of the maternal neutralising antibody response to vaccination and reduction in maternal viraemia5,6,11,23. The use of vectored approaches for the delivery of subunit GPCMV vaccines that provide both cellular and humoral immunity also significantly reduce the incidence of congenital GPCMV infection5,24. Notably, transplacental transmission of GPCMV has been observed in dams that had been previously infected, a feature in common with the evidence of symptomatic HCMV congenital infections resulting from maternal re-infections during pregnancy25.
To date, there have been few RhCMV vaccination studies, confined to immunogenicity, rather than efficacy studies. This is due in part by the paucity of seronegative colonies. Nevertheless, the RhCMV model has been useful to identify optimal vaccination regimes and immunogens that elicit strong cellular and humoral immune responses, using heterologous DNA prime-protein boost approaches5. Notably, recent studies of RhCMV have uncovered novel, diverse and highly promiscuous CD8+ T-cell repertoires from macaques immunised with a live RhCMV vaccine deleted of HCMV counterparts responsible for cell tropism. The results have implications for the use of HCMV deletion mutants in directing the CD8+ T-cell repertoire and for their use as a vector for delivery of other immunogens26.
The MCMV model has been instrumental in our understanding of mechanisms of innate and adaptive mechanisms of host resistance to infection14. The availability of immunological reagents has facilitated the characterisation of both humoral and cell-mediated responses to MCMV using live attenuated, subunit, DNA and vectored vaccines27,28. Although poor transplacental transmission precludes laboratory studies of vaccine efficacy, there is potential for the MCMV model to measure the potency of maternal immunity from vaccinated dams in restricting perinatal infection of newborn pups.
Other animal models
The potentials of rat CMV (RCMV) or porcine CMV (PCMV) as models of HCMV congenital infection have not been explored. PCMV is of interest because natural maternal infection results in transplacental transmission and thus it may provide an authentic evaluation of vaccination efficacy29. There has been a recent report of placental infection of rats with a novel RCMV30. If further studies demonstrate foetal infection, then this model would provide a highly amenable approach to testing intervention strategies.
Future Perspectives
Several advances are facilitating refinement of the above animal models for congenital HCMV:
-
The implementation of chimaeric CMVs that express authentic HCMV immune/drug targets and ‘humanised’ animal models that dissect protective immune responses9,31;
-
The exploitation of viral ‘immune evasion’ proteins either as targets for the immune response or their deletion in live attenuated viral vaccines32,33;
-
The implementation of live imaging technologies to track virus dissemination in vivo9;
-
The identification of viral and cellular determinants that dictate HCMV species-specificity may allow future cross-species studies of HCMV infection5.
References
[1] Carter, A.M. (2007) Animal models of human placentation–a review. Placenta 28, S41–S47.| Animal models of human placentation–a review.Crossref | GoogleScholarGoogle Scholar | 17196252PubMed |
[2] Powers, C. and Früh, K. (2008) Rhesus CMV: an emerging animal model for human CMV. Med. Microbiol. Immunol. (Berl.) 197, 109–115.
| Rhesus CMV: an emerging animal model for human CMV.Crossref | GoogleScholarGoogle Scholar |
[3] Tarantal, A.F. et al. (1998) Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macaca mulatta) J. Infect. Dis. 177, 446–450.
| Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macaca mulatta)Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7is1Sisg%3D%3D&md5=5dbe9f0a73fd6e5e4809801cd73de7a3CAS | 9466534PubMed |
[4] North, T.W. et al. (2004) Rhesus cytomegalovirus is similar to human cytomegalovirus in susceptibility to benzimidazole nucleosides. Antimicrob. Agents Chemother. 48, 2760–2765.
| Rhesus cytomegalovirus is similar to human cytomegalovirus in susceptibility to benzimidazole nucleosides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsFWmu7c%3D&md5=bf9b413d9833553189d4e7ef440eb4a5CAS | 15215146PubMed |
[5] Schleiss, M.R. (2013) Developing a vaccine against congenital cytomegalovirus (CMV) Infection: what have we learned from animal models? Where should we go next? Future Virol. 8, 1161–1182.
| Developing a vaccine against congenital cytomegalovirus (CMV) Infection: what have we learned from animal models? Where should we go next?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVGktbbN&md5=ac7432b9f2c4aa6090431641d786d2f3CAS | 24523827PubMed |
[6] Schleiss, M.R. (2006) Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns. ILAR J. 47, 65–72.
| Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht12nu7nM&md5=b24e44bda0c94447520485353b02309cCAS | 16391432PubMed |
[7] Park, A.H. et al. (2010) Development of cytomegalovirus-mediated sensorineural hearing loss in a Guinea pig model. Arch. Otolaryngol. Head Neck Surg. 136, 48–53.
| Development of cytomegalovirus-mediated sensorineural hearing loss in a Guinea pig model.Crossref | GoogleScholarGoogle Scholar | 20083778PubMed |
[8] Bravo, F.J. et al. (2011) Oral hexadecyloxypropyl-cidofovir therapy in pregnant guinea pigs improves outcome in the congenital model of cytomegalovirus infection. Antimicrob. Agents Chemother. 55, 35–41.
| Oral hexadecyloxypropyl-cidofovir therapy in pregnant guinea pigs improves outcome in the congenital model of cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisVehtb8%3D&md5=33d0c15837ba082f22b81035a2a1fbaaCAS | 21078944PubMed |
[9] McGregor, A. and Choi, K.Y. (2011) Cytomegalovirus antivirals and development of improved animal models. Expert Opin. Drug Metab. Toxicol. 7, 1245–1265.
| Cytomegalovirus antivirals and development of improved animal models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtF2jsLzL&md5=0c84f981f311af5bb40fd584b26e61c8CAS | 21883024PubMed |
[10] Schleiss, M.R. and McVoy, M.A. (2010) Guinea Pig Cytomegalovirus (GPCMV): A model for the study of the prevention and treatment of maternal-fetal transmission. Future Virol. 5, 207–217.
| Guinea Pig Cytomegalovirus (GPCMV): A model for the study of the prevention and treatment of maternal-fetal transmission.Crossref | GoogleScholarGoogle Scholar | 23308078PubMed |
[11] Schleiss, M.R. et al. (2015) Vaccination with a live attenuated cytomegalovirus devoid of a protein kinase R inhibitory gene results in reduced maternal viremia and improved pregnancy outcome in a guinea pig congenital infection model. J. Virol. 89, 9727–9738.
| Vaccination with a live attenuated cytomegalovirus devoid of a protein kinase R inhibitory gene results in reduced maternal viremia and improved pregnancy outcome in a guinea pig congenital infection model.Crossref | GoogleScholarGoogle Scholar | 26178990PubMed |
[12] Tsutsui, Y. et al. (2005) Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev. Med. Virol. 15, 327–345.
| Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFaktrfI&md5=4de884464d1c29d25e586fbb5648bf24CAS | 16100703PubMed |
[13] Schachtele, S.J. et al. (2011) Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J. Neurovirol. 17, 201–211.
| Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice.Crossref | GoogleScholarGoogle Scholar | 21416394PubMed |
[14] Scalzo, A.A. et al. (2007) The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol. Cell Biol. 85, 46–54.
| The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhslOgsbY%3D&md5=63217169ca6ea58c3491437061123effCAS | 17146464PubMed |
[15] Cheeran, M.C. et al. (2009) Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin. Microbiol. Rev. 22, 99–126.
| Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnslKrs7s%3D&md5=b0931df02bd99fd303c775a0d8330596CAS | 19136436PubMed |
[16] Shinmura, Y. et al. (1997) Differential expression of the immediate-early and early antigens in neuronal and glial cells of developing mouse brains infected with murine cytomegalovirus. Am. J. Pathol. 151, 1331–1340.
| 1:CAS:528:DyaK2sXntlOksbc%3D&md5=f7d94a34d324c292a6f2962e3fa78facCAS | 9358759PubMed |
[17] Schraff, S.A. et al. (2007) The role of CMV inflammatory genes in hearing loss. Otol. Neurotol. 28, 964–969.
| 17558342PubMed |
[18] Nassetta, L. et al. (2009) Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J. Antimicrob. Chemother. 63, 862–867.
| Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVejsrs%3D&md5=b3d10dc60642dde056032a713232cd55CAS | 19287011PubMed |
[19] Griffiths, P. and Lumley, S. (2014) Cytomegalovirus. Curr. Opin. Infect. Dis. 27, 554–559.
| Cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvVKrsLvJ&md5=72b4cf5fa11717d74003a45d7b39caf5CAS | 25304390PubMed |
[20] Kimberlin, D.W. et al. (2003) Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J. Pediatr. 143, 16–25.
| Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt1egsLo%3D&md5=53b44f54a2fce6bf20a2ccb9d1f89227CAS | 12915819PubMed |
[21] Meine Jansen, C.F. et al. (2005) Treatment of symptomatic congenital cytomegalovirus infection with valganciclovir. J. Perinat. Med. 33, 364–366.
