Ant mimicry in Australian plant bugs: a new genus (Heteroptera: Miridae: Austromirini: Carenotus gen. nov.), eight new species, myrmecomorphic traits, host plants and distribution
Arlee McMah A * and Gerasimos Cassis
A * and Gerasimos Cassis  A *
A *
A
Abstract
The Australian plant bug tribe Austromirini consists of ant-mimetic taxa which are poorly known, with no information of their phylogenetic relationships and ant-mimetic traits. In this study, we examined nearly 1000 ingroup specimens and developed a comprehensive morphological dataset comprising 37 characters, which was analysed both weighted and unweighted, using ‘Tree analysis using New Technology’ (TNT) software. A single minimal length phylogenetic tree was found, comprising a monophyletic group of ant-mimetic taxa, that included Myrmecoroides rufescens, Myrmecoridea sp., Kirkaldyella spp. and eight species of a new genus, Carenotus gen. nov. The myrmecomorphic traits of Carenotus and allied ant-mimetic taxa are documented and analysed phylogenetically, in conjunction with genitalic characters. Carenotus is defined by the myrmecomorphic colour patterning of the abdominal venter, whereas the ingroup species relationships are supported by genitalic characters alone. Carenotus is described as new with eight included species as follows: C. arltunga sp. nov., C. louthensis sp. nov., C. luritja sp. nov., C. pullabooka sp. nov., C. scaevolaphilus sp. nov., C. schwartzi sp. nov., C. tanami sp. nov. and C. yuendumu sp. nov. Host plant associations are also documented, ranging from host plant specificity and genus-group preferences to host plant generalism. The distribution of Carenotus species is documented with reference to phytogeographic subregions, with all species being semi-arid and arid dwelling. The male and female genitalia of Kirkaldyella pilosa and K. rugosa are described and illustrated, for comparative and phylogenetic purposes. This research expands our knowledge on the plant bug tribe Austromirini and has broader implications for myrmecomorphic research in the suborder Heteroptera.
ZooBank: urn:lsid:zoobank.org:pub:2FF9BE23-38A6-42B4-8488-74F216D8237F
Keywords: ant mimicry, Australia, Bush Blitz, distribution, host plants, myrmecomorphy, Orthotylinae, phylogeny, taxonomy.
Introduction
Batesian ant-mimicry theory postulates that the simulation of unpalatable signal properties of ant model(s) by mimicking species confuses ant-averse predators and thereby enhances mimic survivorship (McIver and Stonedahl 1993; Nelson et al. 2006; Nelson 2011). This has putatively evolved independently in many insect lineages and is characterised by a convergence of morphological and behavioural traits (=myrmecomorphy) to those of either generalised ant facies or specific ant models (McIver and Stonedahl 1993). McIver and Stonedahl (1993) identified the Miridae (Insecta: Heteroptera) as one of the more outstanding family-groups that exhibit myrmecomorphy, recognising its presence in independent mirid lineages.
Carvalho (1952) established a suprageneric classification of the Miridae, which includes ant mimicry as foundational in diagnosing certain tribal groups (e.g. Phylinae: Pilophorini, Mirinae: Herdoniini). Schuh (1974) in a seminal work on ant mimicry in southern African Miridae, recognised its occurrence and independence in the second and third most speciose mirid subfamilies, the Phylinae and Orthotylinae respectively. He described two new tribes composed largely of ant-mimetic taxa, the Leucophoropterini (Phylinae) and Nichomachini (Orthotylinae). Schuh (1984) subsequently described a third new ant-mimetic tribe, the Auricillocorini from the Indo-Pacific (subsequently synonymised with Hallodapini by Wyniger et al. 2023).
The Miridae with 11,200+ described species (Schuh 1995, 2013; Cassis and Schuh 2012), has myrmecomorphic lineages in 5 of the 8 subfamilies and 15 tribes (Mirinae: Herdoniini (Carvalho and Ferreira 1973), Stenodemini (Schwartz 2008), Deraeocorinae: Deraeocorini, Surinamellini (Kim et al. 2023), Bryocorinae: Dicyphini (China and Carvalho 1951); Orthotylinae: Austromirini (Cassis and Moulds 2002; Cassis and Wall 2010), Ceratocapsini (Henry 2015), Halticini (Tatarnic and Cassis 2012), Nichomachini (Schuh 1974), Orthotylini (Schuh 1974; Cassis 2008), Phylinae: Cremnorrhinini: Coatonocapsina (Schuh and Menard 2013), Hallodapini (Wyniger 2010 as Pronotocrepini; Wyniger et al. 2023), Leucophoropterini (Schuh 1974; Menard and Schuh 2011; Schuh and Menard 2013; Menard et al. 2014), Pilophorini (Schuh 1991; Schuh and Menard 2013), Semiini: Exocarpocorina (Weirauch and Schuh 2011; Schuh and Menard 2013).
Schuh (1974) assessed at that time, 17% of Orthotylinae and 34% of Phylinae genera as ant mimetic, but without estimates of how many species are mimetic in the two subfamilies. The prevalence of myrmecomorphic taxa in mirid tribal groups more generally ranges from single occurrences within a suprageneric group, such as Setocoris bybliphilus China & Carvalho (Cassis 1986; Cassis and Gross 1995), to mostly ant mimetic in the Leucophoropterini (Menard and Schuh 2011; Schuh and Menard 2013).
This work is part of a broader study of mirid myrmecomorphic taxa in Australia that belong to the tribe Austromirini (Orthotylinae), as defined by Cassis and Gross (1995), Cassis and Moulds (2002) and Cassis and Wall (2010) (see Austromirini checklist in Cassis and Symonds 2014). We describe herein a new genus of austromirine – Carenotus gen. nov. – a mostly arid-dwelling collection of eight new species found in all states and territories aside from Tasmania and the Australian Capital Territory. This includes two new species collected during the Bush Blitz program (Preece et al. 2015). These species are found on a broad range of host plants (field-based criteria of Cassis and Symonds 2016), including numerous species of Eremophila R.Br. (Scrophulariaceae), Callitris Vent. (Cupressaceae), Acacia Mill. (Fabaceae), Solanum Linnaeus (Solanaceae), Sida Linnaeus (Malvaceae), as well as multiple dryland species of the Amaranthaceae.
We also provide a morphologically based phylogenetic analysis of the Carenotus species relationships, where we explore the phylogenetic transformation of myrmecomorphic characters, including exemplars of three other Australian orthotyline mimics (Kirkaldyella Poppius, 1921; Myrmecoroides Gross; and Myrmecoridea Poppius, 1921) (Poppius and Bergroth 1921; Gross 1964; Cassis and Moulds 2002; Cassis and Wall 2010).
Materials and methods
Materials examined
This study was based on examination of 986 specimens of Carenotus, as well as exemplar samples of outgroup species. Specimens of Carenotus examined in this work belong to the following institutions: Australian Museum (AM); American Museum of Natural History (AMNH), National Museum of Victoria (NMV), the Northern Territory Museum and Art Gallery (NTM), Queensland Museum (QM ex UQIC), South Australian Museum (SAMA), University of New South Wales (UNSW) and Western Australia Museum (WAM).
Genitalic dissections
Male and female abdomens were macerated in 5% potassium hydroxide for 5–10 min and rinsed with distilled water. The genitalia were removed from the abdomen and examined in glycerol. Genitalic structures were illustrated using a camera lucida with a Leica M205C stereomicroscope and Leica DMB5000 compound microscope. Scale bars are listed in the figures. In total, 19 genitalic characters are codified for the phylogenetic analysis, given in Table 1. The data matrix is given in Table 2.
| Character number | Character states | |
|---|---|---|
| 1 | Body colour patterning: (0) mostly green, (1) mostly dark brown, without transverse contrasting cream–white markings, (2) mostly dark brown, with transverse contrasting cream–white markings. | |
| 2 | Pale marking on posterior margin of pronotum: (0) absent, (1) present, continuous, (2) present, broken. | |
| 3 | Clavus colour patterning: (0) uniformly green, (1) dark brown, concolourous with most of corium, (2) dark brown, with contrasting cream–white markings or predominantly cream–white. | |
| 4 | Medial claval margin cream–white stripe: (0) absent, (1) present. | |
| 5 | Anterolateral claval margin cream–white stripe: (0) absent, (1) present. | |
| 6 | Distal region of clavus cream–white spots: (0) absent, (1) present. | |
| 7 | Cream–white marking on proximal exocorium region: (0) absent, (1) present. | |
| 8 | Cream–white on apex of corium: (0) absent, (1) small spot, thin or medium transverse fasciae, (2) broad subtriangular transverse fasciae. | |
| 9 | White spot on membrane next to cuneus: (0) absent, (1) present. | |
| 10 | Hemelytral membrane opacity: (0) transparent, (1) translucent, fumose, (2) translucent to opaque, brown, concolourous or near concolourous with cuneus. | |
| 11 | Male abdominal venter cream–white banding: (0) absent, (1) present, on SIV–SVI. | |
| 12 | Female abdominal venter cream–white banding: (0) absent, (1) present, on SIII–SVI. | |
| 13 | Leg colour: (0) mostly yellow or green, (1) mostly brown, orange–brown or red–brown, concolourous with most of thoracic pleura and dorsum. | |
| 14 | Head shape: (0) weakly triangular, (1) strongly triangular. | |
| 15 | Pronotum shape: (0) subtrapezoidal to weakly campanulate, (1) strongly campanulate. | |
| 16 | Metathoracic glands: (0) peritreme and evaporative areas well developed, (1) peritreme and evaporative areas greatly reduced or absent. | |
| 17 | Male hemelytra costal margin: (0) straight, (1) weakly convex, (2) excavated. | |
| 18 | Median flexion line: (0) strongly arcuate, removed from costal margin, (1) almost straight, narrowly removed from costal margin,(2) contiguous in part with costal margin. | |
| 19 | Pygophore left tergal process: (0) absent, (1) present. | |
| 20 | Pygophore left tergal process: (0) undivided, (1) bifurcate. | |
| 21 | Pygophore left tergal process length: (0) short, (1) long. | |
| 22 | Pygophore right tergal process: (0) absent, (1) present. | |
| 23 | Pygophore right tergal process: (0) short, (1) long. | |
| 24 | Left paramere apophysis position at rest: (0) within genital opening of pygophore, (1) on ventral surface of pygophore. | |
| 25 | Left paramere sensory lobe: (0) small, basal, (1) greatly enlarged, apical. | |
| 26 | Left paramere basal dorsal lobe: (0) absent, (1) present. | |
| 27 | Right paramere medial dorsal margin: (0) absent, (1) present. | |
| 28 | Number of endosomal spicules: (0) two or three, (1) one. | |
| 29 | Proximal endosomal spicule: (0) undivided, (1) bifurcate or trifurcate. | |
| 30 | Medial branch of proximal endosomal spicule: (0) undivided, (1) mesially or apically bifurcate. | |
| 31 | Proximal endosomal spicule margins: (0) smooth, (1) serrate. | |
| 32 | First dorsal endosomal spicule: (0) present, (1) absent. | |
| 33 | First dorsal endosomal spicule keel: (0) present, (1) absent. | |
| 34 | Second dorsal endosomal spicule: (0) present, (1) absent. | |
| 35 | Sclerotised rings: (0) oval, short, (1) elliptical, long. | |
| 36 | Inter-ramal lobes: (0) elongate, apically rounded, (1) broadly mitt: shaped, apically attenuated, (2) narrowly mitt-shaped. | |
| 37 | Inter-ramal lobes length: (0) subequal to inter-ramal sclerite, (1) longer than inter-ramal sclerite, (2) shorter than inter-ramal sclerite. |
| Taxa | Character states | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | ||
| Carenotus arltunga | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | |
| Carenotus louthensis | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | |
| Carenotus luritja | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |
| Carenotus pullabooka | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Carenotus scaevolaphilus | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | ? | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | |
| Carenotus schwartzi | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | ? | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |
| Carenotus tanami | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |
| Carenotus yuendumu | 2 | 2 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |
| Kirkaldyella pilosa | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | ? | ? | 0 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | |
| Kirkaldyella rugosa | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | ? | ? | 0 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | |
| Myrmecoridea sp. | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | ? | 1 | 1 | 1 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | ? | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ? | ? | ? | |
| Myrmecoroides rufescens | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 2 | 0 | ? | ? | 0 | ? | 0 | 0 | ? | ? | 1 | 0 | ? | 1 | 1 | 1 | 1 | 1 | 0 | 0 | |
| Naranjakotta myrtlephila | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ? | 0 | 0 | 1 | 1 | 0 | 0 | ? | 0 | 1 | 1 | 0 | 0 | 0 | 1 | |
| Myrtlemiris astartephila | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | ? | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | ? | ? | ? | |
| Myrtlemiris meanarra | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | ? | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | ? | ? | ? | |
?, unknown or inapplicable.
Distribution
Maps were produced using ArcGis Pro (ver. 3.0.3, see https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview) and ArcGIS online software (see https://www.arcgis.com/index.html), and the Human Geography base map. Phytogeographic subregion boundaries of González-Orozco et al. (2014) and Ebach et al. (2015) were overlaid with the Carenotus species distributions.
Plant associations
Host plant information is given in Table 3. Images of host plants, habitats and vouchers were obtained from the Planetary Bug Inventory database (see https://research.amnh.org/pbi/locality/). Host plants are based on the criteria of Cassis and Symonds (2016), where plant species with ≥5 Carenotus specimens are designated hosts. Confidence in host plant designations is supported by the presence of both sexes and nymphs, and the number of localities for each association. Those with <5 Carenotus specimens are regarded as sitting records. The suprageneric plant classification is based on Stevens (2012).
| Carenotus species | Plant family | Plant species | |
|---|---|---|---|
| C. arltunga | Fabaceae | Acacia aneura (59; ♂; ♀; N; 6L) | |
| Fabaceae | Acacia brachystachya (20; ♂; ♀; N; 3L) | ||
| Fabaceae | Acacia paraneura (2; ♀; 1L) | ||
| Fabaceae | Acacia (unknown sp.) (7 ♂; ♀; N; 4L) | ||
| C. louthensis | Poaceae | Aristida holathera (3; ♂; ♀; 1L) | |
| Scrophulariaceae | Eremophila longifolia (4; ♂; ♀; 1L) | ||
| Scrophulariaceae | Eremophila mitchellii (15; ♂; ♀; 3L) | ||
| Scrophulariaceae | Eremophila sturtii (176; ♂; ♀; N; 6L) | ||
| C. luritja | Amaranthaceae | Atriplex elachophylla (31; ♂; ♀; 2L) | |
| Amaranthaceae | Salsola kali (1; ♂; 1L) | ||
| Amaranthaceae | Sclerolaena holtiana (11; ♀; 1L) | ||
| Fabaceae | Acacia aneura (1; ♀; 1L) | ||
| Malvaceae | Sida fibulifera (10; ♂; ♀; 1L) | ||
| C. pullabooka | Cupressaceae | Callitris glaucophylla (8; ♂; ♀; 3L) | |
| Cupressaceae | Callitris gracilis (7; ♂; ♀; N; 1L) | ||
| Cupressaceae | Callitris preissii (1; ♂; 1L) | ||
| C. scaveolaphilus | Goodeniaceae | Scaevola spinescens (16; ♂; ♀; 1L) | |
| C. schwartzi | Solanaceae | Solanum orbiculatum (48; ♂; ♀; N; 1L) | |
| C. tanami | Scrophulariaceae | Eremophila duttonii (8; ♂; ♀; 1L) | |
| Scrophulariaceae | Eremophila gilesii (9; ♂; ♀; 1L) | ||
| Scrophulariaceae | Eremophila latrobei (2; ♂; ♀; N; 1L) | ||
| Scrophulariaceae | Eremophila sturtii (1; ♀; 1l) | ||
| Scrophulariaceae | Eremophila (unknown sp.) (34; ♂; ♀; 3L) | ||
| C. yuendumu | Scrophulariaceae | Eremophila duttonii (405; ♂; ♀; N; 7L) | |
| Scrophulariaceae | Eremophila forrestii (3; ♂; ♀; 1L) | ||
| Scrophulariaceae | Eremophila freelingii (4; ♂; ♀; 1L) | ||
| Scrophulariaceae | Eremophila gilesii (14; ♂; ♀; N; 3L) | ||
| Scrophulariaceae | Eremophila glabra (12; ♂; ♀; 1L) | ||
| Scrophulariaceae | Eremophila latrobei (7; ♂; ♀; 1L) | ||
| Scrophulariaceae | Eremophila longifolia (1; N; 1L) | ||
| Scrophulariaceae | Eremophila neglecta (16; ♂; ♀; N; 3L) | ||
| Scrophulariaceae | Eremophila scoparia (6; ♂; ♀; N; 1L) | ||
| Scrophulariaceae | Eremophila sturtii (44; ♂; ♀; N; 5L) | ||
| TOTAL | 986 specimens |
Rows in bold font indicate host plant species, with ≥5 Carenotus specimens collected on it. Rows in regular font indicate plant species as sitting records, with <5 Carenotus specimens collected on it. Within brackets after plant species name are: number of specimens; sex collected; nymphs collected; number of localities. Abbreviations: N, nymph; L, locality number.
