Therapeutics to prevent congenital cytomegalovirus during pregnancy: what is available now and in the future?
Stuart T Hamilton A B , Corina Hutterer C and Manfred Marschall CA Virology Division, SEALS Microbiology, Level 3, Clinical Sciences Building, Prince of Wales Hospital, Sydney, NSW 2031, Australia
Tel: +61 2 9382 9096
Email: stuart.hamilton@sesiahs.health.nsw.gov.au
B School of Biotechnology and Biomolecular Sciences, University of New South Wales, Sydney, NSW, Australia
C Institute for Clinical and Molecular Virology, Friedrich-Alexander University of Erlangen-Nürnberg, Germany
Microbiology Australia 36(4) 156-161 https://doi.org/10.1071/MA15057
Published: 20 October 2015
Human cytomegalovirus (CMV) is the leading non-genetic cause of fetal malformation in developed countries. Congenital CMV infection can cause serious clinical sequelae, and in severe cases result in fetal or neonatal death. Despite the clinical and social importance of congenital CMV there is currently no standardised management strategy to prevent or treat maternal/fetal CMV infection during pregnancy and no evidence-based therapeutic for prenatally diagnosed CMV infection or disease. For pregnant women with a primary CMV infection during pregnancy, standard medical practise remains to offer no treatment at all or the option to terminate pregnancy. If intervention is requested, pregnant women may be offered a narrow range of medical therapies with limited evidence for efficacy and some with high risks of toxicity. However, there are several experimental and novel anti-CMV therapeutics currently being investigated that may provide a safe and effective therapeutic for use during pregnancy to prevent both fetal infection and reduce the risk of congenital CMV disease developing in the fetus once infected in utero.
Established anti-CMV therapeutics
Valaciclovir
To date, the only established CMV antiviral to undergo investigations during pregnancy is valaciclovir, the prodrug of the nucleoside analogue aciclovir. The converted active form of acyclovir is incorporated into the replicating viral DNA causing premature chain termination. Valaciclovir is used as a CMV prophylaxis in organ transplant recipients1. A pilot observational study showed maternal treatment with oral valaciclovir to treat confirmed cases of fetal CMV infection was well tolerated and decreased viral load in fetal blood2. Furthermore, treatment resulted in a modest decrease in adverse fetal outcomes (52% of 21 infants) compared with the untreated group (58% of 24 infants). These results led to a French multicentre, nonrandomised, single group assignment, phase IV clinical trial evaluating the efficacy of valaciclovir in the treatment of confirmed fetal CMV infection, which has recently completed3. Results demonstrated a high safety profile with no liver or renal toxicity observed and met the primary endpoint in reducing the number of symptomatic children at birth and number of terminations of pregnancy for fetal anomalies using the Optimal Two-Stage Simon’ design. The outcome of valaciclovir treatment was 34/43 (79%) asymptomatic neonates, 2/41 (5%) terminations of pregnancy and 7/43 (16%) symptomatic neonates. Due to the toxicity associated with the other established CMV antivirals (ganciclovir, valganciclovir, cidofovir and foscarnet) it is unlikely these will ever be investigated in any clinical trials involving pregnant women.
Hyperimmune globulin (HIG)
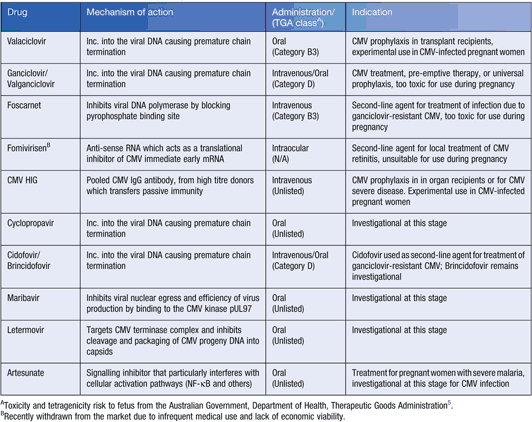
Immunoglobulin therapy has been used safely during pregnancy for passive immunisation against a wide variety of viruses including; cytomegalovirus, rubella, varicella, measles and hepatitis A and B. To date, there have been several clinical trials, observational studies and case series reports on the prophylactic and therapeutic use of HIG during pregnancy, which has been recently reviewed in detail4. While all the various studies on using HIG to prevent or treat congenital CMV to date have shown a beneficial trend, the lack of data from properly designed randomised clinical trials limits the conclusions that can be drawn and prevents recommendation as a standard therapeutic for congenital CMV during pregnancy. Of all the established and experimental CMV antiviral therapies currently available, only HIG and valaciclovir have been investigated in pregnant women (Table 1).