| 16211780PubMed |
[22] Arvin, A.M. et al. (2004) Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39, 233–239.
| Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee.Crossref | GoogleScholarGoogle Scholar | 15307033PubMed |
[23] Pass, R.F. et al. (2009) Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 360, 1191–1199.
| Vaccine prevention of maternal cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtlersbk%3D&md5=37fbcd143155580003a2aef0c548f566CAS | 19297572PubMed |
[24] Hashimoto, K. et al. (2013) Effects of immunization of pregnant guinea pigs with guinea pig cytomegalovirus glycoprotein B on viral spread in the placenta. Vaccine 31, 3199–3205.
| Effects of immunization of pregnant guinea pigs with guinea pig cytomegalovirus glycoprotein B on viral spread in the placenta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXosVOgt7o%3D&md5=d22d529a38ce6e9f38bbf6e2a4f2119bCAS | 23684839PubMed |
[25] Boppana, S.B. et al. (1999) Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104, 55–60.
| Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MzhvV2ntg%3D%3D&md5=39d9b8e571729aa73cb92d438f8b31fbCAS | 10390260PubMed |
[26] Hansen, S.G. et al. (2013) Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340, 1237874.
| Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms.Crossref | GoogleScholarGoogle Scholar | 23704576PubMed |
[27] Morello, C.S. et al. (2005) Systemic priming-boosting immunization with a trivalent plasmid DNA and inactivated murine cytomegalovirus (MCMV) vaccine provides long-term protection against viral replication following systemic or mucosal MCMV challenge. J. Virol. 79, 159–175.
| Systemic priming-boosting immunization with a trivalent plasmid DNA and inactivated murine cytomegalovirus (MCMV) vaccine provides long-term protection against viral replication following systemic or mucosal MCMV challenge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhslemtg%3D%3D&md5=73801de4f4bc3d894bd612a88d6d5c6cCAS | 15596812PubMed |
[28] Liu, G. et al. (2014) Protective MCMV immunity by vaccination of the salivary gland via Wharton’s protection similar to that of MCMV. FASEB J. 28, 1698–1710.
| Protective MCMV immunity by vaccination of the salivary gland via Wharton’s protection similar to that of MCMV.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmtVGrs7c%3D&md5=7d92a7405ba31ce783ded5fbb12c6453CAS | 24391133PubMed |
[29] Edington, N. et al. (1988) Porcine cytomegalovirus (PCMV) in early gestation. Vet. Microbiol. 17, 117–128.
| Porcine cytomegalovirus (PCMV) in early gestation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M%2FitVOgsg%3D%3D&md5=b5455284609c30428a624e951508dad4CAS | 2845635PubMed |
[30] Loh, H.S. et al. (2003) Characterization of a novel rat cytomegalovirus (RCMV) infecting placenta-uterus of Rattus rattus diardii. Arch. Virol. 148, 2353–2367.
| Characterization of a novel rat cytomegalovirus (RCMV) infecting placenta-uterus of Rattus rattus diardii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptleksr4%3D&md5=510accb05db7e22ac0af265a615d0d32CAS | 14648291PubMed |
[31] Zhong, J. et al. (2008) Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus. PLoS One 3, e3256.
| Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 18806877PubMed |
[32] Crumpler, M.M. (2009) A live guinea pig cytomegalovirus vaccine deleted of three putative immune evasion genes is highly attenuated but remains immunogenic in a vaccine/challenge model of congenital cytomegalovirus infection. Vaccine 27, 4209–4218.
| A live guinea pig cytomegalovirus vaccine deleted of three putative immune evasion genes is highly attenuated but remains immunogenic in a vaccine/challenge model of congenital cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvFGgtL4%3D&md5=c2a54fb9f0fe04fe9bcb0a5fc4a0f7a8CAS | 19389443PubMed |
[33] Eberhardt, M.K. et al. (2013) Vaccination against a virus-encoded cytokine significantly restricts viral challenge. J. Virol. 87, 11323–11331.
| Vaccination against a virus-encoded cytokine significantly restricts viral challenge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1KjtL3M&md5=203b3ee0cbfefeffaee2a118030412a1CAS | 23946461PubMed |
Biography
Helen Farrell completed her PhD at the University of Western Australia under the mentorship of Geoff Shellam in 1989, exploring virus-host relationships using the mouse CMV model. She has continued to identify and characterise herpesvirus determinants of pathogenesis and persistence during her postdoctoral career in the UK and in Australia. She is currently a Senior Research Officer at the School of Chemistry and Molecular Biology and the Centre for Children’s Health Research at the University of Queensland.


