Recognition of two new species of freshwater crabs from the Seychelles based on molecular evidence (Potamoidea : Potamonautidae)
Neil Cumberlidge A C and Savel R. Daniels BA Department of Biology, Northern Michigan University, 1401 Presque Isle Ave, Marquette, MI 49855, USA.
B Department of Botany and Zoology, University of Stellenbosch, Private Bag X1, Matieland 7602, South Africa.
C Corresponding author. Email: ncumberl@nmu.edu
Invertebrate Systematics 28(1) 17-31 https://doi.org/10.1071/IS13017
Submitted: 18 April 2013 Accepted: 17 October 2013 Published: 20 March 2014
Abstract
The Afrotropical freshwater crab genus Seychellum is endemic to the granitic Seychelles in the Indian Ocean (Mahé, Silhouette, Praslin, La Digue and Frégate). Here we describe two new cryptic species of Seychellum that represent two evolutionarily separate lineages of a previously monotypic genus. This raises to three the number of species of freshwater crabs known from Seychelles. Each species is endemic to either one island (Silhouette) or to a pair of islands (Mahé and Frégate, or Praslin and La Digue). The three species can be clearly distinguished as separate lineages by DNA analysis, haplotyping and examination of gonopod characters. The recognition of S. silhouette, sp. nov. (endemic to Silhouette) and S. mahefregate, sp. nov. (endemic to Mahé and Frégate) reduces the range of the type species, S. alluaudi (A. Milne-Edwards & Bouvier, 1893) to La Digue and Praslin. Both dispersal and vicariance may have played a role in shaping the present distribution patterns of the Seychellois freshwater crabs.
Additional keywords: cryptic species, Seychellum.
Introduction
The Seychelles are an isolated island group of more than a hundred granitic and coralline islands, cays and atolls in the Indian Ocean up to 1500 km from the east coast of Africa. Freshwater crabs occur only on granitic islands that lie on the undersea shelf that forms the Seychelles bank, and were initially known from Praslin, La Digue and Mahé (Ng et al. 1995). Their distribution was later expanded to four islands with the addition of previously unpublished museum material from Silhouette (Cumberlidge 2008a), and then to five islands with the recent discovery of freshwater crabs on Frégate (Daniels 2011). The freshwater crabs of the Seychelles are not well known and very few specimens have been reported in the 120 years since they were first discovered on Praslin (Milne-Edwards and Bouvier 1893). It is not surprising, therefore, that the first comprehensive survey of five of the major islands of the Seychelles Inner Islands by the second author in 2010 produced several new locality records and two new cryptic taxa (Daniels 2011).
Recent taxonomically significant publications on the freshwater crabs of the Seychelles include Ng et al. (1995), who established the endemic genus Seychellum Ng, Števčić and Pretzmann, 1995 and assigned it to the Asian family Gecarcinucidae Rathbun, 1904. Those authors also clarified the generic assignment of S. alluaudi (Milne-Edwards & Bouvier, 1893), which had previously been regarded as a species of Deckenia by Bott (1955). The other significant publications are those by Daniels et al. (2006), Cumberlidge et al. (2008) and Ng et al. (2008) who assigned Seychellum to the Afrotropical family Potamonautidae and placed this genus in the subfamily Deckeniinae Ortmann, 1897.
Ng et al. (1995) remarked on a series of minor morphological differences between specimens from Praslin, La Digue and Mahé, which they attributed to changes that occur as crabs increase in size, but these differences were judged to be not significant enough to recognise a second taxon. The apparently strong morphological similarity between the crabs found on three widely separated islands in the Seychelles prompted the survey by Daniels (2011), which produced 83 specimens and 15 new locality records, including crabs from Frégate, an island that had never been sampled before.
Daniels (2011) focussed on the phylogeography of S. alluaudi, and used two mitochondrial markers (COI and 16S) to investigate the possibility that there may in fact be one or more evolutionary lineages of freshwater crabs in the Seychelles. The molecular investigations of the new material revealed that the formerly widespread species S. alluaudi actually comprises three cryptic taxa that are apparently almost morphologically identical but genetically distinct. Each of the three distinct well supported clades within Seychellum reported by Daniels (2011: fig. 2) has a strong correlation with one of three distinct regions: either one island or a group of islands. For example, one clade grouped populations on La Digue and Praslin, another grouped populations on Mahé and Frégate and the third grouped populations on Silhouette (Fig. 1). The initial examinations of the new specimens by Daniels (2011) revealed no dramatic morphological differences between specimens of Seychellum from any of the five islands, either between individuals in the same clade, or between those in different clades. However, here we describe two new cryptic species in addition to S. alluaudi in light of the unequivocal molecular findings of Daniels (2011: figs 2, 3), based on a detailed comparative morphological study of the new series of specimens from five different islands in the Seychelles. Details of the habitats at each of the 15 new localities are also provided.
Material and methods
Eighty three specimens of Seychellum were collected by the second author from 15 new localities on five islands on the Seychelles from 17 May to 2 June 2010 (see Daniels 2011; Table 1). A sixth island (North) was surveyed but no evidence of freshwater crabs was found, perhaps because North Island lacks suitable freshwater habitats. On the other five islands, crabs were always associated with freshwater habitats (either streams or rivers) and were typically semi-terrestrial, either living on land, in burrows near water, or under rocks in a stream. Most of the collection localities were in either closed-canopy primary mist forest, palm forest, secondary forest or disturbed land (Walsh 1984). Morphological analyses consisting of a detailed examination of characters of the carapace, sternum, mouthparts, chelipeds, walking legs and gonopods were carried out on specimens from each of the five islands. Specimens are deposited in the South African Museum (Iziko Museums), Cape Town, South Africa (SAM) and the Museum Royale Centrale ‘d’Afrique, Tervuren, Belgium (MRAC). All measurements were made with digital calipers and are given in millimetres. Abbreviations used: a1–a6, abdominal somites 1–6; asl, above sea level; cw, distance across the carapace at the widest point; ch, carapace height, the maximum height of the cephalothorax; cl, carapace length, the distance between the central lobe of the frontal margin and the posterior margin of the carapace; coll., collected by; fw, front width measured between the two lateral lobes of the front; e, thoracic episternite; G1, first gonopod ( = first pleopod of male); G2, second gonopod ( = second pleopod of male); juv., juvenile; ovig., ovigerous; p1–p5, pereiopods 1–5; s, thoracic sternite; s1/s2, s2/ s3, s3/s4, sternal sutures between adjacent thoracic sternites. The terminology is adapted from Cumberlidge (1999), and the higher classification used here follows that of Ng et al. (2008).

|
Results
Careful comparison of the taxonomically significant morphological characters of the carapace, sternum, mouthparts and abdomen yielded no evidence that any of the individuals from any of the five islands were sufficiently different to warrant the recognition of new taxa based on morphological evidence alone. Any morphological differences that were detected were minor in nature and fell within the normal range of intraspecific variation described for S. alluaudi by Ng et al. (1995) based on specimens from Praslin and La Digue (S. alluaudi) and Mahé (S. mahefregate). This lack of conspicuous morphological character variation was also found to be the case in our detailed interspecific comparisons of G1 and G2 of adult male specimens (Figs 2, 3): but here minor differences are recorded for G1 and G2 morphology among individuals representing the three main lineages (clades).