Morphological measurements
Morphometric characters were measured using a Leica MC170HD camera attached to a Leica M165C stereomicroscope. Measurements were recorded from five male and five specimens per species, where possible. Measurements in millimetres were collected for the following traits: body length from clypeus to the posterior margin of the hemelytra; length from apex of clypeus to tip of cuneus; head length; pronotum length; scutellum length; cuneus length; head width; pronotum width; scutellum width; interocular distance; length of four antennal segments (AI–AIV). Measurements are given in Table 4.
| Length | Width | InterOc | AntSegI | AntSegII | AntSegIII | AntSegIV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body | Cun-Clyp | Head | Pron | Scut | Cun | Head | Pron | Scut | ||||||||
| C. arltunga | ||||||||||||||||
| ♂ (n = 5) | Mean | 3.42 | 2.64 | 0.25 | 0.43 | 0.35 | 0.57 | 0.66 | 0.91 | 0.43 | 0.3 | 0.28 | 1.06 | 0.98 | 0.38 | |
| s.d. | 0.48 | 0.05 | 0.04 | 0.14 | 0.07 | 0.04 | 0.24 | 0.18 | 0.12 | 0.1 | 0.06 | 0.31 | 0.16 | 0.02 | ||
| Range | 1.08 | 0.15 | 0.11 | 0.33 | 0.16 | 0.1 | 0.52 | 0.38 | 0.3 | 0.24 | 0.15 | 0.88 | 0.37 | 0.05 | ||
| Min. | 2.99 | 2.58 | 0.2 | 0.28 | 0.27 | 0.5 | 0.45 | 0.76 | 0.31 | 0.18 | 0.19 | 0.61 | 0.71 | 0.36 | ||
| Max. | 4.07 | 2.74 | 0.31 | 0.61 | 0.43 | 0.6 | 0.97 | 1.14 | 0.62 | 0.43 | 0.34 | 1.49 | 1.08 | 0.41 | ||
| ♀ (n = 5) | Mean | 3.7 | 2.69 | 0.4 | 0.6 | 0.38 | 0.45 | 0.91 | 1.09 | 0.54 | 0.45 | 0.25 | 0.44 | 0.82 | 0.37 | |
| s.d. | 0.17 | 0.07 | 0.04 | 0.02 | 0.05 | 0.04 | 0.02 | 0.06 | 0.02 | 0.03 | 0.03 | 0.13 | 0.13 | 0 | ||
| Range | 0.46 | 0.2 | 0.13 | 0.06 | 0.15 | 0.11 | 0.05 | 0.19 | 0.04 | 0.07 | 0.07 | 0.32 | 0.29 | 0 | ||
| Min. | 3.42 | 2.58 | 0.35 | 0.58 | 0.31 | 0.37 | 0.89 | 0.99 | 0.51 | 0.41 | 0.22 | 0.26 | 0.66 | 0.37 | ||
| Max. | 3.87 | 2.79 | 0.48 | 0.64 | 0.46 | 0.48 | 0.94 | 1.18 | 0.56 | 0.48 | 0.29 | 0.59 | 0.95 | 0.37 | ||
| C. louthensis | ||||||||||||||||
| ♂ (n = 5) | Mean | 3.07 | 2.46 | 0.24 | 0.38 | 0.31 | 0.49 | 0.58 | 0.87 | 0.43 | 0.31 | 0.25 | 0.97 | 0.91 | 0.34 | |
| s.d. | 0.06 | 0.17 | 0.02 | 0.09 | 0.03 | 0.09 | 0.14 | 0.13 | 0.12 | 0.12 | 0.09 | 0.33 | 0.14 | 0.06 | ||
| Range | 0.15 | 0.41 | 0.06 | 0.22 | 0.09 | 0.22 | 0.32 | 0.29 | 0.27 | 0.28 | 0.2 | 0.92 | 0.36 | 0.14 | ||
| Min. | 2.99 | 2.22 | 0.2 | 0.28 | 0.27 | 0.38 | 0.45 | 0.76 | 0.31 | 0.18 | 0.13 | 0.57 | 0.71 | 0.26 | ||
| Max. | 3.14 | 2.63 | 0.26 | 0.49 | 0.36 | 0.6 | 0.77 | 1.05 | 0.58 | 0.47 | 0.34 | 1.49 | 1.07 | 0.41 | ||
| ♀ (n = 5) | Mean | 3.33 | 2.5 | 0.37 | 0.54 | 0.34 | 0.38 | 0.77 | 1.06 | 0.52 | 0.45 | 0.16 | 0.43 | 0.87 | 0.24 | |
| s.d. | 0.12 | 0.12 | 0.04 | 0.04 | 0.02 | 0.03 | 0.02 | 0.05 | 0.03 | 0.03 | 0.02 | 0.11 | 0.15 | 0.09 | ||
| Range | 0.33 | 0.34 | 0.11 | 0.1 | 0.04 | 0.08 | 0.06 | 0.13 | 0.08 | 0.08 | 0.07 | 0.33 | 0.4 | 0.21 | ||
| Min. | 3.14 | 2.28 | 0.32 | 0.46 | 0.33 | 0.36 | 0.73 | 1 | 0.49 | 0.4 | 0.12 | 0.27 | 0.62 | 0.16 | ||
| Max. | 3.47 | 2.62 | 0.42 | 0.56 | 0.37 | 0.43 | 0.79 | 1.13 | 0.56 | 0.49 | 0.19 | 0.61 | 1.02 | 0.38 | ||
| C. luritja | ||||||||||||||||
| ♂ (n = 5) | Mean | 3.26 | 2.58 | 0.23 | 0.38 | 0.35 | 0.55 | 0.61 | 0.88 | 0.43 | 0.3 | 0.28 | 1.07 | 1.02 | 0.38 | |
| s.d. | 0.3 | 0.04 | 0.03 | 0.09 | 0.08 | 0.03 | 0.18 | 0.15 | 0.12 | 0.11 | 0.06 | 0.3 | 0.16 | 0.02 | ||
| Range | 0.77 | 0.11 | 0.08 | 0.26 | 0.19 | 0.1 | 0.38 | 0.32 | 0.29 | 0.27 | 0.15 | 0.84 | 0.45 | 0.05 | ||
| Min. | 2.99 | 2.52 | 0.18 | 0.28 | 0.27 | 0.5 | 0.45 | 0.76 | 0.31 | 0.18 | 0.18 | 0.65 | 0.71 | 0.36 | ||
| Max. | 3.76 | 2.63 | 0.27 | 0.54 | 0.46 | 0.6 | 0.84 | 1.08 | 0.6 | 0.45 | 0.34 | 1.49 | 1.16 | 0.41 | ||
| ♀ (n = 5) | Mean | 3.23 | 2.58 | 0.38 | 0.54 | 0.35 | 0.34 | 0.88 | 0.95 | 0.51 | 0.48 | 0.21 | 0.51 | 1.14 | 0.16 | |
| s.d. | 0.1 | 0.07 | 0.03 | 0.02 | 0.04 | 0.04 | 0.01 | 0.02 | 0.04 | 0.03 | 0.05 | 0.12 | 0.32 | 0 | ||
| Range | 0.26 | 0.18 | 0.08 | 0.04 | 0.11 | 0.12 | 0.04 | 0.05 | 0.11 | 0.1 | 0.14 | 0.32 | 0.84 | 0 | ||
| Min. | 3.07 | 2.45 | 0.34 | 0.52 | 0.29 | 0.29 | 0.86 | 0.93 | 0.47 | 0.44 | 0.15 | 0.39 | 0.93 | 0.16 | ||
| Max. | 3.33 | 2.63 | 0.42 | 0.56 | 0.4 | 0.41 | 0.9 | 0.99 | 0.58 | 0.53 | 0.29 | 0.71 | 1.77 | 0.16 | ||
| C. pullabooka | ||||||||||||||||
| ♂ (n = 5) | Mean | 3.03 | 2.48 | 0.26 | 0.41 | 0.32 | 0.49 | 0.62 | 0.89 | 0.42 | 0.3 | 0.28 | 0.88 | 0.83 | 0.38 | |
| s.d. | 0.2 | 0.16 | 0.04 | 0.11 | 0.05 | 0.1 | 0.18 | 0.15 | 0.11 | 0.1 | 0.05 | 0.44 | 0.21 | 0.02 | ||
| Range | 0.6 | 0.38 | 0.13 | 0.27 | 0.14 | 0.29 | 0.4 | 0.32 | 0.26 | 0.26 | 0.13 | 1.24 | 0.53 | 0.05 | ||
| Min. | 2.73 | 2.26 | 0.2 | 0.28 | 0.27 | 0.31 | 0.45 | 0.76 | 0.31 | 0.18 | 0.2 | 0.25 | 0.54 | 0.36 | ||
| Max. | 3.33 | 2.63 | 0.32 | 0.55 | 0.41 | 0.6 | 0.85 | 1.08 | 0.57 | 0.44 | 0.34 | 1.49 | 1.07 | 0.41 | ||
| ♀ (n = 4) | Mean | 3.03 | 2.69 | 0.39 | 0.26 | 0.53 | 0.53 | 0.55 | 0.63 | 0.33 | 0.34 | 0.47 | 0.85 | 0.97 | 0.38 | |
| s.d. | 0.06 | 0.15 | 0.28 | 0.10 | 0.41 | 0.06 | 0.15 | 0.22 | 0.01 | 0.22 | 0.26 | 0.23 | 0.45 | 0.02 | ||
| Range | 0.13 | 0.36 | 0.67 | 0.26 | 0.97 | 0.16 | 0.35 | 0.52 | 0.03 | 0.54 | 0.61 | 0.52 | 1.24 | 0.05 | ||
| Min. | 2.99 | 2.58 | 0.20 | 0.09 | 0.27 | 0.44 | 0.45 | 0.25 | 0.31 | 0.18 | 0.31 | 0.55 | 0.25 | 0.36 | ||
| Max. | 3.11 | 2.95 | 0.87 | 0.35 | 1.24 | 0.60 | 0.80 | 0.77 | 0.35 | 0.72 | 0.92 | 1.07 | 1.49 | 0.41 | ||
| C. scaevolaphilus | ||||||||||||||||
| ♂ (n = 5) | Mean | 3.03 | 2.44 | 0.27 | 0.4 | 0.31 | 0.48 | 0.58 | 0.88 | 0.42 | 0.3 | 0.25 | 1.21 | 0.99 | 0.38 | |
| s.d. | 0.04 | 0.2 | 0.05 | 0.11 | 0.03 | 0.1 | 0.14 | 0.14 | 0.11 | 0.11 | 0.09 | 0.2 | 0.16 | 0.02 | ||
| Range | 0.13 | 0.46 | 0.14 | 0.28 | 0.07 | 0.25 | 0.32 | 0.29 | 0.27 | 0.25 | 0.21 | 0.45 | 0.39 | 0.05 | ||
| Min. | 2.99 | 2.17 | 0.2 | 0.28 | 0.27 | 0.35 | 0.45 | 0.76 | 0.31 | 0.18 | 0.13 | 1.04 | 0.71 | 0.36 | ||
| Max. | 3.11 | 2.63 | 0.34 | 0.56 | 0.33 | 0.6 | 0.77 | 1.05 | 0.58 | 0.44 | 0.34 | 1.49 | 1.1 | 0.41 | ||
| ♀ (n = 5) | Mean | 3.14 | 2.33 | 0.31 | 0.56 | 0.32 | 0.38 | 0.78 | 1.06 | 0.57 | 0.47 | 0.18 | 0 | 0 | 0 | |
| s.d. | 0.08 | 0.07 | 0.04 | 0.04 | 0.03 | 0.03 | 0.02 | 0.06 | 0.06 | 0.03 | 0.01 | 0 | 0 | 0 | ||
| Range | 0.22 | 0.19 | 0.09 | 0.12 | 0.07 | 0.09 | 0.05 | 0.17 | 0.15 | 0.06 | 0.03 | 0 | 0 | 0 | ||
| Min. | 3.03 | 2.28 | 0.27 | 0.5 | 0.29 | 0.32 | 0.76 | 1 | 0.49 | 0.44 | 0.16 | 0 | 0 | 0 | ||
| Max. | 3.25 | 2.47 | 0.37 | 0.62 | 0.36 | 0.41 | 0.81 | 1.17 | 0.64 | 0.5 | 0.19 | 0 | 0 | 0 | ||
| C. schwartzi | ||||||||||||||||
| ♂ (n = 5) | Mean | 3.06 | 2.46 | 0.25 | 0.4 | 0.32 | 0.49 | 0.58 | 0.88 | 0.4 | 0.29 | 0.26 | 0.84 | 0.95 | 0.29 | |
| s.d. | 0.08 | 0.18 | 0.04 | 0.11 | 0.04 | 0.09 | 0.14 | 0.14 | 0.09 | 0.09 | 0.09 | 0.48 | 0.13 | 0.11 | ||
| Range | 0.2 | 0.44 | 0.12 | 0.27 | 0.1 | 0.22 | 0.33 | 0.31 | 0.22 | 0.24 | 0.21 | 1.29 | 0.36 | 0.28 | ||
| Min. | 2.99 | 2.2 | 0.2 | 0.28 | 0.27 | 0.38 | 0.45 | 0.76 | 0.31 | 0.18 | 0.13 | 0.2 | 0.71 | 0.12 | ||
| Max. | 3.18 | 2.63 | 0.32 | 0.55 | 0.37 | 0.6 | 0.78 | 1.07 | 0.54 | 0.42 | 0.34 | 1.49 | 1.07 | 0.41 | ||
| ♀ (n = 5) | Mean | 3.42 | 2.53 | 0.38 | 0.57 | 0.35 | 0.42 | 0.79 | 1.11 | 0.56 | 0.46 | 0.17 | 0.47 | 0.97 | 0.24 | |
| s.d. | 0.12 | 0.06 | 0.04 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.04 | 0.01 | 0.02 | 0.14 | 0.05 | 0.08 | ||
| Range | 0.31 | 0.13 | 0.11 | 0.08 | 0.05 | 0.07 | 0.07 | 0.09 | 0.09 | 0.04 | 0.04 | 0.37 | 0.13 | 0.2 | ||
| Min. | 3.22 | 2.45 | 0.31 | 0.54 | 0.34 | 0.39 | 0.75 | 1.08 | 0.52 | 0.44 | 0.15 | 0.28 | 0.91 | 0.14 | ||
| Max. | 3.53 | 2.59 | 0.42 | 0.62 | 0.39 | 0.46 | 0.81 | 1.17 | 0.61 | 0.48 | 0.19 | 0.65 | 1.05 | 0.34 | ||
| C. tanami | ||||||||||||||||
| ♂ (n = 5) | Mean | 2.98 | 2.41 | 0.24 | 0.39 | 0.3 | 0.48 | 0.57 | 0.88 | 0.4 | 0.27 | 0.25 | 0.88 | 0.91 | 0.31 | |
| s.d. | 0.09 | 0.24 | 0.03 | 0.11 | 0.02 | 0.09 | 0.13 | 0.14 | 0.08 | 0.08 | 0.09 | 0.43 | 0.14 | 0.1 | ||
| Range | 0.29 | 0.59 | 0.08 | 0.29 | 0.06 | 0.24 | 0.29 | 0.35 | 0.22 | 0.21 | 0.21 | 1.2 | 0.36 | 0.24 | ||
| Min. | 2.82 | 2.04 | 0.2 | 0.28 | 0.27 | 0.36 | 0.45 | 0.76 | 0.31 | 0.18 | 0.12 | 0.29 | 0.71 | 0.17 | ||
| Max. | 3.11 | 2.63 | 0.28 | 0.57 | 0.33 | 0.6 | 0.74 | 1.11 | 0.53 | 0.4 | 0.34 | 1.49 | 1.07 | 0.41 | ||
| ♀ (n = 5) | Mean | 3.06 | 2.31 | 0.28 | 0.5 | 0.36 | 0.37 | 0.77 | 1.07 | 0.55 | 0.45 | 0.18 | 0.5 | 0.83 | 0 | |
| s.d. | 0.18 | 0.06 | 0.07 | 0.05 | 0.04 | 0.04 | 0.01 | 0.04 | 0.02 | 0.02 | 0.03 | 0.2 | 0.11 | 0 | ||
| Range | 0.52 | 0.18 | 0.18 | 0.11 | 0.1 | 0.11 | 0.04 | 0.12 | 0.06 | 0.07 | 0.08 | 0.55 | 0.31 | 0 | ||
| Min. | 2.74 | 2.25 | 0.2 | 0.45 | 0.3 | 0.32 | 0.75 | 1.02 | 0.54 | 0.42 | 0.14 | 0.25 | 0.64 | 0 | ||
| Max. | 3.26 | 2.42 | 0.38 | 0.56 | 0.4 | 0.43 | 0.79 | 1.13 | 0.59 | 0.49 | 0.21 | 0.8 | 0.95 | 0 | ||
| C. yuendumu | ||||||||||||||||
| ♂ (n = 5) | Mean | 3.08 | 2.47 | 0.22 | 0.38 | 0.33 | 0.5 | 0.59 | 0.89 | 0.43 | 0.3 | 0.26 | 0.92 | 0.94 | 0.31 | |
| s.d. | 0.08 | 0.17 | 0.02 | 0.09 | 0.06 | 0.08 | 0.15 | 0.16 | 0.12 | 0.11 | 0.09 | 0.39 | 0.13 | 0.08 | ||
| Range | 0.22 | 0.39 | 0.06 | 0.24 | 0.14 | 0.2 | 0.32 | 0.36 | 0.28 | 0.26 | 0.19 | 1.05 | 0.36 | 0.21 | ||
| Min. | 2.99 | 2.25 | 0.2 | 0.28 | 0.27 | 0.4 | 0.45 | 0.76 | 0.31 | 0.18 | 0.14 | 0.44 | 0.71 | 0.2 | ||
| Max. | 3.21 | 2.63 | 0.26 | 0.51 | 0.4 | 0.6 | 0.77 | 1.12 | 0.59 | 0.45 | 0.34 | 1.49 | 1.07 | 0.41 | ||
| ♀ (n = 5) | Mean | 3.27 | 2.49 | 0.34 | 0.55 | 0.34 | 0.39 | 0.78 | 1.11 | 0.54 | 0.45 | 0.16 | 0.54 | 1.04 | 0.32 | |
| s.d. | 0.17 | 0.13 | 0.07 | 0.04 | 0.04 | 0.02 | 0.04 | 0.05 | 0.06 | 0.02 | 0.01 | 0.08 | 0.21 | 0.03 | ||
| Range | 0.5 | 0.35 | 0.21 | 0.09 | 0.1 | 0.04 | 0.12 | 0.13 | 0.16 | 0.06 | 0.04 | 0.22 | 0.64 | 0.08 | ||
| Min. | 2.98 | 2.26 | 0.28 | 0.51 | 0.3 | 0.37 | 0.71 | 1.05 | 0.47 | 0.42 | 0.13 | 0.39 | 0.81 | 0.28 | ||
| Max. | 3.48 | 2.61 | 0.49 | 0.6 | 0.4 | 0.41 | 0.83 | 1.18 | 0.64 | 0.49 | 0.17 | 0.61 | 1.44 | 0.36 | ||
Measurements are given in millimetres. Abbreviations: Cun-Clyp, length between clypeus apex and cuneus tip; Pron, pronotum; Scut, scutellum; InterOc, interocular distance; AntSegI–IV, antennal segments I–IV; s.d., standard deviation, Min., mininum; Max., maximum.