|
Novel therapies with clinical drug candidates
Brincidofovir
Brincidofovir (or CMX001) is an alkoxyalkyl ester analogue of cidofovir (a nucleoside analogue of cytosine), which acts by competitive inhibition of cysteine incorporation into the viral DNA strand and thus causing premature chain termination. While no data are available for the safety and efficacy of brincidofovir to prevent or treat congenital CMV, several studies in animal guinea-pig models show cidofovir and brincidofovir have potential benefits in preventing CMV transmission during pregnancy6–8. A recent phase II study on the efficacy of brincidofovir to prevent CMV disease in haematopoietic-cell transplant (HSCT) recipients showed promising results9. Patients who received brincidofovir twice weekly had significantly fewer incidence of CMV events than those who received placebo (10% v. 37%; P = 0.002). Brincidofovir has now entered phase III trials in HSCT recipients.
Maribavir
Maribavir is an orally bioavailable benzimidazole antiviral that binds to the CMV-encoded protein kinase pUL97 (a viral orthologue of cellular cyclin-dependent kinases) and inhibits viral nuclear egress and the efficiency of virus production in CMV-infected cells. Despite early phase I and II clinical trials showing promising anti-CMV activity observed10,11, this activity could not be confirmed in two subsequent phase III prophylaxis trials performed in allogeneic HSCT12 and liver transplant recipients13. However, there is evidence that supports increasing the dosage of maribavir may improve efficacy14. Shire are currently conducting two Phase II dose-ranging clinical trials in transplant recipients for first line treatment of CMV viremia and treatment of resistant/refractory CMV. Resistance against maribavir has been reported at times, but the clinical significance has still to be investigated in the ongoing Phase II studies. Interestingly, the residues subject to resistance mutation within the pUL97 kinase are distinct from those of ganciclovir/valganciclovir resistance, although all contained within the kinase domain (Figure 1). Notably, other pUL97 inhibitors, presently under intense investigation at the experimental level, do not show detectable resistance formation15,16.
Letermovir
Letermovir (AIC246) represents a new class of non-nucleoside CMV inhibitors known as the 3,4-dihydro-quinazolines. Letermovir acts by targeting the CMV terminase complex and thus interfering with viral DNA concatemer maturation and subsequent cleavage and packaging of CMV progeny DNA into capsids. As there is no mammalian counterpart of the viral heterodimeric terminase enzyme, target-related toxicities, which are observed with the current anti-CMV polymerase inhibitors, are not expected17. Furthermore, the novel mode of action should provide new treatment options for resistant variants; however, the putative frequency of viral drug resistance to letermovir has not been addressed in detail18. Letermovir exhibits potent anti-CMV activity in vitro and in vivo and retains high efficacy against resistant variants. Letermovir has shown promising anti-CMV activity in an open-label, proof-of-concept phase IIa trial involving kidney/pancreas transplant recipients with CMV viremia17. It also met all primary endpoints as a prophylactic drug in a recent phase IIb clinical trial in HSCT recipients and is currently entering phase III trials.