Details of the molecular analyses and results are given in Daniels (2011: figs 2, 3). The 83 specimens of freshwater crabs from five islands in the Seychelles diverged into three monophyletic lineages or clades, one of which (Clade 2, Praslin and La Digue) includes the nominal species S. alluaudi. The two other clades grouped together populations from Mahé and Frégate (Clade 3), and populations from Silhouette (Clade 1). This tree topology summarised here in Fig. 1 had high support, with high Bayesian posterior probabilities and Bootstrap support values for each species clade in the maximum likelihood analysis of both COI and 16S (see Daniels 2011: fig. 2). There is strong evidence that this genetic pattern was generated by independent coalescent processes as evidenced by reciprocal monophyly of all three clades, deep genetic differentiation between the clades, shallow differentiation within each clade and allopatry resulting from isolation on the islands hosting these clades. Therefore, despite the fact that this study could detect only minor differences in the external morphology of specimens belonging to any of the three clades or from any of the five islands, we consider the genetic evidence to be compelling enough to warrant the treatment of all three clades as three valid species. The haplotype network (see Daniels 2011: fig. 3) containing 51 haplotypes was congruent with the phylogenetic analyses in that it also retrieved the same three well supported groups. No haplotypes were shared among islands, although a small number of haplotypes were shared between sample sites within islands (suggesting recent and ongoing dispersal). Haplogroup one corresponds to Clade 1 from Silhouette and contains all individuals from that island, haplogroup two corresponds to Clade 2 and contains all individuals from Praslin and La Digue and haplogroup three corresponds to Clade 3 and contains all individuals from Mahé and Frégate (see Daniels 2011: fig. 3).
Taxonomy
Suborder BRACHYURA Latreille
Superfamily POTAMOIDEA Ortmann
Family POTAMONAUTIDAE Bott
Subfamily DECKENIINAE Ortmann
Genus Seychellum Ng, Števčić, & Pretzmann
Seychellum alluaudi (A. Milne-Edwards & Bouvier)
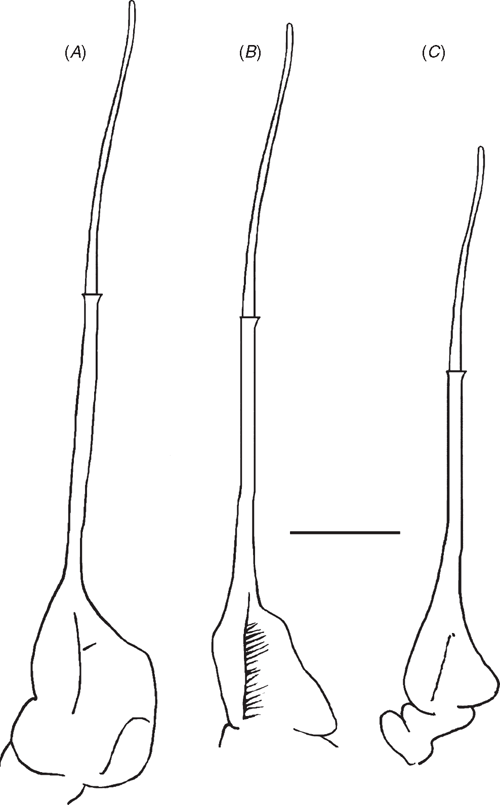
|
Material examined
Diagnosis
G1 terminal article straight, ending in broad tube-like tip, midsection not widened; terminal article and subterminal segment of G1 poorly demarcated on ventral side, clearly separated on dorsal side by dorsal membrane. Medial and lateral margins of dorsal membrane both short; superior margin forming straight diagonal line sloping downward from medial to lateral margins; inferior margin wavy, medial half curving downward, mid region curving upward, lateral region curving downward to meet lateral margin. G2 terminal article with long flagellum-like distal segment distinctly shorter than subterminal segment; distal two-thirds of subterminal segment long, narrow, tube-like, basal third widened both medially and laterally, medial side of base of subterminal segment of G2 slim with medial margin slightly widened in middle, lateral side twice as wide as medial side, rectangular, distal lateral margin sloping outward before turning straight down to meet basal margin.
Redescription
For a detailed description of the lectotype of S. alluaudi, an adult male, cw 42.8 mm, MNHN-BP 152, from Praslin Island, Seychelles, see Ng et al. (1995: 589–599). For illustrations of the lectotype of S. alluaudi see Ng et al. (1995: figs 1B, 4–6). For illustrations of a specimen of S. alluaudi, an adult male, cw 51.8 mm, SMF 12926 from La Digue Island, Seychelles, see Ng et al. (1995: figs 7, 8). Museum voucher identification numbers and GenBank accession numbers for 16S rRNA and COI mitochondrial DNA sequences for specimens of S. alluaudi are listed in Table 1.
Carapace outline quadrangular, dorsal surface rugose, regions well defined, carapace very high (ch/fw 2.0), frontal margin distinctly trilobate with median lobe projecting forward, lateral lobes rounded. Antennules folding obliquely at ~45 degrees from horizontal. Exorbital, epibranchial teeth large, pointed; postfrontal crest incomplete; epigastric, postorbital crests fused, granular, well defined, ending at junction with cervical groove, lateral crests granular, distinct, meeting epibranchial teeth; granular anterolateral margin posterior to epibranchial tooth, curving inward over carapace surface ending after one-third of length of carapace; posterior carapace surface laterally marked by parallel fields of strong carinae; suborbital region with strong field of granules; carapace sidewall vertical sulcus granular, curving, meeting anterolateral margin at base of epibranchial tooth; strong curved line of large rounded granules crossing suborbital region midway between suborbital margin and epimeral suture ending just before exorbital tooth; pterygostomial region covered with dense field of raised carinae. Mandibular palp 2-segmented, basal segment stout, terminal segment distinctly bilobed, with smaller (anterior) lobe exceeding 0.5 times length of larger posterior lobe. Endopod of 1st maxilliped slender, elongated, lateral margins sinuous. Paired efferent respiratory channel openings tube-like, openings at tips of long upwardly directed tubes terminating close to frontal margin medial lobe; respiratory openings level with frontal margin positioned either side of medial frontal lobes; top of respiratory channels formed by shelf on medial lower orbital margin together with closely applied tips of elongated endopods of first maxillipeds. Outer surface of ischium of third maxilliped gently convex, sulcus shallow but distinct. Thoracic sternal sulcus s2/s3 faint, curved, s3/s4 incomplete, short notches at sides, interrupted medially, not close to tip of sternoabdominal cavity. Male abdomen triangular, abdominal segment a1 partially hidden by carapace, lateral margins of abdominal segments a4–a6 distinctly concave. Inferior margins of merus of cheliped granular, distal meral tooth pointed; first carpal tooth medium-sized, slim, pointed, second carpal tooth small, pointed. Ischium of cheliped with low blunt teeth. G1 terminal article straight ending in broad, tube-like tip, midsection not widened, terminal and subterminal segments of G1 poorly demarcated on ventral side, clearly separated on dorsal side by dorsal membrane. Medial and lateral margins of dorsal membrane both short; superior margin forming straight diagonal line sloping downward from medial to lateral margins; inferior margin wavy, medial half curving downward, mid region curving upward then downward to meet lateral margin. G2 terminal article with long flagellum-like distal segment distinctly shorter than subterminal segment; distal two-thirds of subterminal segment long, narrow, tube-like, basal third widened both medially and laterally, medial side of base of subterminal segment of G2 slim with medial margin slightly widened in middle, lateral side twice as wide as medial side, rectangular, distal lateral margin sloping outward before turning straight down to meet basal margin. Large-sized species, recoreded adult size range cw 53.4 to cw 37.3 mm; largest ovigerous female cw 42.4 mm. Pubertal moult between cws 31.2 and 34.7 mm (largest subadult female is cw 31.2 mm). Carapace surface dark brown, ventral surface of sternum cream, chelipeds orange dorsally, white with small dark flecks ventrally.