Imaging
Dorsal and lateral habitus images were taken with a Canon 5Dsr camera attached to a Microptics Visual Imaging System. Multiple images were concatenated with Zerene Stacker software (ver. T2023-06-11-1120, see https://zerenesystems.com/cms/stacker). Final images and plates were processed using Photoshop CC 2019 (ver. 14.0, Adobe). Scanning electron micrographs were taken using a Hitachi TM-3000 desktop scanner, with samples uncoated and examined at low voltage. Scale bars are provided on each figure.
Morphological homology and terminology of non-myrmecomorphic traits
Morphological homologies are based on those given in Cassis and Schuh (2012) and as applied to Naranjakotta Cassis & Symonds, 2016 (Cassis and Symonds 2016). The homologies of the male genitalia follow Cassis (2008), with modifications in Symonds and Cassis (2018) and Cheng and Cassis (2019). Terminology of the female genitalia follows Davis (1955) and Schwartz (2008, 2011), and as applied in Cassis and Symonds (2016) for Naranjakotta (Orthotylinae). The genitalic characters are given in the taxon descriptions as well as the codified characters for the phylogenetic analysis in Table 1.
Myrmecomorphic traits
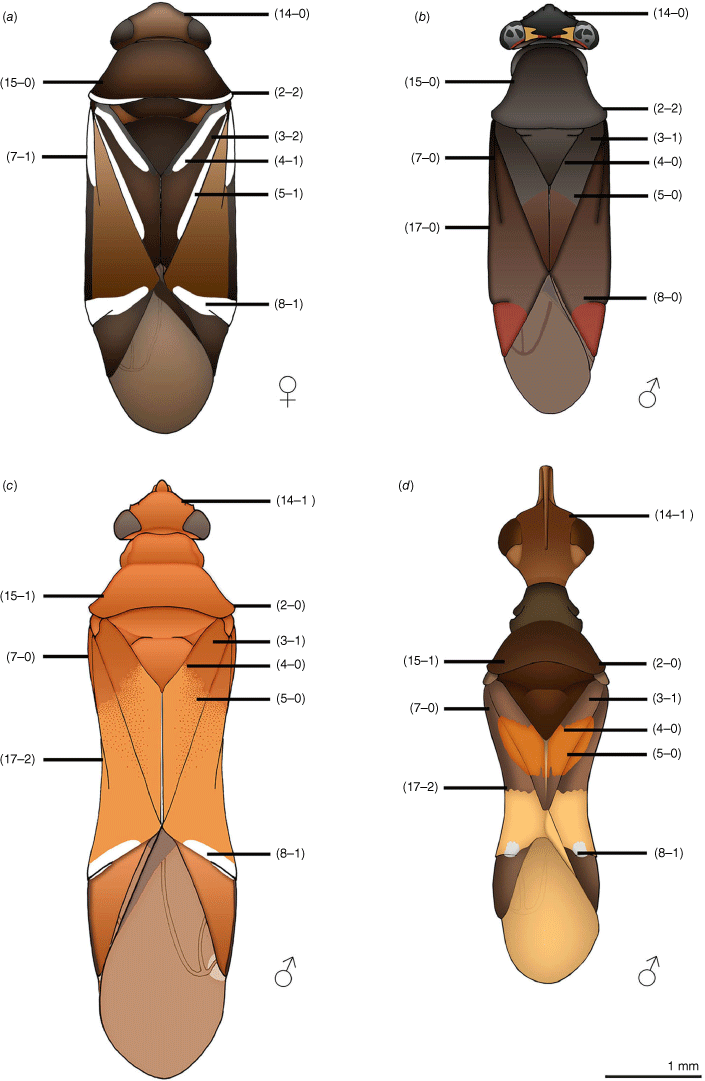
Taxonomic authors refer to ant-mimetic facies or myrmecomorphic traits (e.g. Carvalho 1952; Schuh 1974; Menard and Schuh 2011; Henry 2015; Wyniger et al. 2023), typically without reference to specific ant models. Schuh (1974) proposed a number of myrmecomorphic traits for the Orthotylinae and Phylinae, as follows: (1) carinate genae; (2) concave posterior margin of head; (3) anterior constriction of the pronotum; (4) presence of a flattened pronotal collar; (5) hour-glass shaped pronotum; (6) transverse pale fasciae or maculae contrasting against a mostly dark forewing and body; (7) sinuation of the costal margin of the forewing; and, (8) female forewing shortening (e.g. brachyptery, microptery). McIver and Stonedahl (1993) further recognised that the ant petiole is simulated by patterns of contrasting colour (i.e. pale on dark) or vestiture on the forewings. They also hypothesised antennal and mandibular myrmecomorphic traits in the Miridae, which are not relevant in this Carenotus case study.
In this work we recognise 18 characters that are putatively myrmecomorphic, in alignment with modifications of those of Schuh (1974) and McIver and Stonedahl (1993) (see Table 1, characters and character states 1–18). A selection of the myrmecomorphic character states are illustrated in Fig. 1.
Phylogenetics
The characters and character list are given in Table 1. The data matrix is given in Table 2. Phylogenetic analyses were performed using TNT software (‘Tree analysis using New Technology’, ver. 1.5, see https://www.lillo.org.ar/phylogeny/tnt/; Goloboff et al. 2008). Data were initially analysed with characters unweighted using the New Technology option with 10,000 iterations. Data were subsequently run with implied weighting using sectorial search with RSS and CSS applied with default settings; ratchet with 1000 iterations, drift with 1000 cycles, and default tree fusing. Implied weighting analyses were performed with K = 3–10 and 100. Given the same topologies were found for unweighted and implied weights analyses, symmetric resampling was applied to the K = 3 tree, with 10,000 iterations. The tree file and data matrix were transferred to Winclada software (ver. 1.0000, K. C. Nixon, Ithaca, NY, USA, see www.diversityoflife.org/winclada) and Illustrator (ver. 27.3, Adobe) to produce a graphics file displaying the optimised characters to the minimal tree.
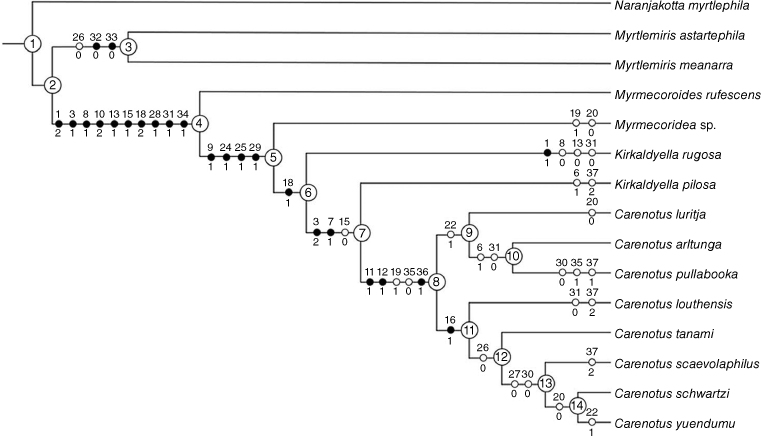
The following seven taxa were assigned as putative outgroups: Naranjakotta myrtlephila Cassis & Symonds, 2016, Myrtlemiris meanarra Cheng, Mututantri & Cassis, 2012, Myrtlemiris astartephila Cheng, Mututantri & Cassis, 2012. Myrmecoroides rufescens Cassis & Wall, 2010, Myrmecoridea sp., Kirkaldyella pilosa Cassis & Moulds, 2002, and Kirkaldyella rugosa Poppius, 1921. Naranjakotta myrtlephila was specified as the root of the tree. The character and character state list and data matrix are given in Tables 1 and 2 respectively, and the phylogenetic tree in Fig. 2.
Results
Phylogeny
The unweighted and implied weights (K = 3–10 and 100) analyses resulted in an identical topology. The unweighted tree had a length of 70, with a consistency index of 0.61 and a retention index of 0.76. Synapomorphies (closed circles) and contradicted synapomorphies (open circles) are displayed in Fig. 2.
The following discussion refers to character distribution from Node 2, accounting for the myrmecomorphic genera, with Naranjakotta myrtlephila as the root of the tree.
Non-myrmecomorphic taxa Myrtlemiris astartephila and M. meanarra are sister groups to the myrmecomorphic taxa at Node 4, with symmetric resampling support of 100%.
The sister-species relationship of Myrtlemiris astartephila and M. meanarra is defined by two synapomorphies: first dorsal endosomal spicule present (32–0) and first dorsal endosomal spicule keel present (33–0). It is further supported with one contradicted synapomorphy (left paramere basal dorsal lobe absent; 26–0). Symmetric resampling support for this node is 84%.
The myrmecomorphic clade consists of the genera Myrmecoroides, Myrmecoridea, Kirkaldyella and Carenotus. It is defined by the following 10 synapomorphies: body colour patterning mostly dark brown, with transverse contrasting cream–white markings (1–2); clavus dark brown, concolourous with most of corium (3–1); cream–white on apex of corium, as small spot or transverse fasciae (8–1); hemelytral membrane translucent to opaque, brown, and concolourous or near concolourous with cuneus (10–2); legs mostly brown, orange–brown or red–brown, concolourous with most of thoracic pleura and dorsum (13–1); pronotum strongly campanulate (15–1); median flexion line contiguous in part with costal margin (18–2); one endosomal spicule present (28–1); proximal endosomal spicule serrate (31–1); and second dorsal endosomal spicule absent (34–1). Symmetric resampling support for this node is 99%.
This clade is defined by the following four synapomorphies: the presence of a white spot on the hemelytral membrane next to the cuneus (9–1); left paramere apophysis position on ventral wall of pygophore (24–1); left paramere sensory lobe greatly enlarged (25–1); and proximal endosomal spicule bifurcate or trifurcate (29–1). Symmetric resampling support for this node is 62%.
This clade clusters the putatively poor myrmecomorphic genera Carenotus and Kirkaldyella. It is defined by one synapomorphy: median flexion line almost straight, narrowly removed from costal margin (18–1). Symmetric resampling support for this node is 60%. It results in non-monophyly of the two included Kirkaldyella species.
This node comprises K. pilosa + Carenotus and is defined by two synapomorphies: clavus dark brown, with contrasting cream–white markings or predominantly cream–white (3–2); and cream–white marking on proximal exocorium region present (7–1). It is supported with one contradicted synapomorphy: the pronotum is subtrapezoidal to weakly campanulate (15–0). Symmetric resampling support for this node is insignificant, at 41%.
Carenotus is recognised as a monophyletic genus, relative to the seven outgroups included. This node consists of two subclades and is defined by the following three synapomorphies: male abdominal venter with cream–white banding (11–1); female abdominal venter with cream–white banding (12–1); and, inter-ramal lobes broadly mitt-shaped (36–1). It is further supported with two contradicted synapomorphies: left tergal process present (19–1) and inter-ramal lobes oval and short (35–0). Symmetric resampling support for this node is 94%
C. luritja (C. arltunga + C. pullabooka) is supported by one contradicted synapomorphy: pygophore right tergal process present (22–1). Symmetric resampling support for this node is insignificant, at 14%.
The sister-species relationship of C. arltunga + C. pullabooka is supported by two homoplasious characters: distal region of clavus with cream–white spots (6–1); and, proximal endosomal spicule smooth (31–0). Symmetric resampling support for this node is insignificant, at 35%.
C. louthensis (C. tanami (C. scaevolaphilus (C. schwartzi + C. yuendumu) is defined by one synapomorphy: metathoracic gland peritreme and evaporative area greatly reduced (16–1). Symmetric resampling support for this node is significant, at 57%.
C. tanami (C. scaevolaphilus (C. schwartzi + C. yuendumu) is supported by one contradicted synapomorphy: left paramere basal dorsal lobe absent (26–0). Symmetric resampling support for this node is significant, at 51%.
Ant-mimetic characters
The myrmecomorphic genera in the phylogenetic study herein share seven non-contradicted myrmecomorphic and three genitalic synapomorphies. Characters 1, 3, 8 and 13 are colour characters of the dorsum, particularly the contrasting patterns on the corium and clavus. The pronotal shape (character 15) is challenging to assess because of the difficulty in differentiating between subtrapezoidal and campanulate character states. The median flexion line orientation is group-defining for the three austromirine genus-groups in this study. This is commensurate with the ‘sinuation of the lateral margin (i.e. costal margin)’ referred to by Schuh (1974).
Myrmecoroides is hypothesised as a ‘good’ myrmecomorphic taxon, with its strongly triangular head, as a result of the exaggerated bicompressed frontal plate (=coplanar frons + clypeus), which is potentially simulating an ant mandible. Schuh (1974) hypothesised the carinate genae in Philippine Leucophoropterini and Pilophorini as indicative of a mandible; this may be analogous in its mandibular simulation to the Myrmecoroides frontal plate. It is noteworthy that the Myrmecoroides frontal plate is also sexually dimorphic in shape (Cassis and Wall 2010). In addition, the females of this genus exhibit microptery, which enhances the simulation of an ant petiole.
