The dark side of an island radiation: systematics and evolution of troglobitic spiders of the genus Dysdera Latreille (Araneae : Dysderidae) in the Canary Islands
Miquel A. Arnedo A D , Pedro Oromí B , Cesc Múrria C , Nuria Macías-Hernández A B and Carles Ribera AA Departament de Biologia Animal, Universitat de Barcelona, Avinguda Diagonal 645, 08028, Barcelona, Spain.
B Departamento de Biología Animal, Universidad de La Laguna, Tenerife, Islas Canarias, Spain.
C Departament d’Ecologia, Universitat de Barcelona, Avinguda Diagonal 645, 08028, Barcelona, Spain.
D Corresponding author. Email: marnedo@ub.edu
Invertebrate Systematics 21(6) 623-660 https://doi.org/10.1071/IS07015
Submitted: 24 April 2007 Accepted: 22 October 2007 Published: 18 December 2007
Abstract
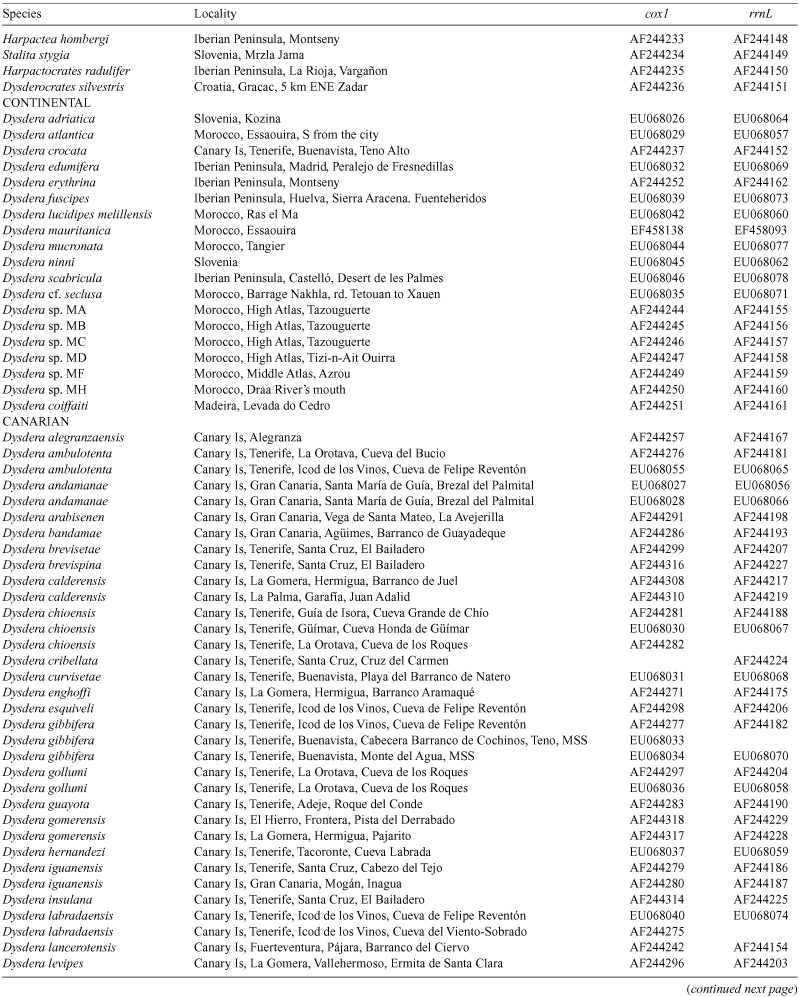
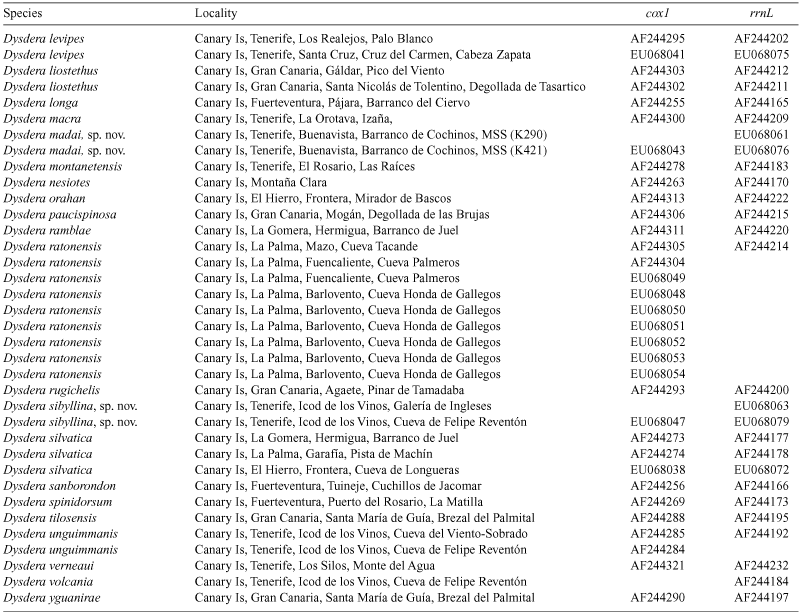
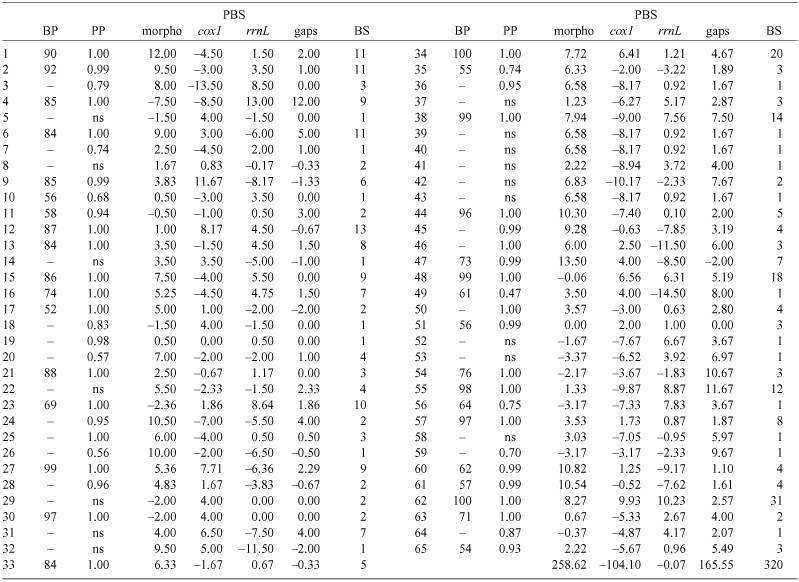
The spider genus Dysdera Latreille is an excellent model for the study of the evolution of cave life: ten species are known to exist exclusively in the subterranean environment of the Canary Islands, where the genus has undergone local diversification. In the present paper, two new troglobitic species (Dysdera madai, sp. nov. and D. sibyllina, sp. nov.) and the previously unknown sex of five additional species are described and illustrated: the males of D. gollumi Ribera & Arnedo, 1994, D. hernandezi Arnedo & Ribera, 1999 and D. labradaensis Wunderlich, 1991; and the females of D. andamanae Arnedo & Ribera, 1997 and D. gibbifera Wunderlich, 1991. The first direct evidence of troglobitic members of Dysdera in micro- and mesocaverns are reported. The evolution of cave life as hypothesised following a combined morphological and molecular phylogeny is investigated. Troglobitic Canarian Dysdera species have colonised the underground on eight independent occasions. The Dysderidae groundplan represents a preadaptation to cave life and has facilitated the colonisation of caves. Canarian members of Dysdera have a predominantly parapatric mode of speciation, although postspeciation changes in distribution may have obscured allopatric processes. Eye regression and, to a lesser extent, larger body size and appendage elongation characterise troglobitic species. The different levels of troglobiomorphism are interpreted as local adaptations to heterogeneous subterranean conditions. The high levels of sympatry among troglobites are explained by trophic segregation and changes in prey capture strategy were involved in the single identified case of subterranean speciation in the group.