Artesunate
Another novel approach focuses on CMV inhibitors derived from natural resources or semi-synthetic derivatives like the antimalaria drug artesunate. Artesunate is derived from arteminisin, a natural product from the Chinese herb Artemisia annua. Meta-analyses of malaria patients treated with artemisinins demonstrated the safety of this class of drugs19–21. For pregnant women with severe malaria, artemisinins are recommended by the World Health Organization as first-line therapy during the second and third trimester whereas less certainty about the safety is given in the first trimester22. In addition to antimalaria activities, artesunate possesses a strong and broad antiviral activity and is particularly efficacious against CMV23–27. Based on promising data obtained in vitro and from an experimental animal model28, a small number of clinical antiviral investigations in transplant recipients have been conducted so far. In some cases, treatment of patients with standard drug-resistant CMV led to an efficient control of infection29,30, whereas other case reports showed an unsatisfying outcome with poor benefit or complete treatment failure31,32. A recent investigation using an ex vivo model of first trimester placental CMV infection showed a reduction of infection by 40% in the presence of artesunate33. Although the mode of action is not fully elucidated, the targeting of artesunate to cellular proteins was demonstrated34–36 and may act as an inhibitor of cellular activation pathways, in particular the NF-κB pathway (C. Hutterer and M. Marschall, unpublished data).
Experimental therapies presently under early development
Kinase inhibitors
Small molecules inhibiting cyclin-dependent kinases (CDKs) or other virus-supportive cellular kinases show promise as a potential tool for host cell-directed antiviral intervention. CDKs are serine/threonine protein kinases characterised by the formation of heterodimeric complexes with cyclins thereby regulating substrate recognition and phosphorylation activity. An obvious advantage of the targeting of CDKs or other cellular kinases over direct antivirals (e.g. viral kinase inhibitor maribavir) may be the reduction, or even complete suppression, of the induction of drug-resistant virus as it is generally difficult to select for resistance to inhibitors that target cellular functions. This may improve the therapeutic quality of a novel drug candidate. The recent identification of a potent selective CDK7 inhibitor with broad anti-herpesviral activity and low cytotoxicity profiles nicely substantiates this concept37 and now awaits proof-of-concept investigations in animal models. Thus, the kinase inhibitor strategy may lead to a novel option for antiviral therapy approaches that already proved to be highly potent in current anticancer therapy.
Inhibitors of viral nuclear egress
In vivo, HCMV production is largely co-regulated by the interaction between viral and cellular proteins and by the formation of virus-host multi-protein complexes. Recently, the viral ‘nuclear egress complex’ (NEC) has attracted the deep interest of researchers, since it represents a regulatory key position of viral replication and a putative target for novel antiviral strategies16,38–41. During the nuclear phase of HCMV replication, viral capsids are packaged and exported to the cytoplasm by transition through the nuclear envelope (nuclear egress) for further virion maturation. Nuclear egress is a multi-step regulatory process that involves a phosphorylation-triggered distortion of the nuclear lamina42–45. Pivotal for nuclear egress is the role of the two viral nuclear egress proteins pUL50 and pUL53 that heterodimerise and form a core for the multimeric viral-cellular NEC46 (Figure 2). For antiviral strategies directed to the NEC as a novel target, two concepts are favoured at present, either (1) the development of small molecules (in silico drug docking analyses combined with high throughput screenings) that sterically interfere with NEC subunit interactions or (2) the identification of kinase inhibitors that potentially inhibit the phosphorylation of NEC components in order to block their ordered core or multimeric association in an indirect manner. First experimental candidates of NEC-inhibiting drugs have already been initially described by several researchers so that an increase of experimental and clinical data in this field is expected for the near future.
Conclusion
In the absence of a CMV vaccine or an evidence-based therapeutic option available to clinicians to prevent or treat fetal CMV infection during pregnancy, further scientific data is urgently needed on the safety and efficacy profiles of experimental and novel antiviral compounds. These investigations are extremely problematic given potential drug toxicities and the fact that CMV tropism and placental physiology are both highly host-specific. We have therefore begun investigating these experimental compounds in our established ex vivo placental explant model systems47 to better inform future directions in clinical trials.