The remaining myrmecomorphic taxa form a monophyletic group based on the presence of a white spot on the forewing membrane adjacent to the cuneus (9–1), plus four male genitalic characters, including the presence of the left paramere apophysis positioned on the ventral wall of the pygophore. In this analysis, Kirkaldyella is non-monophyletic, with K. pilosa sister to Carenotus, inclusive of two putative myrmecomorphic characters, the patterned claval colour patterning (3–2) and the cream–white colour on the proximal region of the exocorium.
Carenotus is grouped together by two colour-based myrmecomorphic traits, namely the cream–white colour of the abdominal venter in both sexes. This is unique within the myrmecomorphic Austromirini. This patterning is also observed in the myrmecomorphic genus Daerlac Signoret, a distantly related ant-mimetic genus in the family Rhyparochromidae (Cassis and Symonds 2012). Thus the abdominal venter colouration represents a remarkable convergence in two infraorders (Cimicomorpha and Pentato-momorpha respectively). Within Carenotus there are no myrmecomorphic characters that are group-defining, aside from the cream–white distal spots on the clavus that support the sister-group relationship of C. arltunga and C. pullabooka. The ingroup relationships of Carenotus are chiefly defined in the phylogenetic analysis by male and female genitalic characters, although none are non-contradicted synapomorphies. Thus, there is no evidence of phylogenetic transformation of the myrmecomorphic traits identified within Carenotus in this study (Table 1, Fig. 1, 2), a result which is to be retested in a future phylogenomic treatment of ant-mimetic austromirines.
Taxonomy
Genus Carenotus, gen. nov.
ZooBank: urn:lsid:zoobank.org:act:6F5A53FC-B669-40CE-873E-A13825D0CBE6
Type species: Carenotus arltunga McMah & Cassis, sp. nov., by original designation.
From the Greek karenon meaning head; suffix -tus in reference to the prominent head. Masculine.
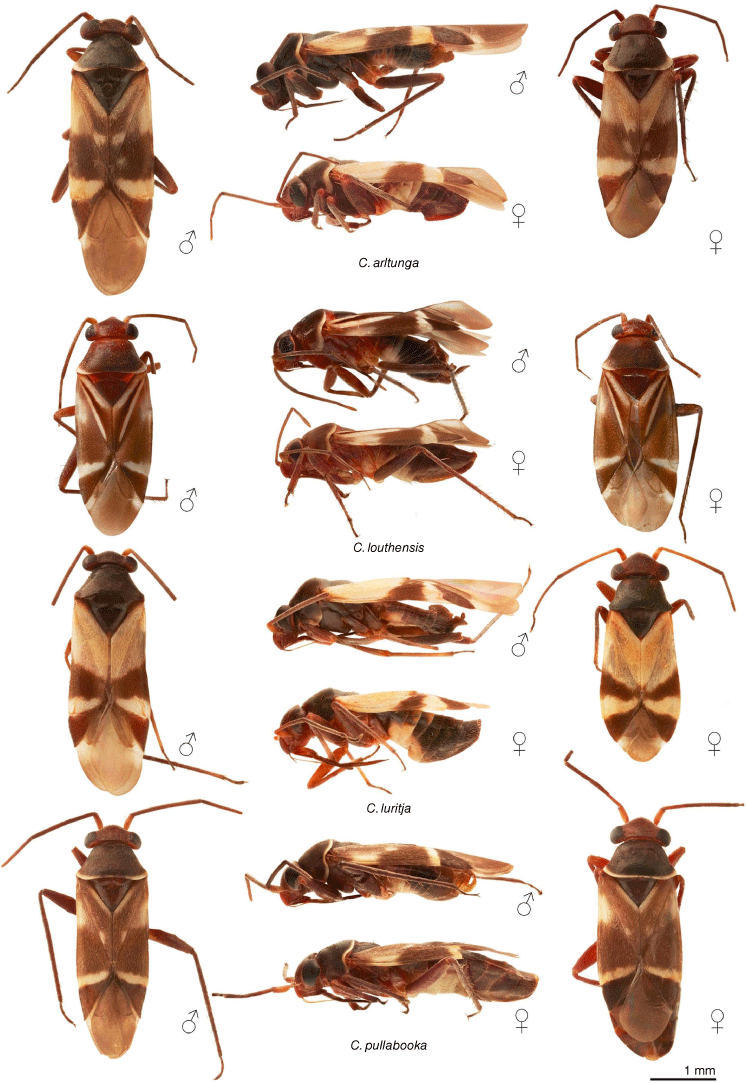
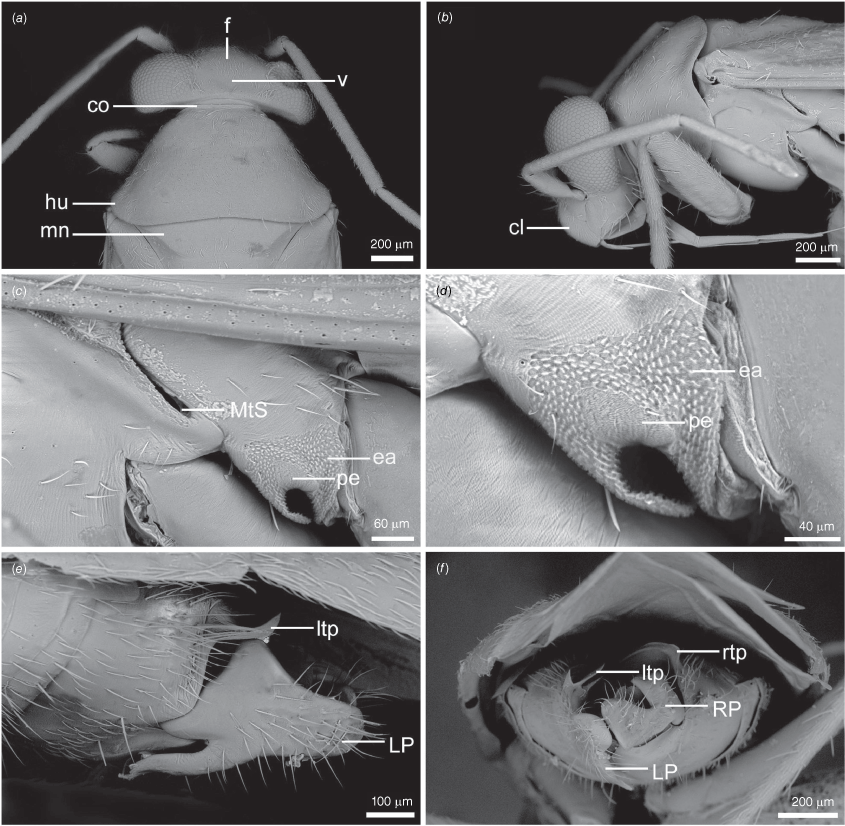
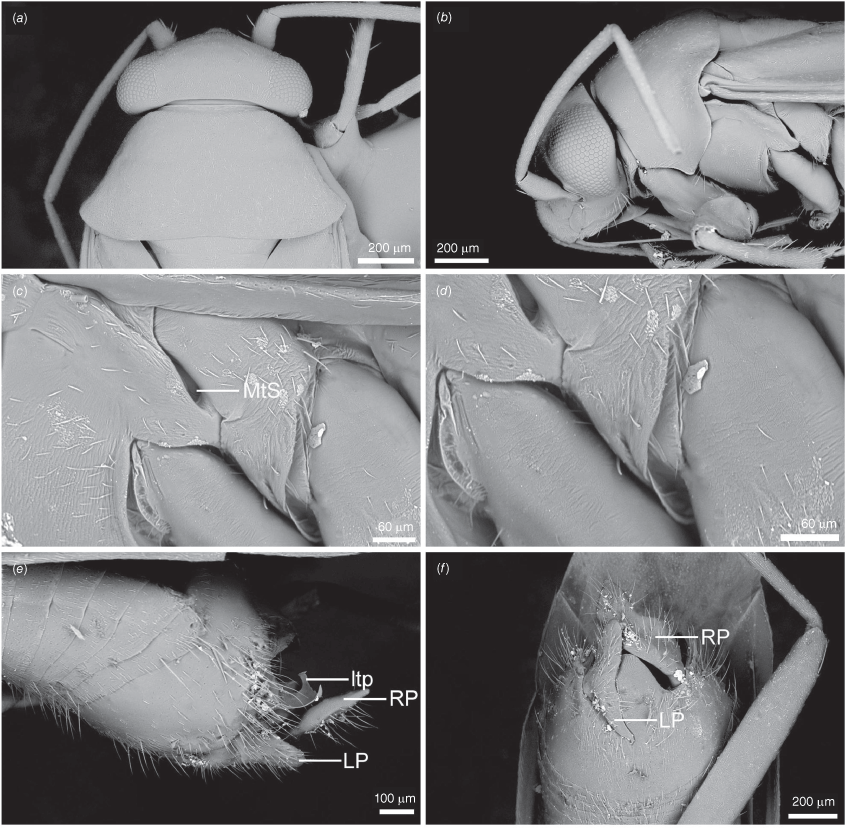
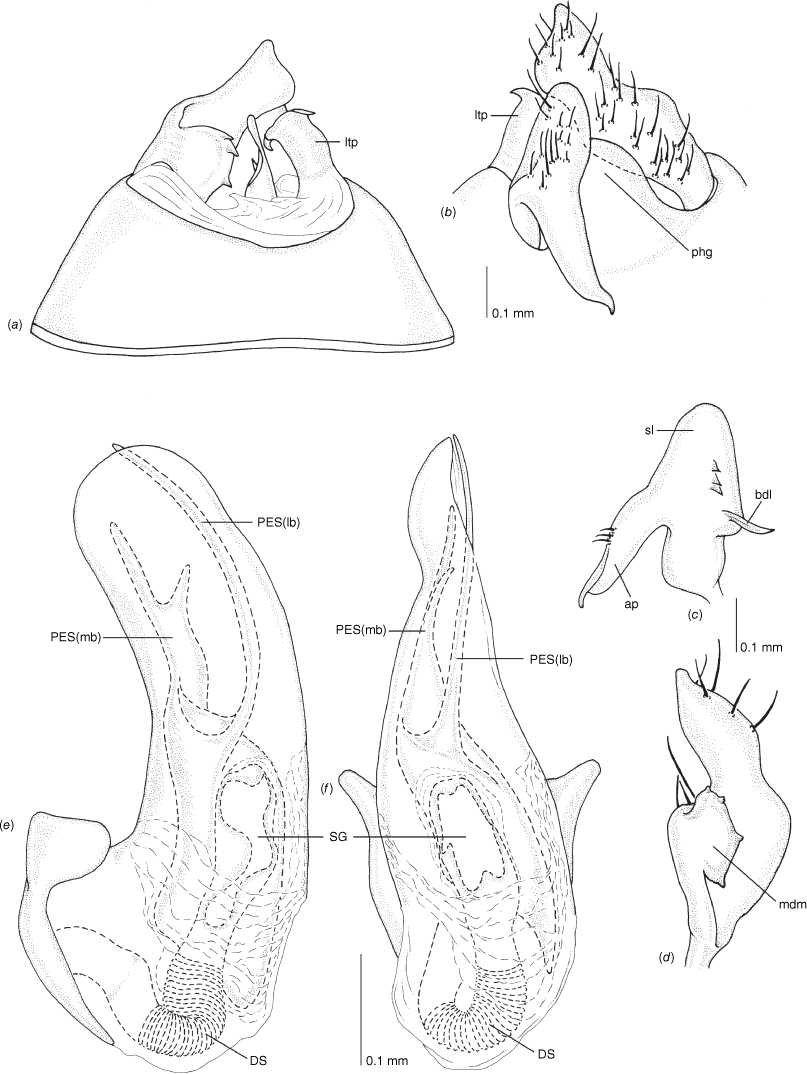
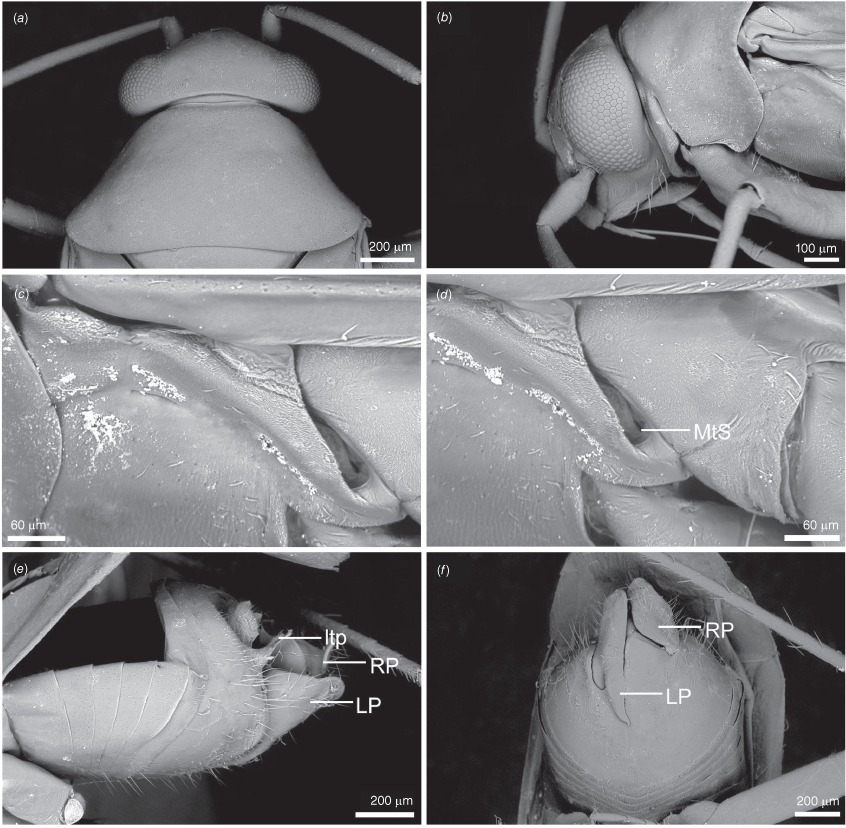
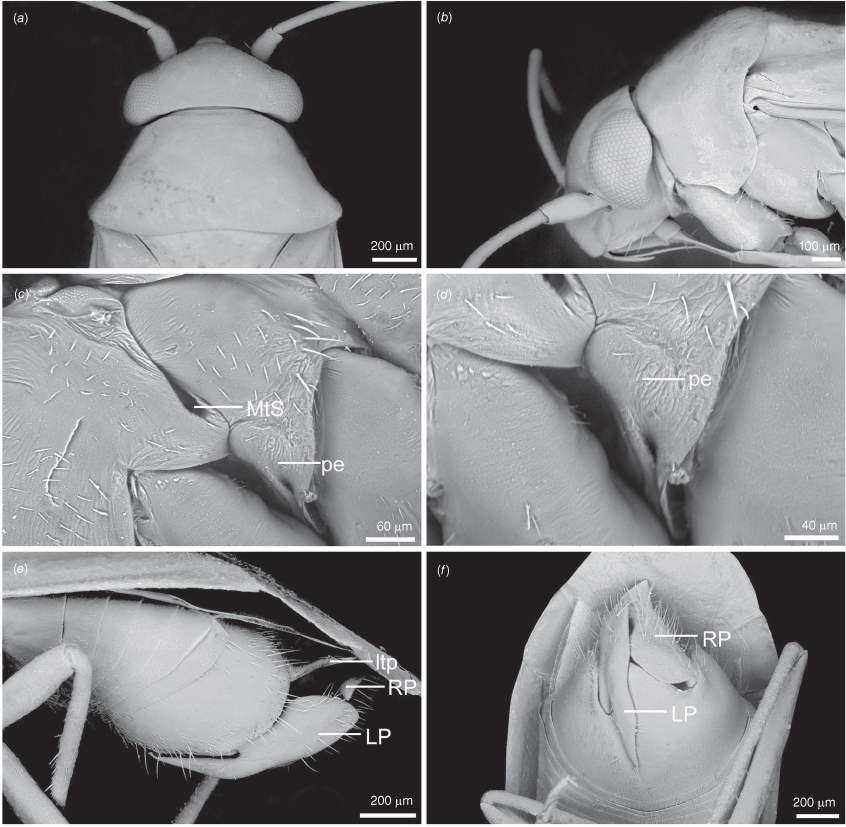
Carenotus is recognised by the following combination of characters: weakly myrmecomorphic (Fig. 6, 7); head subtriangular, clypeus barely visible from above (e.g. Fig. 8a); pronotum subtrapezoidal (e.g. Fig. 8a), to weakly campanulate (e.g. Fig. 14a), with narrow and weakly depressed collar (e.g. Fig. 8a); pronotal calli absent (e.g. Fig. 14a); body mostly dark brown, usually with cream transverse stripes on corium, proximal to cuneus (Fig. 6, 7); clavus usually with cream and brown stripes (Fig. 6, 7); pregenital abdominal sterna with cream banding (e.g. Fig. 6, 7); broad interocular distance, ~4× longer than length of first antennal segment (e.g. Fig. 11a); left paramere with greatly enlarged sensory lobe, apophysis lying on ventral wall of pygophore at rest (e.g. Fig. 8e, f, 9b); left tergal process (ltp) present (e.g. Fig. 8e, 9a, b); single (proximal) endosomal spicule, with deep bifurcation (e.g. Fig. 15e, f), branches serrate (e.g. Fig. 15e, f) or without serration (e.g. Fig. 18e, f).