Haplotypes
Haplogroup two corresponds to Clade 2 (Fig. 1) and contains all individuals from Praslin and La Digue (see Daniels 2011: figs 2, 3).
Distribution
Seychellum alluaudi is endemic to the northern Seychelles islands of Praslin (38 km2) and La Digue (10 km2) (Fig. 1). The known distribution of S. alluaudi is shown in Fig. 1 and is based on the georeferenced localities of the specimens collected by Daniels (2011: table 1, fig. 1) from Praslin (Zimbabwean Highlands and Praslin National Park) and La Digue (Belle Vue and Grand Anse) as well as other museum specimens from La Digue and Praslin. No species of freshwater crabs are known to occur on any of the other islands in this northern group (Aride, Curieuse, Cousin, Cousine, Cocos, Petite Sœur, Grand Sœur, Felicite, Marianne). This species is found from sea level to at least 160 m asl. Praslin and La Digue are both mountainous granitic Gondwanan islands of great age separated by 10 km of shallow seas less than 30 m deep (Rocha et al. 2011). These islands were connected to each other in the past as part of the continuous landmass known as the Seychelles Bank that also included Frégate and Mahé when the seas were 50 m or more below present levels (Fig. 1) (Rocha et al. 2011). Praslin and La Digue are presently separated from Mahé to the south-west by 60 km of shallow seas, and from Frégate to the south by 30 km of shallow seas. Although Praslin and La Digue once shared the same landmass as Mahé and Frégate, the lineages of freshwater crabs found on these two groups of islands fall into a northern group (for S. alluaudi) and a southern group (for S. mahefregate) (see later). The taxonomic conclusions reached here mean that S. alluaudi is no longer recognised to occur on Mahé and Silhouette Islands. This represents a significant reduction in the extent of occurrence of this species from 223 km2 to 48 km2. Ng et al. (1995) established the lectotype for S. alluaudi (MNHN-BP 152), an adult male (cw 42.8 mm) from ‘‘Praslin ‘Island’ and included in this species two other specimens (SMF 12925 and SMF 12926) from a ‘mountain on La ‘Digue’ (4°20″S, 55°50″E).
Ecological notes
This species lives in burrows dug into the banks of streams and rivers, and occurs in clear water streams at elevations from near sea level to 160 m asl in the mountainous parts of its range. Recorded locations are in lowland forest, secondary forest and a residential area, and most details of its ecology are unknown. The mountain/hill slopes in Praslin are covered by secondary mixed forest with a high proportion of native species, mainly palms (Gerlach 1999).
Remarks
Intraspecific character variation of characters between specimens of S. alluaudi from La Digue (adult male cw 51.8 mm) and Praslin (adult male cw 42.8 mm) listed by Ng et al. (1995) include the shape of the exorbital tooth (triangular versus low and rounded), the infraorbital margin (lacking a cleft versus with a cleft), the infraorbital shelf (with two tubercles versus three tubercles), the sub-branchial region (highly inflated versus not inflated), and the basal antennal segment (with a distinctly concave margin versus a slightly concave margin). The above intraspecific character differences between the populations on these two islands were not found to be the case for the additional series of specimens examined here from these two islands. These characters may be attributed to differences in the size of the two specimens compared by Ng et al. (1995), because these characters in a large adult male examined in the present study from the Zimbabwe Highlands of Praslin (cw 53.4 mm) agreed more with the similar-sized male from La Digue (cw 51.5 mm) examined by Ng et al. (1995) than with the smaller specimen from Praslin. The above intraspecific variable characters are not therefore considered here to be a sufficient basis to warrant the formal taxonomic separation of these island populations, especially in view of the shared genetic history of these specimens described by Daniels (2011: figs 2, 3).
Before the study of Daniels (2011), hardly any collections of crabs had been made from the two islands making up Clade 2: just two specimens from one locality in Praslin in 1893, and one specimen from one locality in La Digue in 1991 (Ng et al. 1995). Seychellum alluaudi is apparently well represented, because Daniels (2011) collected 11 specimens from two new localities in Praslin and seven specimens from two new localities in La Digue. Specimens of S. alluaudi from Praslin were included in the molecular phylogenic study of the Afrotropical freshwater crabs by Daniels et al. (2006), where its position as a sister group to the East African genus Deckenia was confirmed. That study also led to the assignment of Seychellum to the subfamily Deckeniinae (Cumberlidge et al. 2008; Ng et al. 2008; Cumberlidge and Ng 2009).
Conservation status
Seychellum alluaudi was listed as least concern (LC) on the Internation Union for the Conservation of Nature Red list (Cumberlidge 2008b; Cumberlidge et al. 2009) in view of its wide distribution (which was then understood to be on four islands in the Seychelles), estimated stable population size, lack of known widespread long-term threats and presence in a protected area in at least part of its range (the Praslin National Park) (Cumberlidge et al. 2009). However, this conservation assessment now needs to be revised in the light of its range reduction from four islands (total area 223 km2) to only two islands (total area 48 km2).
Seychellum silhouette, sp. nov.
(Figs 1, 2A–C, 4C, D, 5B, Table 1)
Material examined
Diagnosis
G1 terminal article straight, ending in broad, tube-like tip, midsection not widened; terminal and subterminal segments of G1 not visible on ventral side, clearly separated on dorsal side by dorsal membrane. Medial and lateral margins of dorsal membrane both short; superior margin forming straight diagonal line sloping downward from medial margin before curving sharply upward to meet lateral margin; inferior margin of dorsal membrane wavy, medial section curving downward medially, mid region curving upward, lateral region curving downward to meet lateral margin. G2 terminal article with long flagellum-like distal segment distinctly shorter than subterminal segment; distal two-thirds of subterminal segment long, narrow, tube-like, basal third widened both medially and laterally; medial side of base of subterminal segment of G2 slim, triangular, with point in middle; lateral side of G2 subterminal segment base twice as wide as medial side, forming right triangle with hypotenuse (diagonal lateral margin) indented in middle.
Description
Seychellum silhouette is a cryptic species recognised mainly on the basis of genetic evidence with an overall morphology that is identical to that described for S. alluaudi by Ng et al. (1995), although the G1 and G2 show species-specific characters (listed above) that can be used in conjunction with genetic evidence to distinguish this species. Museum voucher identification numbers and GenBank accession numbers for 16S rRNA and COI mitochondrial DNA sequences for specimens of S. silhouette are listed in Table 1.