Acknowledgements
We wish to thank Manuel Arechavaleta, Eduardo Muñoz, Nieves Zurita, Heriberto López, Salvador de la Cruz, Helena Morales and Antonio J. Pérez, who collected many of the specimens included in the present study, and Rafael García who provided additional specimens. The paper deeply benefited from patient explanations and insightful discussions with Frank Howarth. We are in debt to Laure Desutter-Grandcolas, whose comments and suggestions on an early draft of the manuscript greatly helped to improve the final version. Gustavo Hormiga kindly provided access to the SEM facility at George Washington University. The Cabildos (local authorities) in Tenerife, La Palma and El Hierro granted collecting permits, and the latter two provided logistic facilities. MA was supported by the Ramón y Cajal Programme funded by the Spanish Ministry of Education and Science and the European Regional Development Fund, and NM was supported by a graduate grant from the Autonomous Government of the Canary Islands. This project was funded by the Spanish Ministry of Education and Science grants PB97–0937 (CR&PO) and BOS2003–05876 (MA&PO) and additional financial support was provided through project 2005SGR00045 of the Autonomous Government of Catalonia. Part of the fieldwork was supported by a LIFE-Nature project on the conservation of cave fauna, co-sponsored by the Consejería de Medio Ambiente (Environmental Department) of the Canarian Autonomous Government, and project P1042004/047 also of the the Canarian Autonomous Government.
Allegrucci G.,
Todisco V., Sbordoni V.
(2005) Molecular phylogeography of Dolichopoda cave crickets (Orthoptera, Rhaphidophoridae): a scenario suggested by mitochondrial DNA. Molecular Phylogenetics and Evolution 37, 153–164.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
[Verified 30 November 2007]
Gould S. J., Vrba E. S.
(1982) Exaptation- a missing term in the science of form. Palaeobiology 8, 4–15.