Body: patterned, with ant-mimetic contrasting bicolouration; body mostly brown, with cream–white stripes and fasciae. Head: mostly dark brown; mandibular plates light reddish-brown; bucculae red brown. Antennae: mostly dark brown, concolourous with body; AI sometimes with yellowish or orange–brown. Pronotum: mostly dark brown, usually with narrow cream–white band on posterior margin, mostly broken medially. Thoracic pleura: mostly dark brown, usually with cream–white band on posterior margin of proepimeron; pterothoracic pleura uniformly dark brown, including evaporative areas of metathoracic glands. Scutellum and mesoscutum: scutellum uniformly dark brown, without other markings; mesoscutum usually dark brown, concolourous with scutellum, sometimes anteriorly paler, orange–brown. Hemelytra: diagnostic colour patterning; clavus either mostly cream–white or dark brown, if latter with cream–white stripes or spots; corium proximally either mostly cream–white or mostly dark brown with white spots, mostly on exocorium adjacent to base of claval commissure, medially with subquadrate to subtriangular dark brown fasciae, distally with narrow or broad cream–white transverse fasciae (Fig. 6, 7); cuneus uniformly brown; membrane mostly opaque to translucent brown, cream–white spot adjacent to apex of cuneus; membrane veins dark brown, concolourous with remainder of membrane. Legs: mostly dark brown, concolourous with antennae; pterothoracic coxae and trochanter cream–white; apices of pterothoracic femora sometimes partly orange–brown, sometimes with minor cream–white tint. Abdominal venter: dark brown with cream–white fasciae on abdominal SIV–SVI in males and SIII–SVI in females.
Head: with sparse distribution of decumbent silvery, short hairlike setae; sparse distribution of longer and semierect hairlike setae near eyes and bucculae. Antennae: with sparsely distributed short, fine hairlike setae, pale setae intermixed with sparse distribution of long pale setae, longer on AI. Pronotum and scutellum: with sparsely distributed short, pale silvery or yellowish setae, intermixed with very sparse distribution of a few long, pale silvery hairlike setae. Hemelytra: with densely distributed semi-erect to decumbent short, silvery or dark brown hairlike setae, more concentrated on clavus and corium. Legs: femora and tibiae with adpressed silvery hairlike setae, intermixed on femora with scattered elongate semierect hairlike setae, intermixed with alternating pattern of bristlelike setae on tibiae; pterothoracic femoral trichobothria obsolete. Abdominal venter: with moderately distributed semierect silvery setae, denser near genitalia.
Macropterous, forewings elongate, tip of abdomen subequal in length to apex of cuneus, not deflexed (Fig. 6, 7); body small, length males 2.73–4.07 mm, females 2.74–3.87 mm (see Table 4). Head: subtriangular in dorsal view (e.g. Fig. 8a), and lateral view (e.g. Fig. 8b), rounded anteriorly; interocular distance ~1/3rd width of anterior margin of pronotum (e.g. Fig. 8a); bucculae margins strongly rounded in ventral view, weakly raised; posterior margin of head carinate, strongly excavate (e.g. Fig. 8a). Antennae: AI small, ~1/4 length of interocular distance; AII cylindrical elongate (e.g. Fig. 8a, b). Labrum: elongate, dorsoventrally flattened, triangular (e.g. Fig. 14b). Labium: reaching between meso-and metacoxae (Fig. 6, 7). Pronotum: subtrapezoidal (e.g. Fig. 8a), to weakly campanulate (e.g. Fig. 14a); short and weakly depressed collar (e.g. Fig. 8a); callosite region obsolete (e.g. Fig. 8a); lateral margins sublinear to weakly excavate (e.g. Fig. 8a); humeral angles strongly rounded (e.g. Fig. 8a); posterior margin weakly excavated (e.g. Fig. 8a). Scutellum and mesonotum: mesonotum exposed; scutellum triangular, weakly convex (Fig. 6, 7). Thoracic pleura: metathoracic spiracular vestibule present, lachrymiform, with evaporative bodies (e.g. Fig. 8c, d), or without evaporative bodies (e.g. Fig. 26c, d). Metathoracic glands: well developed (e.g. Fig. 14d), to greatly reduced (e.g. Fig. 26d); ostiole present, with short canal; peritreme short, tongue-like, flattened (e.g. Fig. 8d) or obsolete (e.g. Fig. 20d). Hemelytra: elongate, reaching well beyond tip of abdomen in males, subequal in females (Fig. 6, 7); costal margins straight to weakly rounded; cuneus triangular; membrane with single cell. Abdomen: dorsoventrally flattened (Fig. 6, 7). Legs: slender and elongate; procoxae elongate, subcylindrical, meeting in midline; femora elongate, narrowly fusiform, oval in cross-section; metatibiae elongate, cylindrical; pretarsi with convergent lamellate parempodia; pulvilli absent. Abdomen: dorsoventrally flattened (Fig. 6, 7).
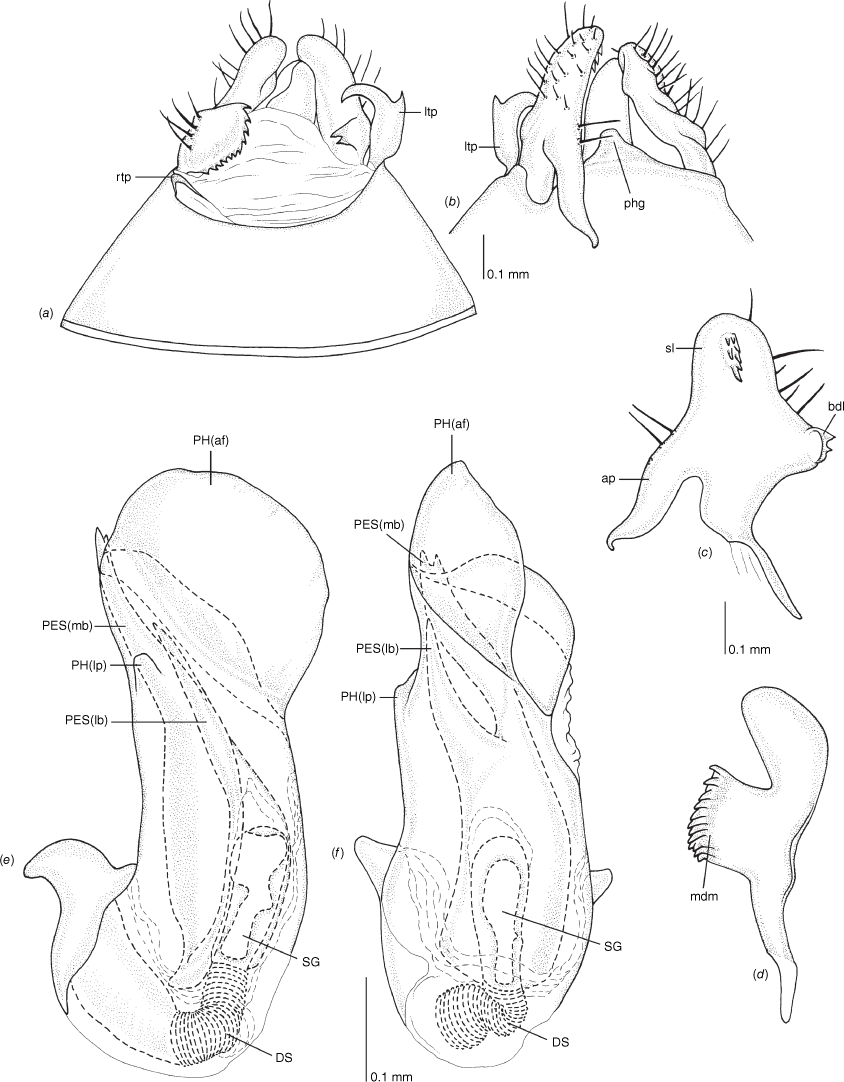
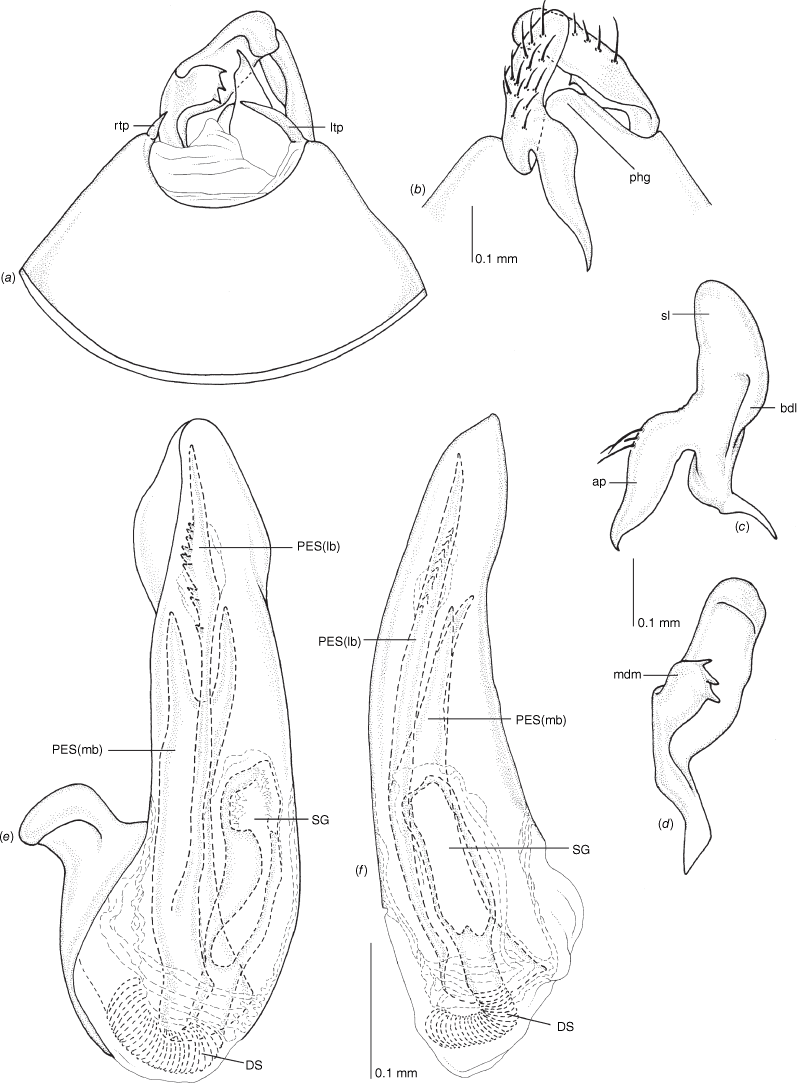
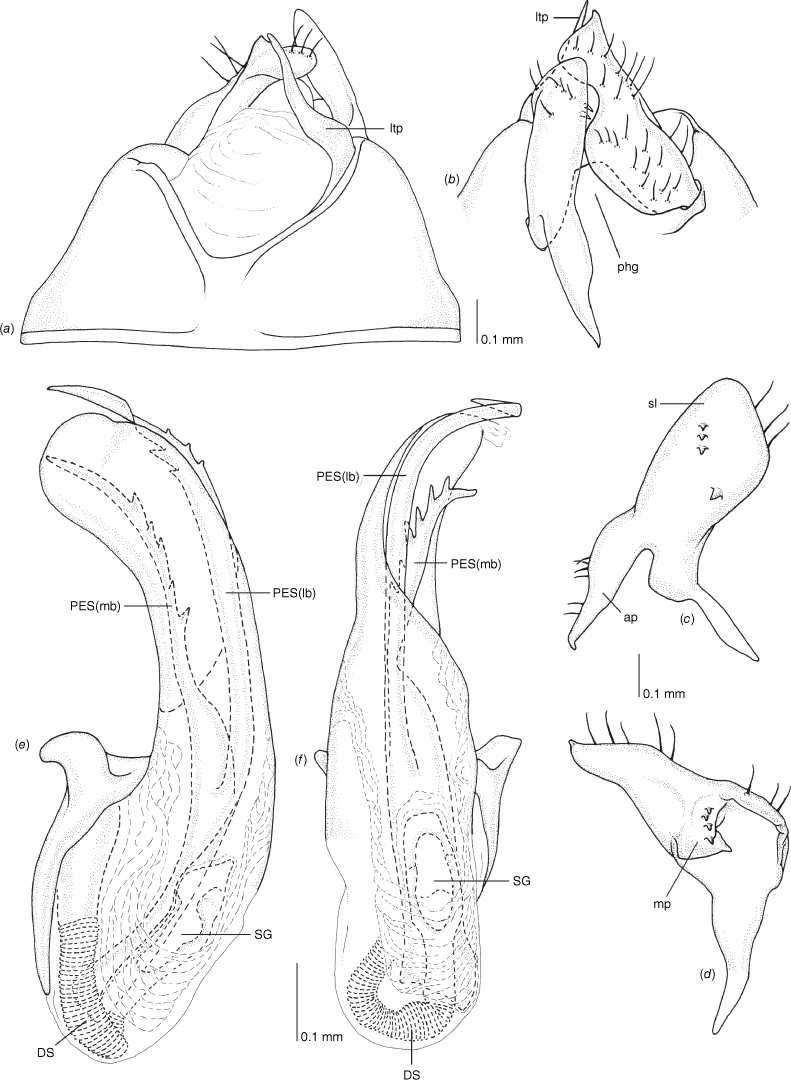
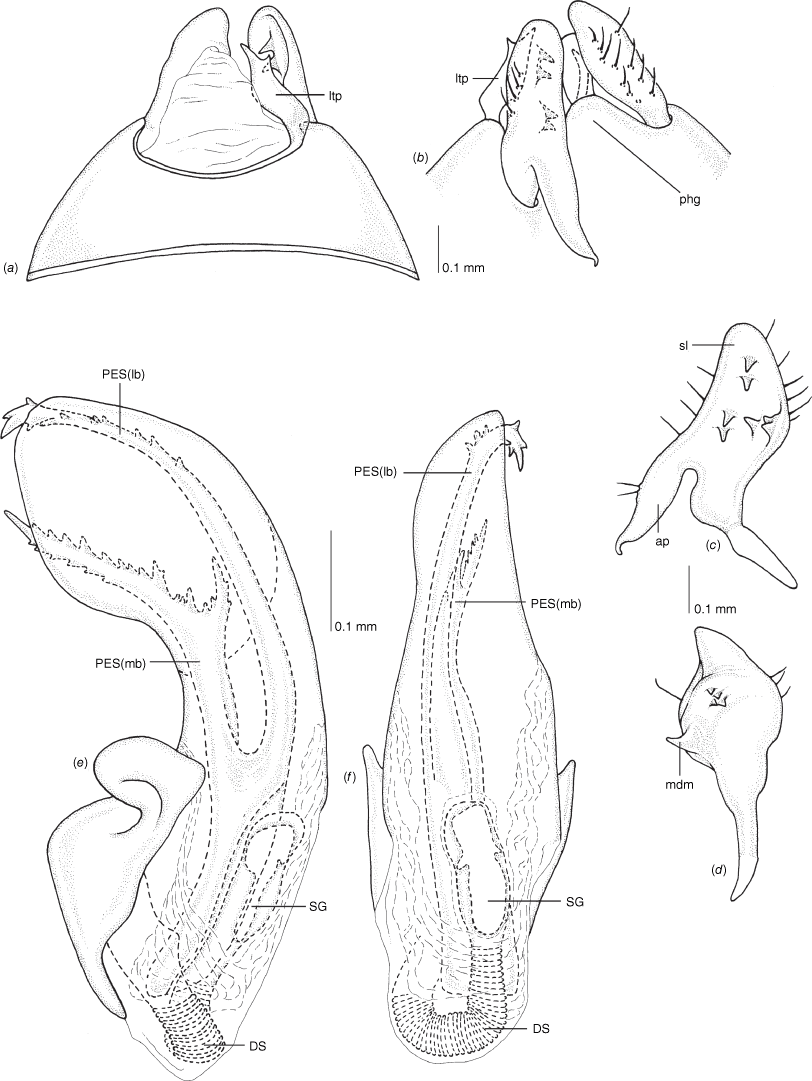
Pygophore: greatly enlarged, >1/2 length of pregenital abdomen (e.g. Fig. 8e); conical (e.g. Fig. 9a); genital opening large, dorsal to terminal in orientation (e.g. Fig. 8e, f), with small subtriangular phalloguide (e.g. Fig. 9b); without medial tergal process (mtp) (e.g. Fig. 8e, 9a, 15a), and with or without right tergal process (rtp) (e.g. Fig. 9b); parameres contiguous at midline at rest (e.g. Fig. 9b). Left paramere: triangular shaped, with large oval sensory lobe, elongate, hooked apex of apophysis, extending along ventral wall of pygophore (e.g. Fig. 9b, c). Right paramere: club-shaped, apically rounded or tapered, sometimes with shelf-like medial process, with or without teeth (e.g. Fig. 9d). Aedeagus: phallotheca arcuate, with or without flanges or processes (e.g. Fig. 9e, f); single proximal endosomal specular spicule (PES), bifurcate or trifurcate, margins smooth or serrated (e.g. Fig. 18e, f); secondary gonopore short with laterally oriented opening (e.g. Fig. 9e, f).