Carapace outline quadrangular, dorsal surface rugose, regions well defined, carapace very high (ch/fw 2.0), frontal margin distinctly trilobate with median lobe projecting forward, lateral lobes rounded. Antennules folding obliquely at ~45 degrees from horizontal. Exorbital, epibranchial teeth large, pointed; postfrontal crest incomplete, epigastric, postorbital crests fused, granular, well defined, ending at junction with cervical groove, lateral crests granular, distinct, meeting epibranchial teeth; granular anterolateral margin posterior to epibranchial tooth, curving inward over carapace surface ending after one-third of length of carapace; posterior carapace surface laterally marked by parallel fields of strong carinae; carapace sidewall vertical sulcus granular, curving, meeting anterolateral margin at base of epibranchial tooth; strong curved line of large rounded granules crossing suborbital region midway between suborbital margin and epimeral suture; pterygostomial region covered with dense field of raised carinae. Mandibular palp 2-segmented, basal segment stout, terminal segment distinctly bilobed, with smaller (anterior) lobe exceeding 0.5 times length of larger posterior lobe. Endopod of 1st maxilliped slender, elongated, lateral margins sinuous. Paired efferent respiratory channel openings tube-like, openings at tips of long upwardly-directed tubes terminating close to frontal margin medial lobe; respiratory openings level with frontal margin positioned either side of medial frontal lobes; top of respiratory channels formed by shelf on medial lower orbital margin together with closely applied tips of elongated endopods of first maxillipeds. Outer surface of ischium of third maxilliped gently convex, sulcus shallow but distinct. Thoracic sternal sulcus s2/s3 faint, curved, s3/s4 incomplete, short notches at sides, interrupted medially, not close to tip of sternoabdominal cavity. Male abdomen triangular, abdominal segment a1 partially hidden by carapace, lateral margins of abdominal segments a4-a6 distinctly concave. Inferior margins of merus of cheliped granular, distal meral tooth pointed; first carpal tooth medium-sized, slim, pointed, second carpal tooth small, pointed. Ischium of cheliped with low blunt teeth. Large species, recorded adult size range cw 50.4 to cw 37.3 mm; largest ovigerous female cw 40.2 mm. Carapace surface dark brown, ventral surface of sternum cream, chelipeds orange dorsally, white with small dark flecks ventrally.
Distribution
Seychellum silhouette is endemic to the Seychelles and has only been recorded from three localities on Silhouette where it is found from sea level to at least 500 m asl. The known distribution of S. silhouette is shown in Fig. 1 and is based on the georeferenced localities of the specimens collected by Daniels (2011: table 1, fig. 1) from La Passe and Anse Patate, and on the museum specimens from Mare aux Cochons. Silhouette is a volcanic mountainous island 20 km north-west of Mahé and although Silhouette only has an area of 20 km2, it is the third largest island in the Seychelles with a human population of less than 150. The entire island of Silhouette has recently been designated a National Park. Silhouette is separated from Mahé by a deep ocean and these two islands would not have formed a single landmass even during the Pleiocene (2.5 to 5.3 MY ago) when sea levels oscillated between 30 m below and 20 m above their present levels. However, Silhouette would have been connected to the rest of the Seychelles Bank during the Pleistocene (2.5 MY ago to 11 700 years ago) when sea levels oscillated between 80 m and 30 m below their present levels (Rocha et al. 2011).
Ecological notes
This species lives in burrows dug into the banks of clear water streams and rivers. The mountainous nature of this island means that this species occurs at higher altitudes at elevations from near sea level to at least 500 m asl in closed-canopy primary mist forests and disturbed secondary forests. Most details of its ecology are unknown.
Haplotype
Haplogroup one corresponds to Clade 1 (Fig. 1) and contains all individuals from Silhouette (Daniels 2011: figs 2, 3).
Remarks
Seychellum silhouette can be distinguished from S. alluaudi as follows. The junction between the terminal and subterminal segments of G1 of S. silhouette are not visible on the ventral side, whereas this junction is faint but visible in S. alluaudi. The superior margin of the dorsal membrane of the G1 of S. silhouette forms a straight diagonal line that slopes downward from the medial margin and curves sharply upward just before meeting the lateral margin, whereas in S. alluaudi this margin runs diagonally to meet the lateral margin without turning upward. The basal third of the G2 subterminal segment of S. silhouette is slim. The medial side is a slim triangle with a point in the middle while the lateral side is twice as wide as the medial side, and forms a right triangle whose hypotenuse (the lateral margin) is indented in the middle. In contrast, the G2 subterminal segment of S. alluaudi does not have a triangular medial region, but the lateral side is twice as wide as the medial side and is rectangular, with a lateral margin that slopes outward before turning straight down to meet the basal margin.
Two specimens collected from Silhouette in 1972 from an endemic forest at Mare aux Cochons (MRAC 53.638, MRAC 54.053) at a high altitude (500 m asl) in a remote area of the island did not appear in the literature until recently (Cumberlidge 2008a). Daniels (2011) collected seven other specimens from two new localities in Silhouette.
Etymology
This species is named for Silhouette Island in the Seychelles, which is the only place it is found. The name is used as a noun in apposition.
Seychellum mahefregate, sp. nov.
(Figs 1, 3A–C, 4E, F, 5C, Table 1)
Material examined
Diagnosis
G1 terminal article straight, ending in broad, tube-like tip, midsection not widened; G1 subterminal segment with rounded shoulder on medial margin at distal end; terminal and subterminal segments of G1 not visible on ventral side, clearly separated on dorsal side by dorsal membrane. Medial margin of dorsal membrane short, lateral margin twice as long; superior margin forming even downward-curving line from medial margin to lateral margin; inferior margin wavy, medial section curving downward, mid region curving upward, lateral region curving downward then upward to meet lateral margin. G2 terminal article with long flagellum-like distal segment distinctly shorter than subterminal segment; distal two-thirds of subterminal segment long, narrow, tube-like, basal third widened both medially, laterally; medial side of base of subterminal segment of G2 slim slightly widened in middle; lateral side of G2 subterminal segment base as wide as medial side, forming slim right triangle with hypotenuse (diagonal lateral margin) straight, not indented in the middle, curving sharply inwards just before meeting basal margin.
Description
Seychellum mahefregate is a cryptic species recognised mainly on the basis of genetic evidence with an overall morphology that is identical to that described for S. alluaudi by Ng et al. (1995), although the G1 and G2 show species-specific characters (listed above) that can be used in conjunction with genetic evidence to distinguish this species. Museum voucher identification numbers and GenBank accession numbers for 16S rRNA and COI mitochondrial DNA sequences for specimens of S. mahefregate are listed in Table 1.
Carapace outline quadrangular, dorsal surface rugose, regions well defined, carapace very high (ch/fw 2.0), frontal margin distinctly trilobate with median lobe projecting forward, lateral lobes rounded. Antennules folding obliquely at ~45 degrees from horizontal. Exorbital, epibranchial teeth large, pointed; postfrontal crest incomplete, epigastric, postorbital crests fused, granular, well defined, ending at junction with cervical groove, lateral crests granular, distinct, meeting epibranchial teeth; granular anterolateral margin posterior to epibranchial tooth, curving inward over carapace surface ending after one third of length of carapace; posterior carapace surface laterally marked by parallel fields of strong carinae; carapace sidewall vertical sulcus granular, curving, meeting anterolateral margin at base of epibranchial tooth; strong curved line of large rounded granules crossing suborbital region midway between suborbital margin and epimeral suture; pterygostomial region covered with dense field of raised carinae. Mandibular palp 2-segmented, basal segment stout, terminal segment distinctly bilobed, with smaller (anterior) lobe exceeding 0.5 times length of larger posterior lobe. Endopod of 1st maxilliped slender, elongated, lateral margins sinuous. Paired efferent respiratory channel openings tube-like, openings at tips of long upwardly-directed tubes terminating close to frontal margin medial lobe; respiratory openings level with frontal margin positioned either side of medial frontal lobes; top of respiratory channels formed by shelf on medial lower orbital margin together with closely applied tips of elongated endopods of first maxillipeds. Outer surface of ischium of third maxilliped gently convex, sulcus shallow but distinct. Thoracic sternal sulcus s2/s3 faint, curved, s3/s4 incomplete, short notches at sides, interrupted medially, not close to tip of sternoabdominal cavity. Male abdomen triangular, abdominal segment a1 partially hidden by carapace, lateral margins of abdominal segments a4-a6 distinctly concave. Inferior margins of merus of cheliped granular, distal meral tooth pointed; first carpal tooth medium-sized, slim, pointed, second carpal tooth small, pointed. Ischium of cheliped with low blunt teeth. Large sized species, recorded adult size range cw 42.4 to cw 33.1 mm; largest ovigerous female cw 42.4 mm. Pubertal moult cw 31.2–34.7 mm (females adult at cw 34.7 mm, largest subadult female cw 31.2 mm). Chelipeds orange dorsally, white with small dark flecks ventrally.