References
[1] Biron, K.K. (2006) Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 71, 154–163.| Antiviral drugs for cytomegalovirus diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XotlGkt7o%3D&md5=22f7242ae769dc8f7726b007f8bbc13dCAS | 16765457PubMed |
[2] Jacquemard, F. et al. (2007) Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG 114, 1113–1121.
| Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFWqtr7I&md5=49007b3d28e5fae58ff6aee9a4d13bb4CAS | 17617198PubMed |
[3] Leruez-Ville, M. et al. (2015) In UTERO treatment of cytomegalovirus congenital infection with valacyclovir (CYMEVAL II) NCT01651585. J. Clin. Virol. 70, S6.
| In UTERO treatment of cytomegalovirus congenital infection with valacyclovir (CYMEVAL II) NCT01651585.Crossref | GoogleScholarGoogle Scholar |
[4] Hamilton, S.T. et al. (2014) Prevention of congenital cytomegalovirus complications by maternal and neonatal treatments: a systematic review. Rev. Med. Virol. 24, 420–433.
| Prevention of congenital cytomegalovirus complications by maternal and neonatal treatments: a systematic review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvVWnsrvF&md5=2bdf687575307ac7bb522f300fae234aCAS | 25316174PubMed |
[5] Australian Government Department of Health, Therapeutic Goods Administration (2015) Prescribing medicines in pregnancy database. www.tga.gov.au/prescribing-medicines-pregnancy-database (accessed 23 September 2015).
[6] Bravo, F.J. et al. (2006) Effect of maternal treatment with cyclic HPMPC in the guinea pig model of congenital cytomegalovirus infection. J. Infect. Dis. 193, 591–597.
| Effect of maternal treatment with cyclic HPMPC in the guinea pig model of congenital cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xhsleqt7w%3D&md5=f3f6eb86454bd8f6a64608de293d6c75CAS | 16425139PubMed |
[7] Schleiss, M.R. et al. (2006) Cyclic cidofovir (cHPMPC) prevents congenital cytomegalovirus infection in a guinea pig model. Virol. J. 3, 9.
| Cyclic cidofovir (cHPMPC) prevents congenital cytomegalovirus infection in a guinea pig model.Crossref | GoogleScholarGoogle Scholar | 16509982PubMed |
[8] Bravo, F.J. et al. (2011) Oral hexadecyloxypropyl-cidofovir therapy in pregnant guinea pigs improves outcome in the congenital model of cytomegalovirus infection. Antimicrob. Agents Chemother. 55, 35–41.
| Oral hexadecyloxypropyl-cidofovir therapy in pregnant guinea pigs improves outcome in the congenital model of cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisVehtb8%3D&md5=33d0c15837ba082f22b81035a2a1fbaaCAS | 21078944PubMed |
[9] Marty, F.M. et al. (2013) CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N. Engl. J. Med. 369, 1227–1236.
| CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFGktLjL&md5=4321f2bee3ad0618162d6577fefa1045CAS | 24066743PubMed |
[10] Wang, L.H. et al. (2003) Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 47, 1334–1342.
| Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXivVSmsr0%3D&md5=b496c2e4bda72d92fde876031a95b19fCAS | 12654667PubMed |
[11] Winston, D.J. et al. (2008) Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood 111, 5403–5410.
| Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFWnsLo%3D&md5=c692788c822ee048d90c10905fa7d641CAS | 18285548PubMed |
[12] Marty, F.M. et al. (2011) Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect. Dis. 11, 284–292.
| Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkt1aksrw%3D&md5=d6c41d3865449a414e138c712b72f089CAS | 21414843PubMed |
[13] Winston, D.J. et al. (2012) Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial. Am. J. Transplant. 12, 3021–3030.
| Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvV2gurjE&md5=65b6420e87df4ed05ecd82b5b88ca0fbCAS | 22947426PubMed |
[14] Avery, R.K. et al. (2010) Oral maribavir for treatment of refractory or resistant cytomegalovirus infections in transplant recipients. Transpl. Infect. Dis. 12, 489–496.
| Oral maribavir for treatment of refractory or resistant cytomegalovirus infections in transplant recipients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1Sqtrg%3D&md5=92a8d8d06765eee1cfd69e7bceff2409CAS | 20682012PubMed |
[15] Steingruber, M. et al. (2015) The interaction between cyclin B1 and cytomegalovirus protein kinase pUL97 is determined by an active kinase domain. Viruses 7, 4582–4601.