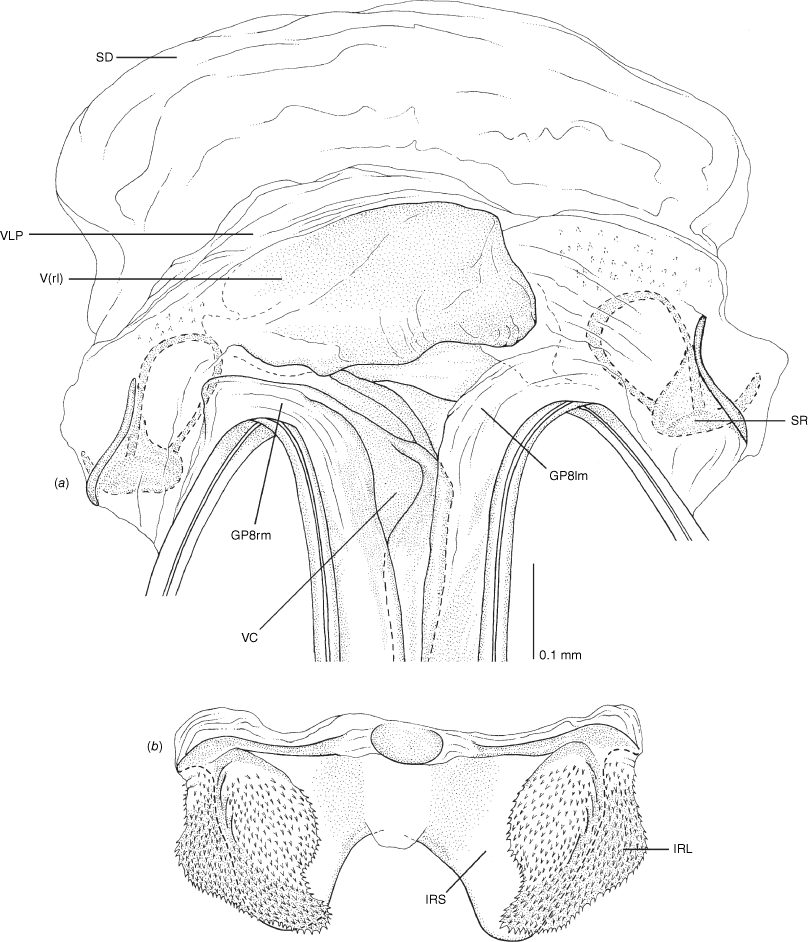
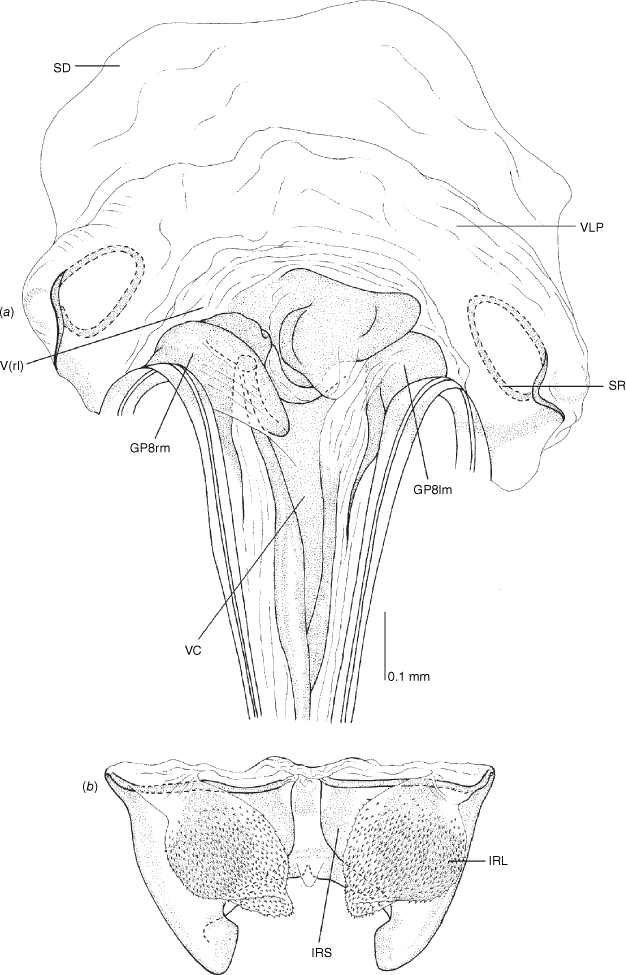
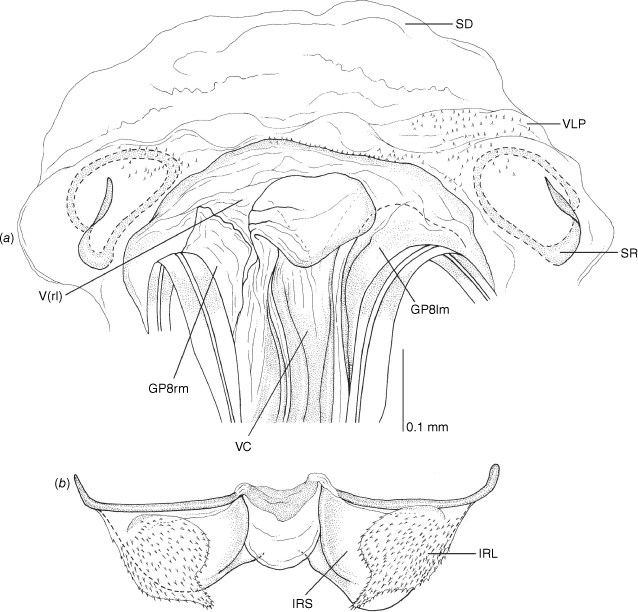
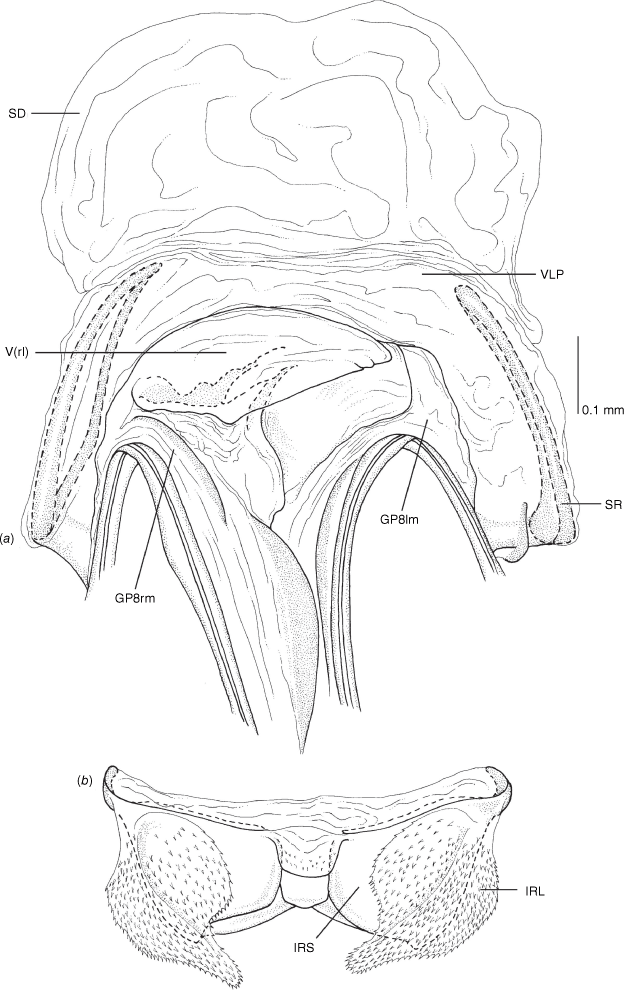
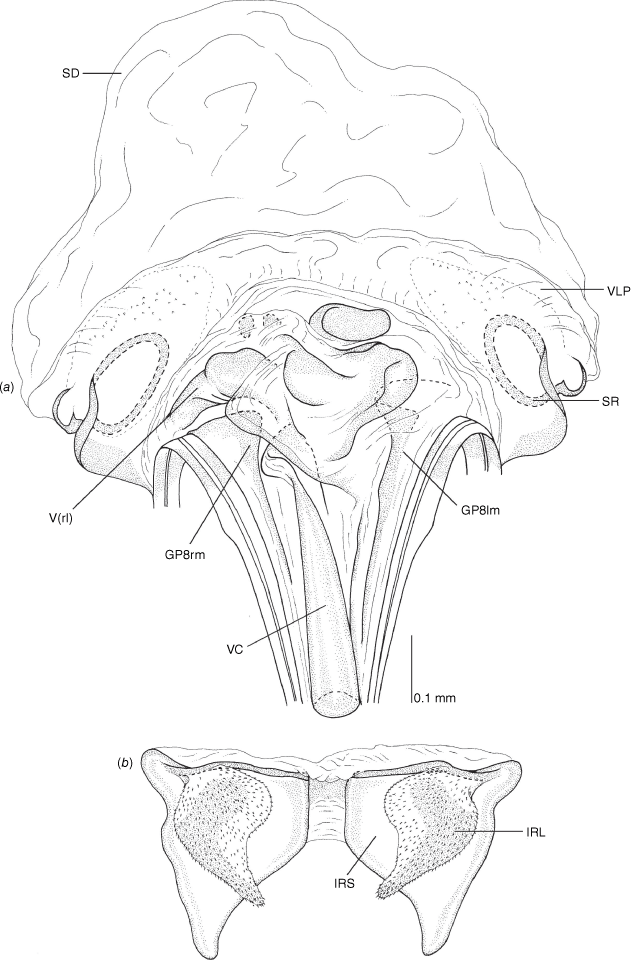
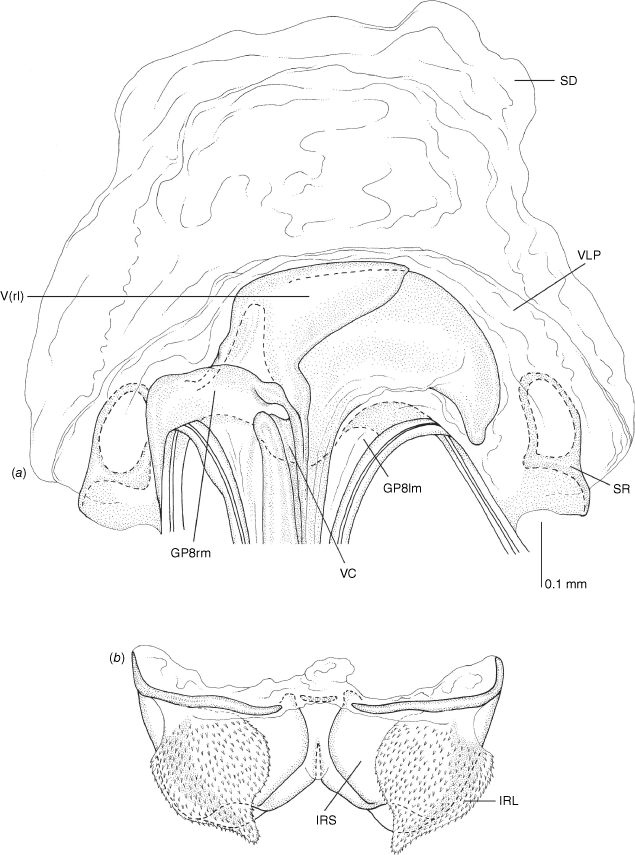
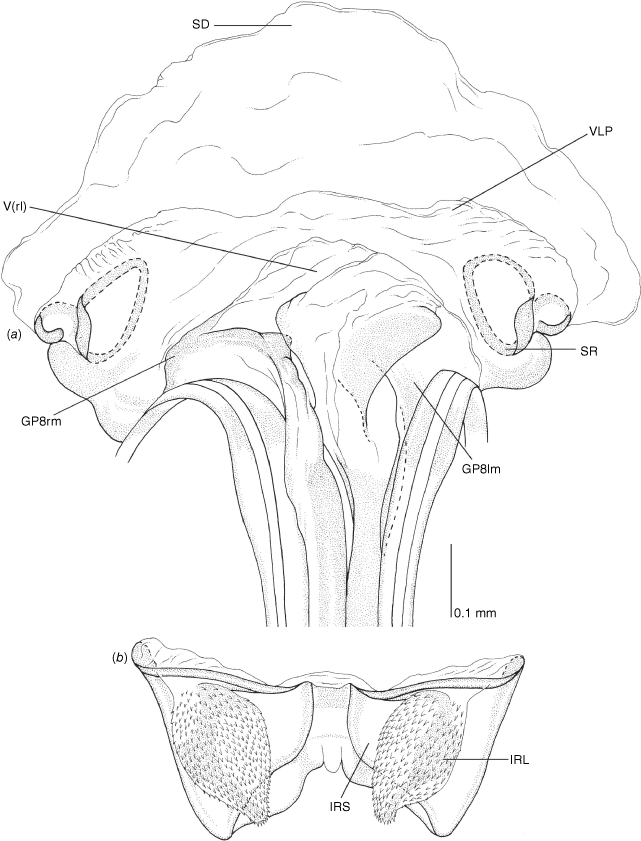
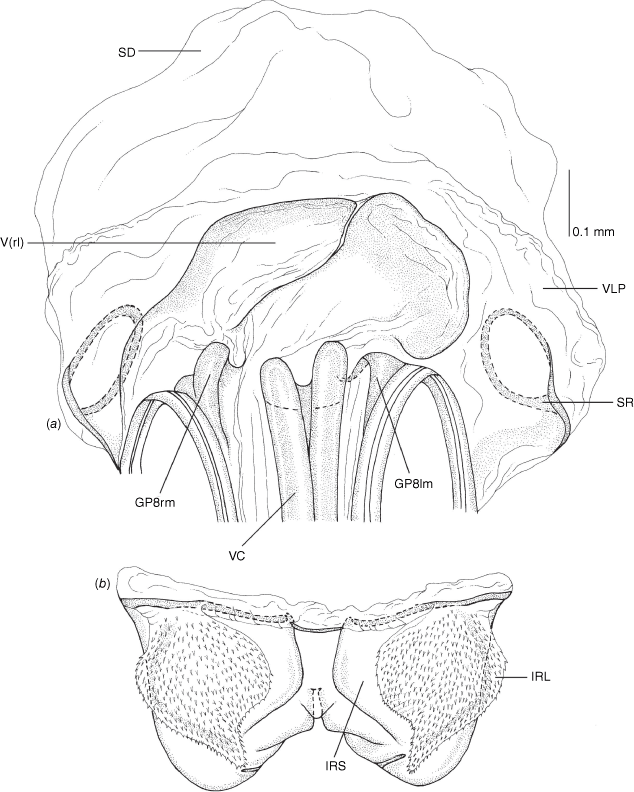
Vestibulum: opening not visible, overlapped by large vestibular right lobe (V(rl), usually extending to left side of the midline (e.g. Fig. 10a), with an elongate vestibular channel (VC), attached mesially on right side of midline (e.g. Fig. 10a). Gonapophyses 8: with asymmetrical basal membraneous to weakly sclerotised medial margins (GP8rm and GP8lm) (e.g. Fig. 10a). Seminal depository: large and membraneous (e.g. Fig. 10a). Sclerotised rings: usually small, oval, with margins thin (e.g. Fig. 10a). Posterior wall of bursa copulatrix: with broad inter-ramal sclerite (IRS), medially excavate, with weak sclerotisation (e.g. Fig. 10b); IRL large, mitt-shaped, attenuated apically, with dense distribution of spines, subequal, shorter or longer than IRS (e.g. Fig. 10b).