Haplotypes
The haplotype network for S. mahefregate from Mahé and Frégate (see Daniels 2011: fig. 3) included all individuals from these islands and revealed three distinct clusters with a large number of mutational step differences that coincide with three distinct areas within Mahé. These are: central Mahé (Le Niol, Morne Seychellois National Park, La Misere, Chemin Montagne Posée, and Du Riz River), northern Mahé (La Gogue), and southern Mahé (Intendance, Chemin Val D’Endor and Chemin Montagne Posée). The locality at Chemin Montagne Posée represents an area of sympatry between the central and southern haplotypes. The southern region has the lowest mountains (<400 m asl), the central region has the highest mountains (up to 900 m asl), and the northern region has both highlands (up to 480 m asl) and lowlands. This varied geographical topology within Mahé, with each region having a separate river basin, has presumably limited crab dispersal and isolated populations on this island.
Distribution
Seychellum mahefregate is endemic to the Seychelles where it has been recorded only on the widely separated southern islands of Mahé and Frégate; it is found from sea level to at least 764 m asl. The known distribution of S. mahefregate is shown in Fig. 1 and is based on the georeferenced localities of the specimens collected by Daniels (2011: table 1, fig. 1) from Mahé (La Gouge, Le Niol, Du Riz River, Morne Seychellois National Park, La Misere, Chemin Montagne Posée, Chemin Val D’Endor, and Intendance) and Frégate (Anse Park). Mahé (with a length of 27 km and an area of 155 km2) is the largest of the Seychelles islands and has a backbone of forested mountains including Morne Seychellois (in the Morne Seychellois National Park), which at 905 m asl is the highest in the island group. Clade 3 also includes specimens from the tiny and remote island of Frégate (3 km2), which lies some 57 km east of Mahé. The populations of freshwater crabs found on Frégate live in lowland palm forest drained by streams. It is noteworthy that the crabs on Frégate are most closely related to those on Mahé (almost 60 km away) rather than to S. alluaudi on the closer northern islands of Praslin or La Digue (33 km and 29 km from Frégate respectively). Mahé and Frégate would have been connected to Praslin and La Digue as part of the Seychelles Bank during the Pleistocene (2.5 MY ago to 11 700 years ago), when sea levels oscillated between 80 m and 30 m below their present levels (Rocha et al. 2011). However, the existence of former land connections alone does not explain why the populations of freshwater crabs from the southern islands of Mahé and Frégate form one clade and why crabs from the northern islands of Praslin and La Digue form a different clade (Fig. 1).
Ecological notes
This species lives in burrows dug into streams and river banks and occurs in clear water streams at elevations from near sea level to above 720 m asl in the Morne Seychellois National Park. Recorded locations are in lowland forest and secondary forest. Most details of its ecology are unknown. Frégate is the easternmost of the granitic Seychelles Islands and comprises two hills reaching up to 125 m asl, and two low-lying coastal plateaus where these freshwater crabs were collected.
Remarks
Seychellum mahefregate can be distinguished from S. alluaudi as follows. The junction between the terminal article and subterminal segment of G1 of S. mahefregate is not visible on the ventral side, whereas this junction is faint but visible in S. alluaudi. The superior margin of the dorsal membrane of the G1 of S. mahefregate curves downward, whereas in S. alluaudi this margin runs diagonally to meet the lateral margin. The medial side of the basal region of the G2 subterminal segment of S. mahefregate is slim (the lateral side is as wide as the medial side) and triangular. In contrast, the medial side of the basal region of the G2 subterminal segment of S. alluaudi is slim and rounded and the lateral side is twice as wide as the medial side and forms a rectangular shape.
Seychellum mahefregate can be distinguished from S. silhouette as follows. The superior margin of the dorsal membrane of the G1 of S. mahefregate curves evenly downward, whereas in S. silhouette this margin is indented in the middle. The medial side of the basal region of the G2 subterminal segment of S. mahefregate is a slim while the lateral side is as wide as the medial side. In contrast, the medial side of the basal region of the G2 subterminal segment of S. silhouette is slim and triangular and the lateral side is twice as wide as the medial side.
Before the study of Daniels (2011), hardly any collections of crabs had been made from the two islands making up Clade 3: two specimens from two high-altitude localities on Mahé (Morne Seychellois, 671 m asl and Cascade river, 244 m asl in 1907); and a single specimen from a third locality in Mahé in 1991. These specimens were all included in S. alluaudi by Ng et al. (1995). In contrast, Daniels (2011: table 1, fig. 1) collected 36 specimens from 8 new localities in Mahé and 8 specimens from one new locality in Frégate. Specimens from these two islands agreed in the morphological characteristics described above for this species.
Etymology
This species is named for the islands of Mahé and Frégate in the Seychelles that are the only two places where it is found. The name is used as a noun in apposition.
Discussion
Accurate species recognition is essential both for the quantification of biodiversity and for conservation assessments and planning, and is especially difficult in the case of cryptic species (Pfenninger and Schwenk 2007; Cook et al. 2008a; Jesse et al. 2010). Daniels (2011) demonstrated that the freshwater crabs on five islands in the Seychelles represent a cryptic species complex within the genus Seychellum that comprises three reproductively isolated allospecies. Allopatric isolation is the most likely primary mechanism of inter-island speciation within the Seychelles given the relatively recent divergence times (Daniels 2011), and the low probability of gene flow between recently separated widely spaced populations that are genetically isolated on widely-spaced islands.
The three Seychelles species are virtually identical morphologically and the species described here have distributional ranges that do not overlap (Fig. 1). Moreover, crabs from all five islands share a similar habitat (lowland and upland streams and rivers in forested areas), similar food preferences (mainly vegetation), and a similar body size range. The vegetation cover and overall topology of the islands are not entirely consistent with the genetic grouping indicated by the three clades. For example, Silhouette (Clade 1) has high mountains with mist forests, Praslin and La Digue (Clade 2) have lowland palm forests, while in Clade 3 Mahé has high mountains with mist forests but Frégate has lowland palm forests.