| The interaction between cyclin B1 and cytomegalovirus protein kinase pUL97 is determined by an active kinase domain.Crossref | GoogleScholarGoogle Scholar | 26270673PubMed |
[16] Marschall, M. et al. (2011) Regulatory roles of protein kinases in cytomegalovirus replication. Adv. Virus Res. 80, 69–101.
| Regulatory roles of protein kinases in cytomegalovirus replication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1ajsL%2FK&md5=b64e9514f71b4aa8674e5aa6f130d0bfCAS | 21762822PubMed |
[17] Stoelben, S. et al. (2014) Pre-emptive treatment of cytomegalovirus infection in kidney transplant recipients with letermovir: results of a phase 2a study. Transpl. Int. 27, 77–86.
| Pre-emptive treatment of cytomegalovirus infection in kidney transplant recipients with letermovir: results of a phase 2a study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFyrtLvP&md5=8917336584a2ac98a43f887de14a9247CAS | 24164420PubMed |
[18] Goldner, T., Hempel, C. et al. (2014) Geno- and phenotypic characterization of human cytomegalovirus mutants selected in vitro after letermovir (AIC246) exposure. Antimicrob Agents Chemother. , .
| 24752278PubMed |
[19] Adjuik, M. et al. (2004) Artesunate combinations for treatment of malaria: meta-analysis. Lancet 363, 9–17.
| Artesunate combinations for treatment of malaria: meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c%2FisF2mtg%3D%3D&md5=32b59bef4265e30006f2fb0a6af67290CAS | 14723987PubMed |
[20] Aweeka, F.T. and German, P.I. (2008) Clinical pharmacology of artemisinin-based combination therapies. Clin. Pharmacokinet. 47, 91–102.
| Clinical pharmacology of artemisinin-based combination therapies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjs1ejt74%3D&md5=a563fd9da1e2de4296e82e25a14de3a5CAS | 18193915PubMed |
[21] Ribeiro, I.R. and Olliaro, P. (1998) Safety of artemisinin and its derivatives. A review of published and unpublished clinical trials. Medecine tropicale: revue du Corps de sante colonial 58, 50–53.
| 1:STN:280:DyaK1M3ivFelug%3D%3D&md5=d3137e1f0f3710cbfd8643f19e4457abCAS |
[22] Clark, R.L. (2009) Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. Reprod. Toxicol. 28, 285–296.
| Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlWhsLzO&md5=bd71371c959f8c733b540b23ef37447bCAS | 19447170PubMed |
[23] Efferth, T. et al. (2002) Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J. Mol. Med. 80, 233–242.
| Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XksFGmsro%3D&md5=e797de70b82bb99690b94be1c61e05cbCAS | 11976732PubMed |
[24] Cai, H. et al. (2014) In vitro combination of anti-cytomegalovirus compounds acting through different targets: role of the slope parameter and insights into mechanisms of action. Antimicrob. Agents Chemother. 58, 986–994.
| In vitro combination of anti-cytomegalovirus compounds acting through different targets: role of the slope parameter and insights into mechanisms of action.Crossref | GoogleScholarGoogle Scholar | 24277030PubMed |
[25] Flobinus, A. et al. (2014) Stability and antiviral activity against human cytomegalovirus of artemisinin derivatives. J. Antimicrob. Chemother. 69, 34–40.
| Stability and antiviral activity against human cytomegalovirus of artemisinin derivatives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFegtrnP&md5=fbf459939273338cffa355247eb83504CAS | 24003183PubMed |
[26] Chou, S. et al. (2011) The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antiviral Res. 92, 364–368.
| The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlKku7jI&md5=2bedba33d903fd3a5f08a1ca1b65790aCAS | 21843554PubMed |
[27] Efferth, T. et al. (2008) The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 47, 804–811.
| 1:CAS:528:DC%2BD1cXhtFyqurbN&md5=88282d0f218af7e48b2860faff856175CAS | 18699744PubMed |
[28] Kaptein, S.J. et al. (2006) The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antiviral Res. 69, 60–69.