Carenotus species are found on a broad range of host plants (Table 3), with three host plant specific species (C. louthensis, C. scaevolaphilus and C. schwartzi), three oligophagous species (C. arltunga, C. pullabooka and C. schwartzi), one species found on three unrelated host plants (C. luritja) and one species found on eight Eremophila species (C. yuendumu). Carenotus pullabooka is found on two non-flowering species of the cupressaceous genus Callitris Vent. The remaining Carenotus species are found on plant species belonging to the core eudicot clade of the Angiosperm Phylogeny Group classification (sensu Stevens 2012). Three Carenotus species are restricted to the woody shrub genus Eremophila; C. louthensis and C. yuendumu prefer E. sturtii, whereas C. tanami prefers E. duttonii (Table 3). Carenotus arltunga specialises on two Acacia Mill. species (sensu Maslin 2001 classification; A. aneura F.Muell. and A. brachystachya Benth.) (Table 3). The least collected species of Carenotus, C. scaevolaphilus is only known from Scaevola spinescens at one location (Table 3). Carenotus luritja is known from two of the saltbush genera Atriplex Linnaeus (A. elachophylla) and Sclerolaena R.Br. (S. holtiana (L.)W.T. Aiton), as well as the malvaceous genus Sida Linnaeus (S. fibulifera Lindl.).
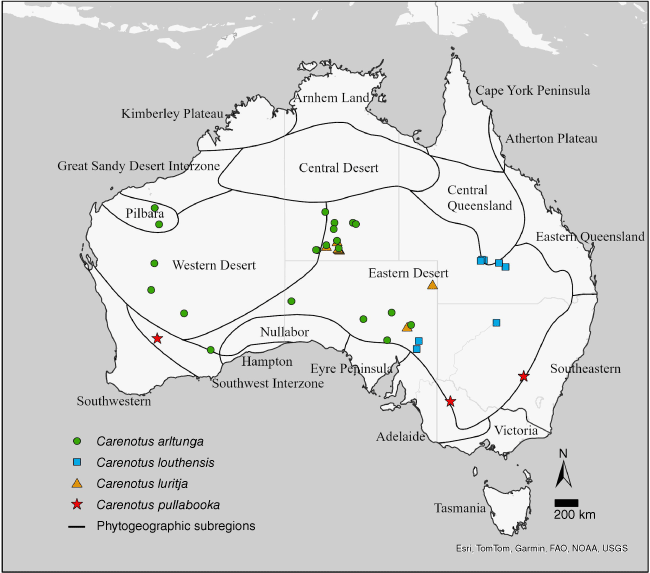
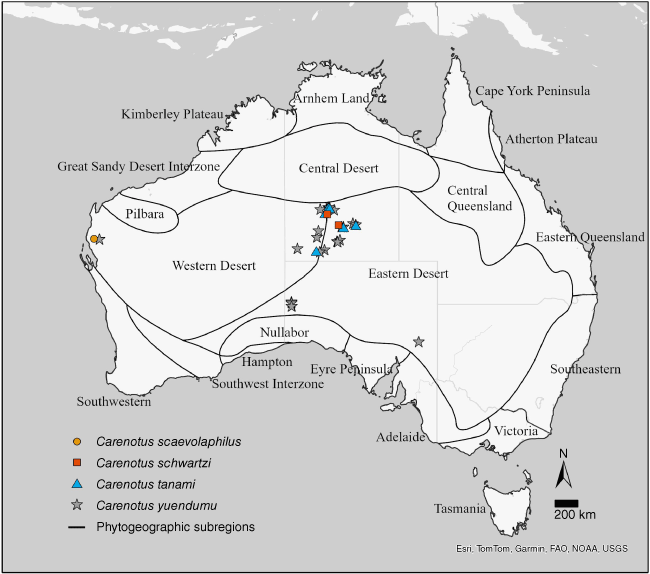
Carenotus is distributed in semi-arid and arid Australia, and is found in all states and territories, aside from the Australian Capital Territory and Tasmania. Five species are found in the central deserts of the Northern Territory (Fig. 4, 5). Seven of the eight Carenotus species are found in the Eastern Desert phytogeographic subregion (Fig. 4, 5).
Carenotus is most similar to Kirkaldyella (see Fig. 1a, b), based primarily on characters of the male genitalia. In particular, the shape and orientation of the left paramere are alike, with an enlarged sensory lobe and elongate apophysis, bearing an apical hook, that is oriented along the ventral wall of the pygophore (e.g. Fig. 9a–c, 32a–c). The aedeagus is also very similar between the two genera, with a single bifurcate proximal endosomal spicule, with the division occurring basally on the spicule (e.g. Fig. 9e, f, 32e). The two genera can also be considered poor ant mimics, with the costal margin of the hemelytra not deeply excavated as in Myrmecoridea and Myrmecoroides (cf. Fig. 1a–d), and the absence of microptery as in Myrmecoroides females (Cassis and Wall 2010).
Carenotus is readily distinguished from Kirkaldyella by bicolouration (brown and cream–white) on the hemelytra (cf. Fig. 1b) and the cream–white banding on the abdominal venter in both sexes (see Fig. 6, 7 in Carenotus spp. – lateral view). Externally, the head is more triangular (cf. Fig. 1a, b) and the pronotum is rugopunctate in Kirkaldyella (cf. smooth in Carenotus gen. nov., e.g. Fig. 8a).
Host plants of Carenotus species by plant family and species. (a) Scaevola spinescens – host of C. scaevolaphilus; (b) Eremophila freelingii possible host of C. yuendumu; (c) Acacia aneura – host of C. arltunga; (d) Eremophila sturtii – host of C. louthensis and C. yuendumu; (e) Callitris glaucophylla – host of C. pullabooka; and (f) Eremophila duttonii – host of C. yuendumu.

Distribution of Carenotus species: C. arltunga, C. louthensis, C. luritja and C. pullabooka. Phytogeographic subregions based on González-Orozco et al. (2014) and Ebach et al. (2015).
Distribution of Carenotus species: C. scaevolaphilus, C. schwartzi, C. tanami and C. yuendumu. Phytogeographic subregions based on González-Orozco et al. (2014) and Ebach et al. (2015).
Dorsal habitus images of Carenotus species: C. arltunga, C. louthensis, C. luritja and C. pullabooka. Scale bar: 1 mm.
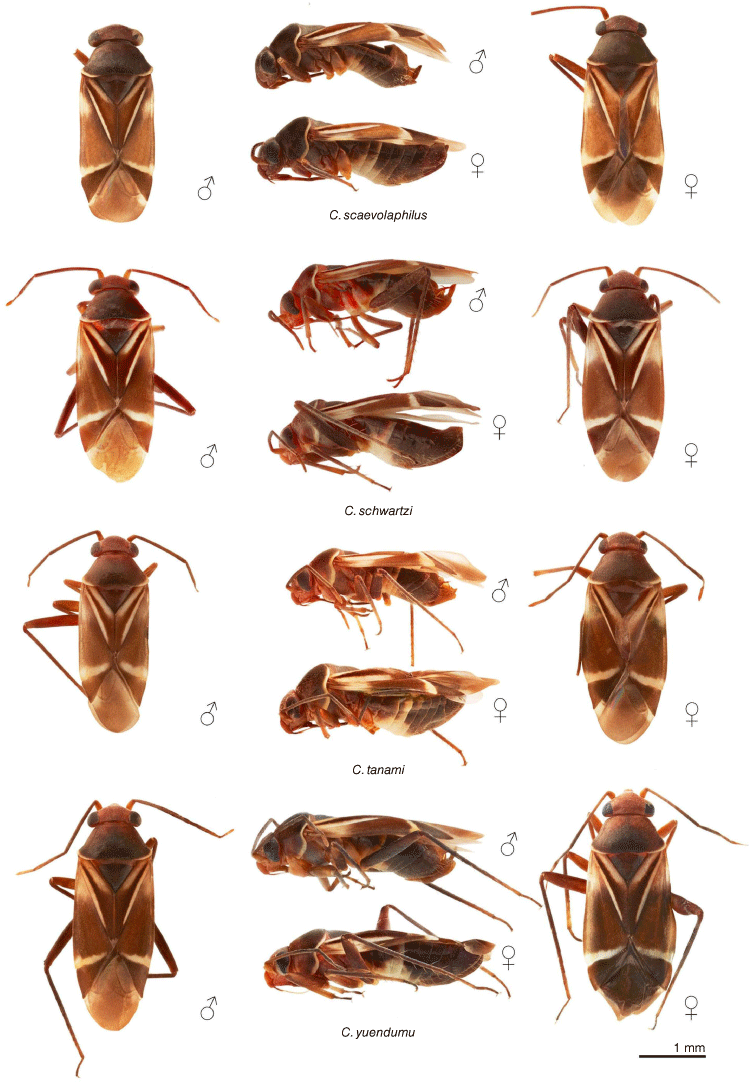
Dorsal habitus images of Carenotus species: C. scaevolaphilus, C. schwartzi, C. tanami and C. yuendumu. Scale bar: 1 mm.
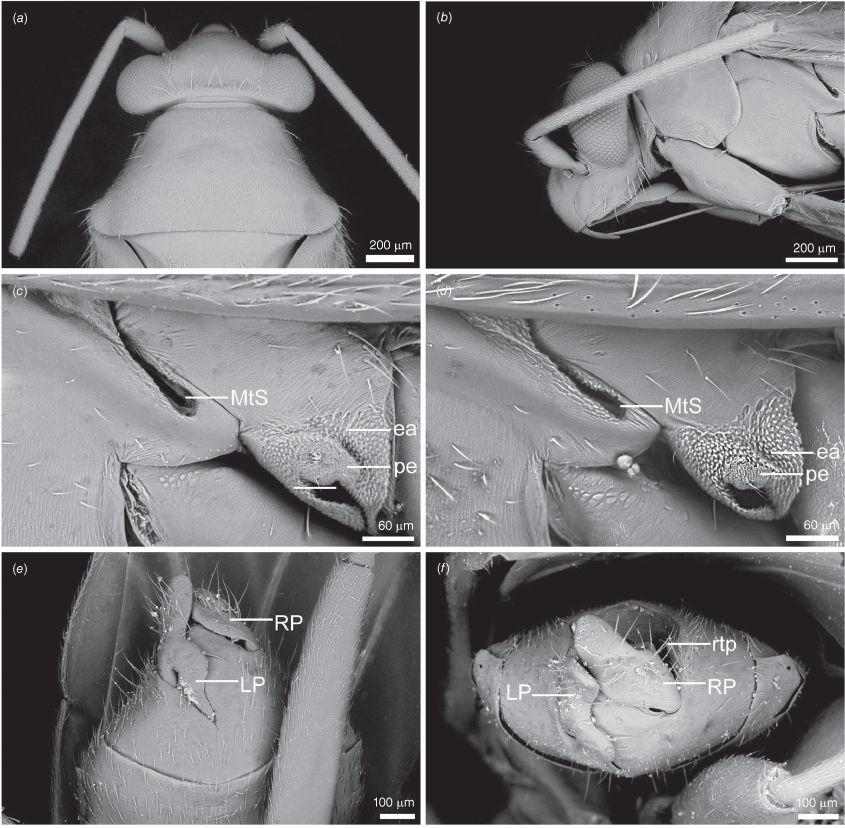
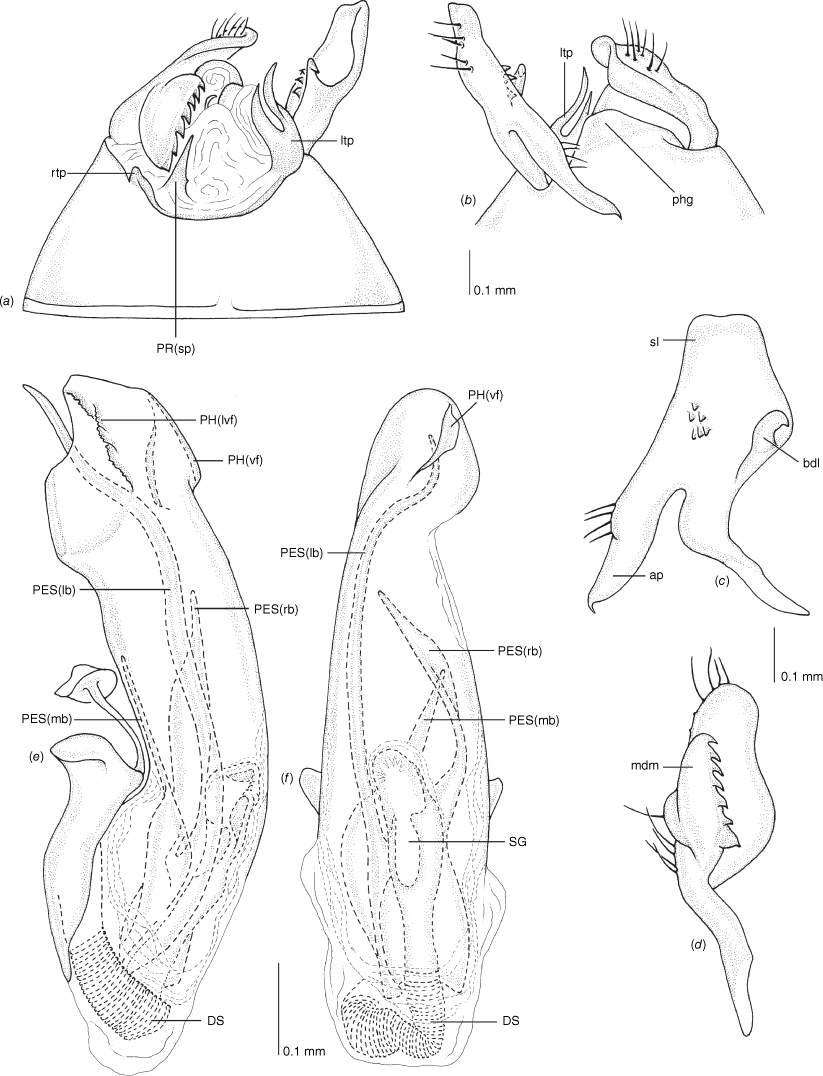
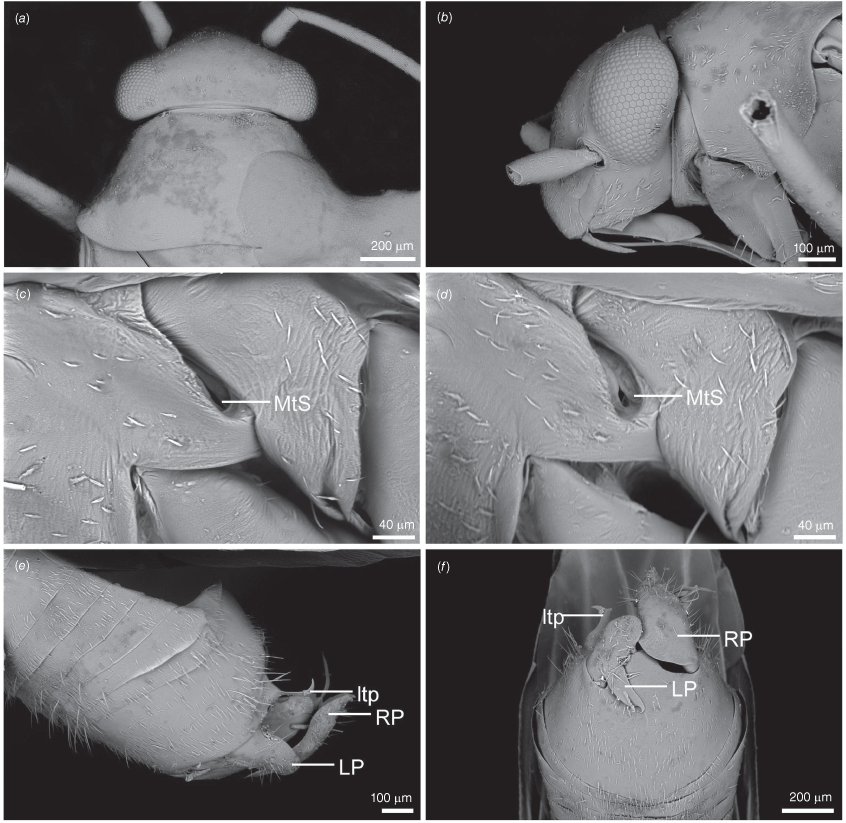
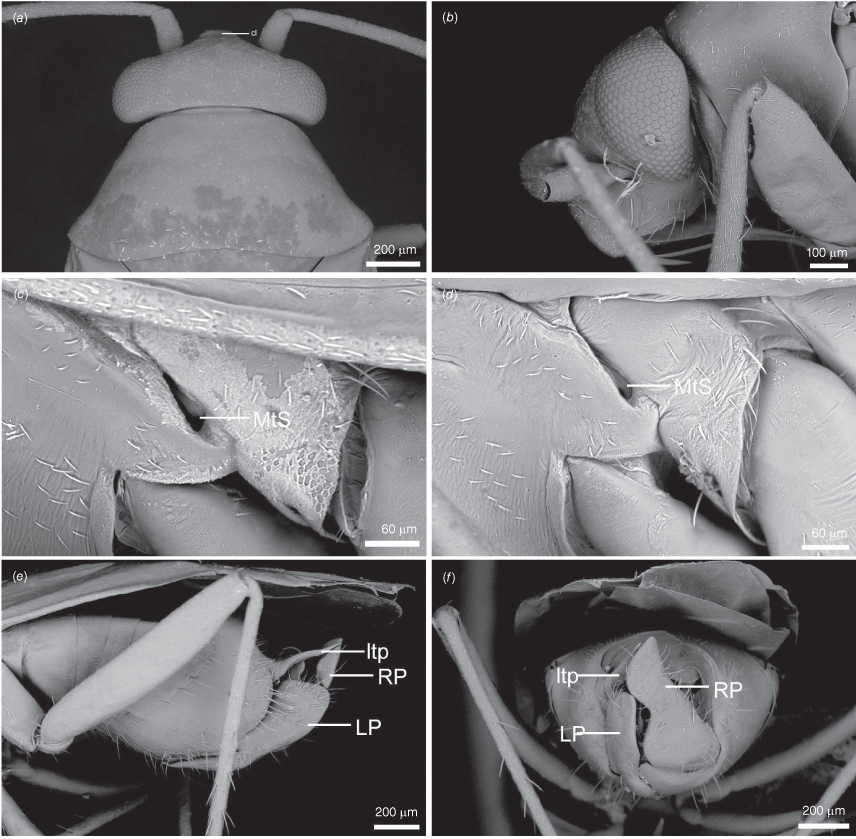
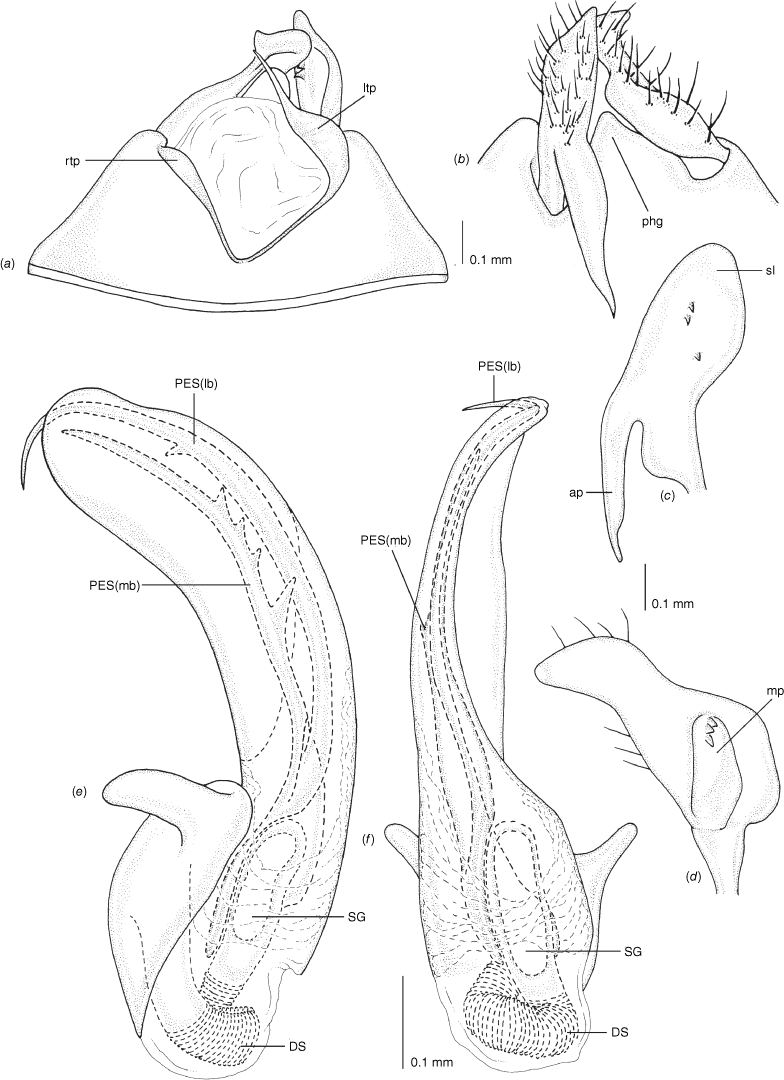
Scanning electron micrographs of Carenotus arltunga, sp. nov. (a) head and pronotum, dorsal view; (b) head and pronotum, lateral view; (c) metathoracic scent gland, lateral view; (d) peritreme and evaporative area, lateral view; (e) pygophore, lateral view; (f) pygophore, terminal view. Abbreviations: cl, clypeus; co, pronotal collar; ea, evaporative area; f, frons; hu, humeral angles of pronotum; LP, left paramere; ltp, left tergal process; mn, mesonotum; MtS, metathoracic spiracle; pe, peritreme; RP, right paramere; rtp, right tergal process; v, vertex.