The successful and unequivocal recognition of species boundaries in freshwater crabs usually depends on a combination of different somatic and gonopod characters (Cumberlidge 1999). The availability of a good series of specimens from five different islands in the Seychelles for the first time has allowed a thorough examination of the species-level somatic morphological characters of the carapace, sternum, mouthparts, and pereiopods. The morphological similarities among S. alluaudi S. silhouette, and S. mahefregate include the form of the antennae, antennules, epistome, endostome, and the mouthparts, including the form of the mandibular palp. These characters do not vary significantly either between the three clades or between the five islands. We were only able to identify small differences in G1 and G2 morphology between S. alluaudi S. silhouette, and S. mahefregate and the subtle nature of some of these gonopod characters make it necessary to bring in additional genetic and locality data to confirm the identification. Nevertheless, the separation of species based on species-specific G1 morphology has proved to be a powerful tool for recognising species boundaries in the potamonautids as well as other freshwater crab families (Cumberlidge 1999; Brandis et al. 1999; Brandis et al. 2000). This approach assumes that the detailed morphology of the terminal article of G1 is specific to each species and is necessary for successful sperm transfer within a species. It follows that a different G1 morphology of one species would not be expected to lead to successful sperm transfer in another species, which would then result in reproductive isolation. However, the genetic evidence of Daniels (2011) indicates that although the G1 and G2 of all three species of Seychellum are undoubtedly under strong selection pressure, dramatic morphological differentiation of the external male reproductive structures has not apparently occurred, and the character differences described here on their own may not be sufficient to define species delimitation in this genus.
The use of genetic data to determine species boundaries is becoming increasingly commonplace, but so far is relatively rare in freshwater crab taxonomy (Jesse et al. 2010). To date only the potamid Potamon pelops Jesse et al., 2010, from the Peloponnesus Peninsula in Greece has been described as an separate evolutionary lineage distinct from its morphologically close relative Potamon fluviatile (Herbst, 1785) on the basis of mitochondrial and nuclear DNA sequence data.
Biogeographic considerations
From Africa to Seychelles: overseas dispersal more likely than vicariance
Despite the strict ecological preference of all freshwater crabs for fresh water habitats (they are never found naturally in water with even low levels of salt), a significant number of species in the Afrotropical and the Oriental regions are found on oceanic islands separated from the mainland by deep seas (Cumberlidge 2008a). The presence of freshwater crabs on oceanic islands is usually explained by overseas dispersal across a saltwater barrier (Rodríguez and López 2003; Cook et al. 2008b; Cumberlidge 2008a; Cumberlidge and Ng 2009; Yeo et al. 2008; Cumberlidge et al. 2009; Klaus et al. 2009; Klaus et al. 2010; Jesse et al. 2010), while the presence of freshwater crabs on continental shelf islands is explained by overland dispersal during past periods of low sea levels (Cumberlidge 2008a; Rocha et al. 2011). The discovery by Daniels (2011) that the colonization of the Seychelles by deckeniine freshwater crabs from Africa happened relatively recently in the late Miocene/Pliocene (between 8.7–2.3 MY ago) supports hypotheses of recent overseas dispersal (Daniels et al. 2006; Cumberlidge et al. 2008; Cumberlidge 2008a; Cumberlidge and Ng 2009) over hypotheses of ancient Gondwanan vicariance (over 180 MY ago) (Ng et al. 1995). The arrival of Seychellum on the remote Seychelles Archipelago in the late Miocene/Pliocene was most likely the result of a single dispersal event (Daniels et al. 2006; Daniels 2011) that involved the forced introduction of ancestral crabs into ocean currents (perhaps during a powerful storm). Crabs clinging to floating vegetation carried out to sea by surface currents could conceivably have reached one of the islands in the Seychelles (probably Silhouette). A recent laboratory study of the survival times and physiological capabilities of related Asian freshwater crabs in saltwater provided experimental support for transoceanic dispersal hypotheses by demonstrating that these animals can indeed survive extended saltwater exposure for at least two weeks (Esser and Cumberlidge 2011).
Dispersal within Seychelles: vicariance more likely than overseas dispersal
The Seychelles Islands were part of the ancient granitic Gondwanan continental landmass (over 500 MY old) that has undergone a series of fragmentations and movements over time, which have resulted in their present location in the Indian Ocean. Mahé, Praslin, La Digue, and Frégate are today separated by the shallow seas of the Seychelles Bank, while the outlying western island of Silhouette is of volcanic origin (~63 MY old), and is separated from the other islands by a deep water channel (Fig. 1). During the past 6 million years these islands have been subjected to a series of oscillating sea levels that have either exposed or submerged substantial parts of the land area of the Seychelles, with only the highest points remaining continuously dry (Rocha et al. 2011). Daniels (2011) estimated that freshwater crabs first arrived on the archipelago (on Silhouette) between 2.73 and 6.0 MY ago and dispersed to the other islands within the Seychelles during the Pleistocene (1.18–1.41 MY ago). Seychellum subsequently diverged into a Silhouette group (Clade 1), a Praslin-La Digue group (Clade 2), and a Mahé-Frégate group (Clade 3) (Daniels 2011). The rapid divergence of freshwater crabs in the Seychelles in a relatively short time took place in a similar timeframe reported for speciation in the genus Platythelphusa that produced nine endemic species in Lake Tanganyika, East Africa (Marijnissen et al. 2006).
The oscillating sea level changes during the Pliocene and Pleistocene epochs in the Seychelles were characterised by cyclic periods of contact and isolation between different groups of islands (Rocha et al. 2011). When freshwater crabs first reached the Seychelles between 2.5 and 5.3 MY ago sea levels oscillated between 30 m below and 20 m above their present levels, but Silhouette was separated from Mahé throughout this time by a deep ocean channel (Fig. 1; Daniels 2011). Freshwater crabs on Silhouette would have had brief land connections to the rest of the islands in the Seychelles Bank several times during the past 1 million years when sea levels fell substantially to between 80 to120 m below their present levels (Colonna et al. 1996; Siddall et al. 2003; Miller et al. 2005; Rocha et al. 2011). During the sea level minima in the Pleistocene two lineages of freshwater crabs emerged on the exposed Seychelles Bank. Clade 2 (with a divergence date of ~0.41 MY ago) spread north and eventually gave rise to S. alluaudi that is today found only on Praslin and La Digue, and Clade 3 (with a divergence date of ~0.21 MY ago) spread throughout the southern part of the exposed Seychelles Bank and gradually evolved into S. mahefregate that is today found only on Mahé and Frégate (Fig. 1).
It is not perhaps surprising that freshwater crabs on some closely positioned islands such as Praslin and La Digue (today separated by 10 km of shallow seas) belong to the same species (S. alluaudi). However, elsewhere in the Seychelles Archipelago, crabs found on neighbouring islands separated by shallow seas do not belong to the same species. For example, S. alluaudi is found on Praslin and La Digue but not on nearby Frégate (where S. mahefregate occurs) despite the fact that these three islands were formerly part of the same landmass during the Pleistocene and are separated by only 30 km of shallow seas. In addition, only 20 km of ocean separates S. silhouette on Silhouette from S. mahefregate on Mahé, and the evolution of these two species presumably reflects the long periods of isolation separating Silhouette from the rest of the Seychelles Bank during the Pliocene.
Finally, it is noteworthy that the distribution patterns of the freshwater crabs of Praslin, La Digue, Mahé, and Frégate (the former single landmass of the Seychelles Bank) fall into two groups within this area. The first includes S. alluaudi, which is part of the northern group represented by Praslin and La Digue, while the second includes S. mahefregate that is part of the southern group represented by Mahé and Frégate. Similar north–south distribution patterns have also been reported for some groups of Seychellois vertebrates and invertebrates (Scott 1933; Gardner 1987; Nussbaum and Wu 1995; Radtkey 1996; Gerlach and van Bruggen 1999; Rocha et al. 2011).