Guillou H.,
Carracedo J. C.,
Torrado F. P., Badiola E. R.
(1996) K-Ar ages and magnetic stratigraphy of a hotspot-induced, fast grown oceanic island: El Hierro, Canary Islands. Journal of Volcanology and Geothermal Research 73, 141–155.
| Crossref | GoogleScholarGoogle Scholar |

Hall T.
(1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98NT. Nucleic Acids Symposium Series 41, 95–98.

Hedin M. C.
(1997a) Molecular phylogenetics at the population/species interface in cave spiders of the Southern Appalachians (Araneae: Nesticidae: Nesticus). Molecular Biology and Evolution 14, 309–324.
| PubMed |

Hedin M. C.
(1997b) Speciational history in a Diverse clade of habitat-specialized spiders (Araneae: Nesticidae: Nesticus): Inferences from geographic-based sampling. Evolution 51, 1929–1945.
| Crossref | GoogleScholarGoogle Scholar |

Hoch H., Howarth F. G.
(1999) Multiple cave invasion by the species of the cixiid planthopper Oliarus in Hawaii. Zoological Journal of the Linnean Society 127, 453–475.
| Crossref | GoogleScholarGoogle Scholar |

Holsinger J. R.
(1988) Troglobites: the evolution of cave-dwelling organisms. American Scientist 76, 146–153.

Hopkin S. P., Martin M. H.
(1985) Assimilation of zinc, cadmium, lead, copper, and iron by the spider Dysdera crocata, a predator of woodlice. Bulletin of Environmental Contamination and Toxicology 34, 183–187.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Howarth F. G.
(1972) Cavernicoles in lava tubes on the island of Hawaii. Science 175, 325–326.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Howarth F. G.
(1973) The cavernicolous fauna of Hawaiian lava tubes, 1. Introduction. Pacific Insects 15, 139–151.