| The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtV2lsbw%3D&md5=2148951e941a45da8516e935fff68a99CAS | 16325931PubMed |
[29] Germi, R. et al. (2014) Success and failure of artesunate treatment in five transplant recipients with disease caused by drug-resistant cytomegalovirus. Antiviral Res. 101, 57–61.
| Success and failure of artesunate treatment in five transplant recipients with disease caused by drug-resistant cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFylurrE&md5=6d158353a72197101943d86771eab006CAS | 24184983PubMed |
[30] Shapira, M.Y. et al. (2008) Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin. Infect. Dis. 46, 1455–1457.
| 1:CAS:528:DC%2BD1cXlvVGnsr4%3D&md5=2b91e23c5a1594ac2ea3201a6eb96f89CAS | 18419454PubMed |
[31] Lau, P.K. et al. (2011) Artesunate is ineffective in controlling valganciclovir-resistant cytomegalovirus infection. Clin. Infect. Dis. 52, 279.
| 21288859PubMed |
[32] Wolf, D.G. et al. (2011) Human cytomegalovirus kinetics following institution of artesunate after hematopoietic stem cell transplantation. Antiviral Res. 90, 183–186.
| Human cytomegalovirus kinetics following institution of artesunate after hematopoietic stem cell transplantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmvFSksbc%3D&md5=e1e367ec4b0bd96d6b673160ea7491daCAS | 21443904PubMed |
[33] Morère, L. et al. (2015) Ex vivo model of congenital cytomegalovirus infection and new combination therapies. Placenta 36, 41–47.
| Ex vivo model of congenital cytomegalovirus infection and new combination therapies.Crossref | GoogleScholarGoogle Scholar | 25479789PubMed |
[34] Bork, P.M. et al. (1997) Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett. 402, 85–90.
| Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXptlSitw%3D%3D&md5=b92b68a925f735436c82df3b0912a2ebCAS | 9013864PubMed |
[35] Siedle, B. et al. (2004) Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-kappaB. J. Med. Chem. 47, 6042–6054.
| Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-kappaB.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXos1Wis7o%3D&md5=f1de4407d7a0562194951f45519590c6CAS | 15537359PubMed |
[36] Souza, M.C. et al. (2012) Artesunate exerts a direct effect on endothelial cell activation and NF-kappaB translocation in a mechanism independent of plasmodium killing. Malar. Res. Treat. 2012, 679090.
| Artesunate exerts a direct effect on endothelial cell activation and NF-kappaB translocation in a mechanism independent of plasmodium killing.Crossref | GoogleScholarGoogle Scholar | 23097741PubMed |
[37] Hutterer, C. et al. (2015) A novel CDK7 inhibitor of the Pyrazolotriazine class exerts broad-spectrum antiviral activity at nanomolar concentrations. Antimicrob. Agents Chemother. 59, 2062–2071.
| A novel CDK7 inhibitor of the Pyrazolotriazine class exerts broad-spectrum antiviral activity at nanomolar concentrations.Crossref | GoogleScholarGoogle Scholar | 25624324PubMed |
[38] Marschall, M. and Stamminger, T. (2009) Molecular targets for antiviral therapy of cytomegalovirus infections. Future Microbiol. 4, 731–742.
| Molecular targets for antiviral therapy of cytomegalovirus infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpslOksLo%3D&md5=c9c48e884f1b2210dd81298f9dcddc7cCAS | 19659428PubMed |
[39] Lee, C.P. and Chen, M.R. (2010) Escape of herpesviruses from the nucleus. Rev. Med. Virol. 20, 214–230.
| Escape of herpesviruses from the nucleus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVegsL7J&md5=a094b0c74c84269e0db99bb28fc49e81CAS | 20069615PubMed |
[40] Tandon, R. and Mocarski, E.S. (2012) Viral and host control of cytomegalovirus maturation. Trends Microbiol. 20, 392–401.