Acknowledgements
The South African Biosystematic Initiative (SABI) via the National Research Foundation is thanked for funding a visit by the first author to the University of Stellenbosch. Dr David Rowat, Michelle Etienne, Linda Vanherck, Justin Gerlach, Pat Matyot, Ron Gerlach, Stella Vale, Greg Canning, Dane McDonald and Jurgens Wilson are thanked for logistic and infrastructural support for the second author at various stages of specimen collection in the Seychelles. Rodney Quatre is thanked for issuing a permit that allowed for the collection of freshwater crabs in the National Parks on Mahé. Finally, Ronley Fanchette, Department of Environmental Affairs of the Seychelles, Mahé is thanked for issuing a permit for the exportation of specimens.
References
Borradaile, L. A. (1907). Land freshwater Decapoda. Reports of the Percy Sladen Trust Expedition to the Indian Ocean in 1905. Transactions of the Linnean Society of London, 2, (Zoology) 13, 63–68.Bott, R. (1955). Die Süßwasserkrabben von Afrika (Crust., Decap.) und ihre Stammesgeschichte. Annales du Musée du Congo belge, (Tervuren, Belgique) C-Zoologie, Serie 3 3, 209–352.
Brandis, D., Storch, V., and Türkay, M. (1999). Morphology and function of the reproductive system in the freshwater crab genus Potamon. Journal of Morphology 239, 157–166.
| Morphology and function of the reproductive system in the freshwater crab genus Potamon.Crossref | GoogleScholarGoogle Scholar |
Brandis, D., Storch, V., and Türkay, M. (2000). Taxonomy and zoogeography of the freshwater crabs of Europe, North Africa and the Middle East. Senckenbergiana Biologica 80, 5–56.
Colonna, M. J., Casanova, J., Dullo, W. C., and Camoin, G. (1996). Sea-level changes and δ18O record for the past 34,000 yr from Mayotte reef, Indian Ocean. Quaternary Research 46, 335–339.
| Sea-level changes and δ18O record for the past 34,000 yr from Mayotte reef, Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Cook, B. D., Page, T. J., and Hughes, J. H. (2008a). Importance of cryptic species for identifying representative units of biodiversity for freshwater conservation. Biological Conservation 141, 2821–2831.
| Importance of cryptic species for identifying representative units of biodiversity for freshwater conservation.Crossref | GoogleScholarGoogle Scholar |
Cook, B. D., Pringle, C. M., and Hughes, J. M. (2008b). Phylogeography of an island endemic, the Puerto Rican freshwater crab (Epilobocera sinuatifrons). The Journal of Heredity 99, 157–164.
| Phylogeography of an island endemic, the Puerto Rican freshwater crab (Epilobocera sinuatifrons).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsVCrt7c%3D&md5=703b72d01ec0340a631522bde0b50734CAS | 18252729PubMed |
Cumberlidge, N. (1999). The freshwater crabs of West Africa, family Potamonautidae. Collection Faune et Flore Tropicales Vol. 36. (Orstom: Paris.)
Cumberlidge, N. (2008a). Insular species of Afrotropical freshwater crabs (Crustacea: Decapoda: Brachyura: Potamonautidae and Potamidae) with special reference to Madagascar and the Seychelles. Contributions to Zoology (Amsterdam, Netherlands) 77, 71–81.
Cumberlidge, N. (2008b). Seychellum alluaudi. In ‘IUCN Red List of Threatened Species. Version 2013.2’. Available at www.iucnredlist.org [Verified 25 November 2013]
Cumberlidge, N., and Ng, P. K. L. (2009). Systematics, evolution, and biogeography of the freshwater crabs. In ‘Crustacean Issues: Decapod Crustacean Phylogenetics’. pp. 491–504. (CRC: Leiden.)
Cumberlidge, N., Sternberg, R. v., and Daniels, S. R. (2008). A revision of the higher taxonomy of the Afrotropical freshwater crabs (Decapoda: Brachyura) with a discussion of their biogeography. Biological Journal of the Linnean Society. Linnean Society of London 93, 399–413.
| A revision of the higher taxonomy of the Afrotropical freshwater crabs (Decapoda: Brachyura) with a discussion of their biogeography.Crossref | GoogleScholarGoogle Scholar |
Cumberlidge, N., Ng, P. K. L., Yeo, D. C. J., Magalhaes, C., Campos, M. R., Alvarez, F., Naruse, T., Daniels, S. R., Esser, L. J., Attipoe, F. Y. K., Clotilde-Ba, F. L., Darwall, W., McIvor, A., Ram, M., and Collen, B. (2009). Freshwater crabs and the biodiversity crisis: importance, threats, status, and conservation challenges. Biological Conservation 142, 1665–1673.
| Freshwater crabs and the biodiversity crisis: importance, threats, status, and conservation challenges.Crossref | GoogleScholarGoogle Scholar |
Daniels, S. R. (2011). Reconstructing the colonization and diversification history of the endemic freshwater crab (Seychellum alluaudi) in the granitic and volcanic Seychelles Arichpelago. Molecular Phylogenetics and Evolution 61, 534–542.
| Reconstructing the colonization and diversification history of the endemic freshwater crab (Seychellum alluaudi) in the granitic and volcanic Seychelles Arichpelago.Crossref | GoogleScholarGoogle Scholar | 21824522PubMed |
Daniels, S. R., Cumberlidge, N., Pérez-Losada, M., Marijnissen, S. A. E., and Crandall, K. A. (2006). Evolution of Afrotropical freshwater crab lineages obscured by convergence. Molecular Phylogenetics and Evolution 40, 227–235.
| Evolution of Afrotropical freshwater crab lineages obscured by convergence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlvF2nu70%3D&md5=c168caa05f8a901519981d98f757cc6dCAS | 16621611PubMed |
Esser, L., and Cumberlidge, N. (2011). Evidence that salt water may not be a barrier to the dispersal of Asian freshwater crabs (Decapoda: Brachyura: Gecarcinucidae and Potamidae). The Raffles Bulletin of Zoology 59, 259–268.
Gardner, A. S. (1987). The systematics of the Phelsuma madagascariensis species group of day geckos (Reptilia: Gekkonidae) in the Seychelles. Zoological Journal of the Linnean Society 91, 93–105.
| The systematics of the Phelsuma madagascariensis species group of day geckos (Reptilia: Gekkonidae) in the Seychelles.Crossref | GoogleScholarGoogle Scholar |
Gerlach, J. (1999). Snails of the genus Pachnodus (Mollusca; Gastropoda; Enidae): their origins and evolution. Journal of Biogeography 26, 251–255.
| Snails of the genus Pachnodus (Mollusca; Gastropoda; Enidae): their origins and evolution.Crossref | GoogleScholarGoogle Scholar |
Gerlach, J., and van Bruggen, A. C. (1999). Streptaxidae (Mollusca: Gastropoda: Pulmonata) of the Seychelles Islands, western Indian Ocean. Zoologische Verhandelingen 328, 1–60.
Haig, J. (1984). Land and freshwater crabs of the Seychelles and neighbouring islands. In ‘Biogeography and Ecology of the Seychelles Islands’. (Ed. D. R. Stoddart.) pp. 123–139. (W. Junk: The Hague.)