Howarth F. G.
(1983) Ecology of cave arthropods. Annual Review of Entomology 28, 365–389.
| Crossref | GoogleScholarGoogle Scholar |

Howarth F. G.
(1987) The evolution of non-relictual tropical troglobites. International Journal of Speleology 16, 1–16.

Howarth F. G.
(1993) High stress subterranean habitats and evolutionary change in cave-inhabiting arthropods. American Naturalist 142, 565–577.

Jarrige J.
(1957) Coleopteres Brachelytra de l’ile de la Reunion. Memoires Institut Sciences Madagascar. Serie E 8, 103–118.

Juberthie C.
(1984) La colonisation du mileu souterrain; théories et méthodes, relations avec la spéciation et l’evolution souterraine. Memoires de Biospeologie 11, 65–102.

Kumar S.,
Tamura K., Nei M.
(2004) MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefings in Bioinformatics 5, 150–163.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Kuntner M.,
Sket B., Blejec A.
(1999) A comparison of the respiratory systems in some cave and surface species of spiders (Araneae, Dysderidae). The Journal of Arachnology 27, 142–148.

Leys R.,
Watts C. H.,
Cooper S. J., Humphreys W. F.
(2003) Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution 57, 2819–2834.
| PubMed |

Losos J. B.,
Jackman T. R.,
Larson A.,
De Queiroz K., Rodriguez Schettino L.
(1998) Contingency and determinism in replicated adaptive radiations of island lizards. Science 279, 2115–2118.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Martín J. L., Oromí P.
(1986) An ecological study of Cueva de los Roques lava tube (Tenerife, Canary Islands). Journal of Natural History 20, 375–388.
| Crossref | GoogleScholarGoogle Scholar |

Miller J. A.
(2005) Cave adaptation in the spider genus Anthrobia (Araneae, Linyphiidae, Erigoninae). Zoologica Scripta 34, 565–592.
| Crossref | GoogleScholarGoogle Scholar |

Miller J. S., Wenzel J. W.
(1995) Ecological characters and phylogeny. Annual Review of Entomology 40, 389–415.
| Crossref | GoogleScholarGoogle Scholar |

Moulds T. A.,
Murphy N.,
Adams M.,
Reardon T.,
Harvey M. S.,
Jennings J., Austin A. D.
(2007) Phylogeography of cave pseudoscorpions in southern Australia. Journal of Biogeography 34, 951–962..
| Crossref |

Peck S. B.
(1975) The invertebrate fauna of tropical American caves, Part III: Jamaica, an introduction. International Journal of Speleology 7, 303–326.

Peck S. B.
(1990) Eyeless arthropods of the Galapagos Islands, Ecuador: Composition and origin of the cryptozoic fauna of a young, tropical, oceanic archipelago. Biotropica 22, 366–381.
| Crossref | GoogleScholarGoogle Scholar |

Peck S. B., Finston T. L.
(1993) Galapagos islands, troglobites: the questions of tropical toglobites, parapatric distribution with eyed-sister-species, and the origin of parapatric speciation. Memoires de Biospeologie 20, 19–37.

Petit-Maire N.,
Delibrias G.,
Meco J.,
Pomel S., Rosso J. C.
(1986) Paleoclimatologie des Canarie Orientales (Fuerteventura). Comptes Rendues Academie Sciences Paris, t. 303, Serie II 13, 1241–1246.

Pollard S. D.
(1986) Prey capture in Dysdera crocata (Araneae: Dysderidae), a long fanged spider. New Zealand Journal of Zoology 13, 149–150.

Pollard S. D.,
Jackson R. R.,
Van Olphen A., Robertson M. W.
(1995) Does Dysdera crocata (Araneae Dysderidae) prefer woodlice as prey? Ethology Ecology and Evolution 7, 271–275.