| Viral and host control of cytomegalovirus maturation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xns1Sqtrw%3D&md5=cf12e26b11fa609e116d0c4771d67d75CAS | 22633075PubMed |
[41] Leigh, K.E. et al. (2015) Structure of a herpesvirus nuclear egress complex subunit reveals an interaction groove that is essential for viral replication. Proc. Natl. Acad. Sci. USA 112, 9010–9015.
| Structure of a herpesvirus nuclear egress complex subunit reveals an interaction groove that is essential for viral replication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtFajsr%2FF&md5=1481d0a0fa2d894c6f7bcb0e6b711569CAS | 26150520PubMed |
[42] Muranyi, W. et al. (2002) Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297, 854–857.
| Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlvV2jsbY%3D&md5=e7dacff61c1582c3c8590304484b7795CAS | 12161659PubMed |
[43] Hamirally, S. et al. (2009) Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 5, e1000275.
| Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress.Crossref | GoogleScholarGoogle Scholar | 19165338PubMed |
[44] Milbradt, J. et al. (2010) Novel mode of phosphorylation-triggered reorganization of the nuclear lamina during nuclear egress of human cytomegalovirus. J. Biol. Chem. 285, 13 979–13 989.
| Novel mode of phosphorylation-triggered reorganization of the nuclear lamina during nuclear egress of human cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltFOgtLk%3D&md5=1ec21664da841b19828ffa08a5d2cb36CAS |
[45] Leach, N.R. and Roller, R.J. (2010) Significance of host cell kinases in herpes simplex virus type 1 egress and lamin-associated protein disassembly from the nuclear lamina. Virology 406, 127–137.
| Significance of host cell kinases in herpes simplex virus type 1 egress and lamin-associated protein disassembly from the nuclear lamina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtV2gtbbJ&md5=89b8e849b72f2170eb3137b310e0d736CAS | 20674954PubMed |
[46] Milbradt, J. et al. (2014) Proteomic analysis of the multimeric nuclear egress complex of human cytomegalovirus. Mol. Cell. Proteomics 13, 2132–2146.
| Proteomic analysis of the multimeric nuclear egress complex of human cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht1CksL3N&md5=eb28a8eb7d7b73ebe4b2a2e6edaa3521CAS | 24969177PubMed |
[47] Hamilton, S.T. et al. (2012) Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One 7, e52899.
| Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXptVSnsg%3D%3D&md5=c3f1ee2af9930cb0c81336a5232f2847CAS | 23300810PubMed |
Biographies
Dr Stuart Hamilton is a postdoctoral scientist in the Virology Research Laboratory, SEALS Microbiology at the Prince of Wales Hospital and University of New South Wales. His research is focussed on: (1) understanding transmission modes of intrauterine CMV infection; (2) examining the adverse effects CMV infection has on placental development/function and subsequent pregnancy outcomes; and (3) investigating safety and efficacy profiles of experimental CMV antiviral compounds in human ex vivo placental explant histoculture and cell culture models.
Dr Corina Hutterer is a senior postdoctoral scientist at the Institute for Clinical and Molecular Virology, Friedrich-Alexander University of Erlangen-Nürnberg, with a strong interest in drug development and novel intervention strategies directed against herpesviruses. She completed her PhD in virology at the Institute of Medical Virology at the Humboldt University of Berlin in 2010 and then joined the research laboratory of Manfred Marschall focusing on virus-host interactions and the development of better therapeutic options for the treatment of cytomegalovirus infections.
Professor Manfred Marschall is group leader and international expert in herpesviruses at the Institute for Clinical and Molecular Virology, Friedrich-Alexander University of Erlangen-Nürnberg. His major research interest is human cytomegalovirus and is particularly focussed on three major topics: (1) the functional and structural characterisation of the CMV-encoded protein kinase pUL97 and CMV nuclear egress complex; (2) the cross-talk between herpesviral and cellular kinases (i.e. the regulation of viral nuclear egress, the virus-CDK interregulation, as well as questions of virus-induced cellular signalling and pathogenesis); and (3) the development of kinase inhibitors (and others) as putative broad-spectrum antiviral therapeutics in collaboration with international biotech and pharmaceutical companies.