Jesse, R., Schubart, C. D., and Klaus, S. (2010). Identification of a cryptic lineage within Potamon fluviatile (Herbst) (Crustacea: Brachyura: Potamidae). Invertebrate Systematics 24, 348–356.
| Identification of a cryptic lineage within Potamon fluviatile (Herbst) (Crustacea: Brachyura: Potamidae).Crossref | GoogleScholarGoogle Scholar |
Klaus, S., Brandis, D., Ng, P. K. L., Yeo, D. C. J., and Schubart, C. D. (2009). Phylogeny and biogeography of Asian freshwater crabs of the family Gecarcinucidae (Brachyura: Potamoidea). In ‘Crustacean Issues 18: Decapod Crustacean Phylogenetics’. (Eds J. W. Martin, K. A. Crandall, and D. L. Felder.) pp. 509–532 (Taylor & Francis/CRC Press: Boca Raton, FL.)
Klaus, S., Schubart, C. D., Streit, B., and Pfenninger, M. (2010). When Indian crabs were not yet Asian – biogeographic evidence for Eocene contact of India and Southeast Asia. BMC Evolutionary Biology 10, 287.
| When Indian crabs were not yet Asian – biogeographic evidence for Eocene contact of India and Southeast Asia.Crossref | GoogleScholarGoogle Scholar | 20849594PubMed |
Marijnissen, S. A. E., Michel, E., Daniels, S. R., Erpenbeck, D., Menken, S. B. J., and Schram, F. R. (2006). Molecular evidence for recent divergence of Lake Tanganyika endemic crabs (Decapoda: Platythelphusidae). Molecular Phylogenetics and Evolution 40, 628–634.
| Molecular evidence for recent divergence of Lake Tanganyika endemic crabs (Decapoda: Platythelphusidae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntFWqsbk%3D&md5=430a470310c395e7f22fb708e1371193CAS |
Miller, K. G., Kominz, M. A., Browning, J. V., Wright, J. D., Mountain, G. S., Katz, M. E., Sugarman, P. J., Cramer, B. S., Christie-Blick, N., and Pekar, S. F. (2005). The Phanerozoic record of global sea-level change. Science 310, 1293–1298.
| The Phanerozoic record of global sea-level change.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1GqsrrF&md5=8a8a06e0f7ae4026f0d7c86b9d50fba7CAS | 16311326PubMed |
Milne-Edwards, A., and Bouvier, E. L. (1893). Sur une nouvelle espèce du genre Deckenia (Hilgendorf) recueilli par M. Alluaud aux îles Seychelles. Annales Des Sciences Naturelles. Zoologie Paris 15, 325–336.
Ng, P. K. L., Števčić, Z., and Pretzmann, G. (1995). A revision of the family Deckeniidae Ortmann, 1897 (Crustacea: Decapoda: Brachyura: Potamoidea), with description of a new genus (Gecarcinucoidea, Gecarcinucidae) from the Seychelles, Indian Ocean. Journal of Natural History 29, 581–600.
| A revision of the family Deckeniidae Ortmann, 1897 (Crustacea: Decapoda: Brachyura: Potamoidea), with description of a new genus (Gecarcinucoidea, Gecarcinucidae) from the Seychelles, Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Ng, P. K. L., Guinot, D., and Davy, P. (2008). Systema Brachyuorum: Part I. An annotated checklist of extant Brachyuran crabs of the world. The Raffles Bulletin of Zoology 17, 1–286.
Nussbaum, R. A., and Wu, S. H. (1995). Distribution, variation, and systematics of the Seychelles treefrog, Tachycnemis seychellensis (Amphibia: Anura: Hyperoliidae). Journal of Zoology 236, 383–406.
| Distribution, variation, and systematics of the Seychelles treefrog, Tachycnemis seychellensis (Amphibia: Anura: Hyperoliidae).Crossref | GoogleScholarGoogle Scholar |
Ortmann, A. E. (1897). Carcinologische studien. Zoologische Jahrbucher (Systematics) 10, 256–372.
Ortmann, A. E. (1902). The geographical distribution of freshwater decapods and its bearing upon ancient geography. Proceedings of the American Philosophical Society 41, 267–400.
Pfenninger, M., and Schwenk, K. (2007). Cryptic animal species are homogenously distributed among taxa and biogeographical regions. BMC Evolutionary Biology 7, 121.
| Cryptic animal species are homogenously distributed among taxa and biogeographical regions.Crossref | GoogleScholarGoogle Scholar | 17640383PubMed |
Radtkey, R. R. (1996). Adaptive radiation of day-geckos (Phelsuma) in the Seychelles archipelago: a phylogenetic analysis. Evolution 50, 604–623.
| Adaptive radiation of day-geckos (Phelsuma) in the Seychelles archipelago: a phylogenetic analysis.Crossref | GoogleScholarGoogle Scholar |
Rathbun, M. J. (1894). Crabs from the Indian Ocean. Proceedings of the United States National Museum 17, 1–7.
Rathbun, M. J. (1905). Les crabes d’eau douce. Nouvelles Archives du Museum d’Histoire Naturelle, Paris 4 7, 159–323.
Rathbun, M. J. (1906). Les crabes d’eau douce. Nouvelles Archives du Musdum d’Histoire Naturelle, Paris 4 8, 33–122.
Rocha, S., Harris, D. J., and Posada, D. (2011). Cryptic diversity within the endemic prehensile-tailed gecko Urocotyledon inexpectata across the Seychelles Islands: patterns of phylogeographical structure and isolation at the multilocus level. Biological Journal of the Linnean Society. Linnean Society of London 104, 177–191.
| Cryptic diversity within the endemic prehensile-tailed gecko Urocotyledon inexpectata across the Seychelles Islands: patterns of phylogeographical structure and isolation at the multilocus level.Crossref | GoogleScholarGoogle Scholar |
Rodríguez, G., and López, B. (2003). Insular species of Neotropical freshwater crabs (Crustacea: Brachyura). Journal of Natural History 37, 2599–2614.
| Insular species of Neotropical freshwater crabs (Crustacea: Brachyura).Crossref | GoogleScholarGoogle Scholar |
Scott, H. (1933). General conclusions regarding the insect fauna of the Seychelles and adjacent islands. Transactions of the Linnean Society of London, 2nd series (Zoology) 19, 307–391.
| General conclusions regarding the insect fauna of the Seychelles and adjacent islands.Crossref | GoogleScholarGoogle Scholar |
Siddall, M., Rohling, E. J., Almogi-Labin, A., Hemleben, C., Meischner, D., Schmelzer, I., and Smeed, D. A. (2003). Sea-level fluctuations during the last glacial cycle. Nature 423, 853–858.
| Sea-level fluctuations during the last glacial cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXks1Kmtbw%3D&md5=843cc055d5c403fc195774a524b2ebe4CAS | 12815427PubMed |
Walsh, R. P. D. (1984). Climate of the Seychelles. In ‘Biogeography and Ecology of the Seychelles Islands’. (Ed. D. R. Stoddart.) pp. 39–61. (W. Junk: The Hague.)
Yeo, D. C. J., Ng, P. K. L., Cumberlidge, N., Magalhaes, C., Daniels, S. R., and Campos, M. (2008). A global assessment of freshwater crab diversity (Crustacea: Decapoda: Brachyura). Hydrobiologia 595, 275–286.
| A global assessment of freshwater crab diversity (Crustacea: Decapoda: Brachyura).Crossref | GoogleScholarGoogle Scholar |