Pons J., Vogler A.
(2006) Size, frequency, and phylogenetic signal of multiple-residue indels in sequence alignment introns. Cladistics 22, 144–156.
| Crossref | GoogleScholarGoogle Scholar |

Posada D., Buckley T. R.
(2004) Model selection and model averaging in phylogenetics: advantages of Akaike Information Criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53, 793–808.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Posada D., Crandall K. A.
(1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14, 817–818.
| Crossref |
PubMed |

Rivera M. A.,
Howarth F. G.,
Taiti S., Roderick G. K.
(2002) Evolution in Hawaiian cave-adapted isopods (Oniscidea: Philosciidae): vicariant speciation or adaptive shifts? Molecular Phylogenetics and Evolution 25, 1–9.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Rognon P.,
Coude-Gaussen G.,
Le Costumer M. N.,
Balouet J. C., Ochietti S.
(1989) Le massif dunaire de Jandia (Fuerteventura, Canaries): evolution des paleoenvironments de 20.000 BP a l’actuel. Bulletin de l’Association francaise pour l’Etude du Quaternaire 1, 31–37.

Ronquist F., Huelsenbeck J. P.
(2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574.
| Crossref |
PubMed |

Rovner J. S.
(1986) Nests of terrestrial spiders maintain a physical gill flooding and the evolution of silk constructions. The Journal of Arachnology 14, 327–338.

Sanger F.,
Nicklen S., Coulsen A. R.
(1977) DNA sequencing with chain terminating inhibitors. Proceedings of the National Academy of Sciences of the United States of America 74, 5463–5468.
| Crossref |
PubMed |

Sarthein M.,
Tetzlaff G.,
Koopmann B.,
Wolter K., Pflaumann U.
(1981) Glacial and interglacial wind regimes over the eastern subtropical Atlantic and North-west Africa. Nature 293, 193–196.
| Crossref |

Schilthuizen M.,
Cabanban A. S., Haase M.
(2005) Possible speciation with gene flow in tropical cave snails. Journal of Zoological Systematics and Evolutionary Research 43, 133–138.
| Crossref | GoogleScholarGoogle Scholar |

Simmons M. P., Ochoterena H.
(2000) Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49, 369–381.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Simmons M. P.,
Ochoterena H., Carr T. G.
(2001) Incorporation, relative homoplasy, and effect of gap characters in sequence-based phylogenetic analyses. Systematic Biology 50, 454–462.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Simon C.,
Frati F.,
Beckenbach A.,
Crespi B.,
Liu H., Flook P.
(1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America 87, 651–701.

Strecker U.,
Bernatchez L., Wilkens H.
(2003) Genetic divergence between cave and surface populations of Astyanax in Mexico (Characidae, Teleostei). Molecular Ecology 12, 699–710.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Thompson J. D.,
Gibson T. J.,
Plewniak F.,
Jeanmougin F., Higgins D. G.
(1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25, 4876–4882.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Trajano E.
(1995) Evolution of tropical troglobites: applicability of the model of quaternary climatic fluctuations. Memoires de Biospeologie 22, 203–210.

Wheeler W. C., Hayashi C. Y.
(1998) The phylogeny of the extant chelicerate orders. Cladistics 14, 173–192.
| Crossref | GoogleScholarGoogle Scholar |

Wiens J. J.,
Chippindale P. T., Hillis D. M.
(2003) When are phylogenetic analyses misled by convergence? A case study in Texas cave salamanders. Systematic Biology 52, 501–514.
| PubMed |

Wilkens H., Hüppop K.
(1986) Sympatric speciation in cave fishes? Studies on a mixed population of epi- and hypogean Astyanax (Characidae, Pisces). Zeitschrift fuer Zoologische Systematik und Evolutionsforschung 24, 223–230.

Wunderlich J.
(1991) Die Spinnen-fauna der Makaronesischen Inseln. Beiträge zur Araneologie 1, 1–619.

Young N. D., Healy J.
(2003) GapCoder automates the use of indel characters in phylogenetic analysis. BMC Bioinformatics 4, 6.
| Crossref |
PubMed |

|
|