Male genitalia of Carenotus arltunga, sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view; (f) aedeagus, ventral view. Abbreviations: ap, apophysis; bdl, basal dorsal lobe; DS, ductus seminis; ltp, left tergal process; mdm, medial dorsal margin; PH(af), phallotheca apical flange; PH(lp), phallotheca lateral process; PES(lb), proximal endosomal spicule, lateral branch; PES(mb) proximal endosomal spicule, medial branch; phg, phalloguide; rtp, right tergal process; sl, sensory lobe; SG, secondary gonopore.
Female genitalia of Carenotus arltunga, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate; V(rl), vestibular right lobe.
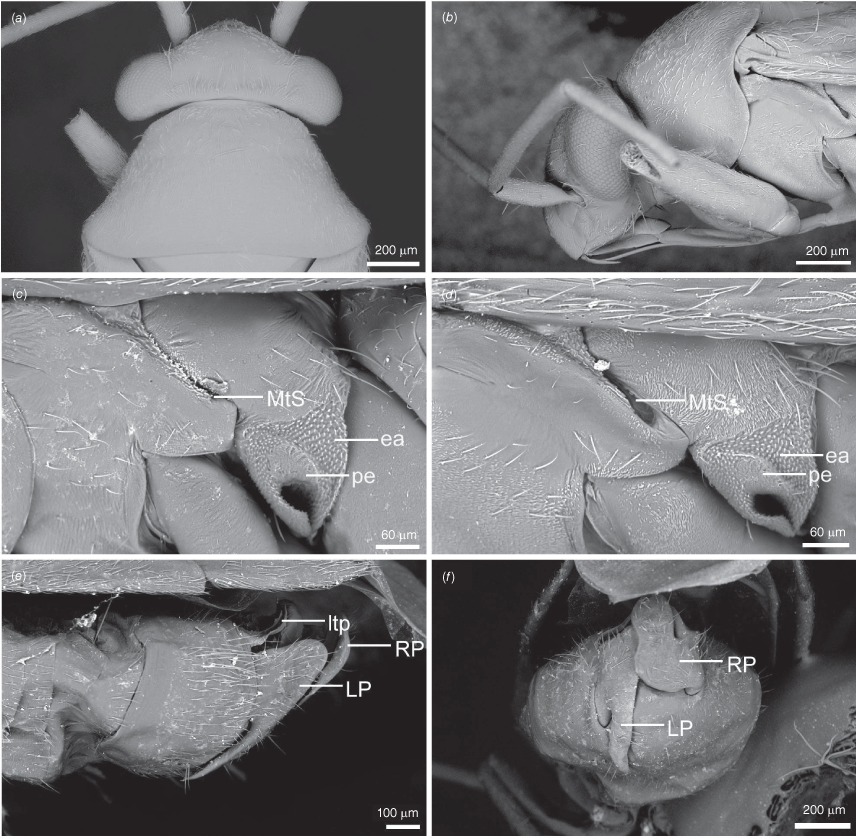
Scanning electron micrographs of Carenotus louthensis, sp. nov. (a) head and pronotum, dorsal view; (b) head and pronotum, lateral view; (c) metathoracic scent gland, lateral view; (d) metathoracic scent gland, close-up, lateral view; (e) pygophore, lateral view; (f) pygophore, ventral view. Abbreviations: LP, left paramere; ltp, left tergal process; MtS, metathoracic spiracle; RP, right paramere.
Male genitalia of Carenotus louthensis, sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view; (f) aedeagus, ventral view. Abbreviations: ap, apophysis; bdl, basal dorsal lobe; DS, ductus seminis; ltp, left tergal process; mdm, medial dorsal margin; PES(lb), proximal endosomal spicule, lateral branch; PES(mb) proximal endosomal spicule, medial branch; phg, phalloguide; sl, sensory lobe; SG, secondary gonopore.
Female genitalia of Carenotus louthensis, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate; V(rl), vestibular right lobe.
Scanning electron micrographs of Carenotus luritja, sp. nov. (a) head and pronotum, dorsal view; (b) head and pronotum, lateral view; (c) metathoracic scent gland, lateral view; (d) peritreme and evaporative area, lateral view; (e) pygophore, ventral view; (f) pygophore, terminal view. Abbreviations: ea, evaporative area; LP, left paramere; MtS, metathoracic spiracle; pe, peritreme; RP, right paramere; rtp, right tergal process.
Male genitalia of Carenotus luritja, sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view; (f) aedeagus, ventral view. Abbreviations: ap, apophysis; bdl, basal dorsal lobe; DS, ductus seminis; ltp, left tergal process; mdm, medial dorsal margin; PES(lb), proximal endosomal spicule, lateral branch; PES(mb) proximal endosomal spicule, medial branch; phg, phalloguide; sl, sensory lobe; SG, secondary gonopore.
Female genitalia of Carenotus luritja, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate; V(rl), vestibular right lobe.
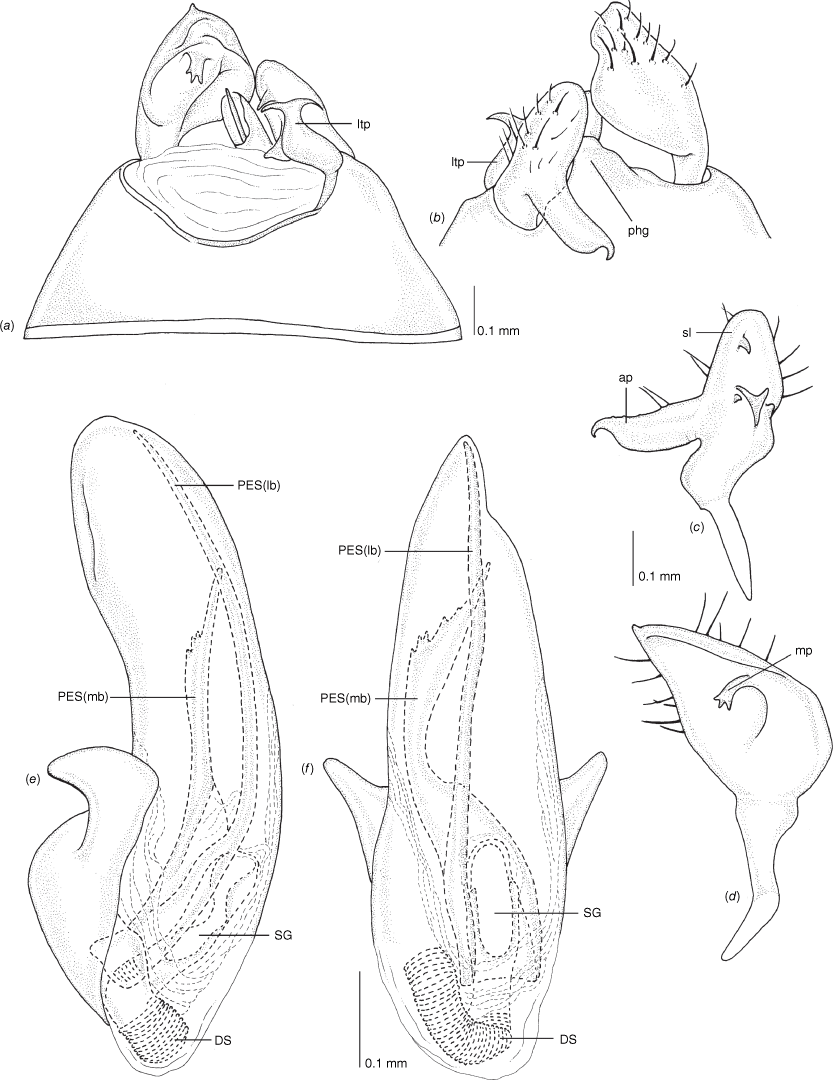
Scanning electron micrographs of Carenotus pullabooka, sp. nov. (a) head and pronotum, dorsal view; (b) head and pronotum, lateral view; (c) metathoracic scent gland, lateral view; (d) metathoracic scent gland, lateral view; (e) pygophore, lateral view; (f) pygophore, terminal view. Abbreviations: ea, evaporative area; LP, left paramere; ltp, left tergal process; MtS, metathoracic spiracle; pe, peritreme; RP, right paramere.
Male genitalia of Carenotus pullabooka, sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view; (f) aedeagus, ventral view. Abbreviations: ap, apophysis; bdl, basal dorsal lobe; DS, ductus seminis; ltp, left tergal process; mdm, medial dorsal margin; PES(lb), proximal endosomal spicule, lateral branch; PES(mb) proximal endosomal spicule, medial branch; PES(rb) promixmal endosomal spicule, right branch; phg, phalloguide; PH(lvf), phallotheca, left ventral flange; PH(vf), phallotheca, ventral flange; PR, proctiger spine; rtp, right tergal process; sl, sensory lobe; SG, secondary gonopore.
Female genitalia of Carenotus pullabooka, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate; V(rl), vestibular right lobe.
Scanning electron micrographs of Carenotus scaevolaphilus, sp. nov. (a) head and pronotum, dorsal view; (b) head and pronotum, lateral view; (c) metathoracic scent gland, lateral view; (d) etathoracic scent gland, lateral view; (e) pygophore, lateral view; (f) pygophore, ventral view. Abbreviations: LP, left paramere; ltp, left tergal process; MtS, metathoracic spiracle; RP, right paramere.
Male genitalia of Carenotus scaevolaphilus, sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view; (f) aedeagus, ventral view. Abbreviations: ap, apophysis; DS, ductus seminis; ltp, left tergal process; mp, medial process; PES(lb), proximal endosomal spicule, lateral branch; PES(mb) proximal endosomal spicule, medial branch; phalloguide; sl, sensory lobe; SG, secondary gonopore.
Female genitalia of Carenotus scaevolaphilus, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate; V(rl), vestibular right lobe.
Scanning electron micrographs of Carenotus schwartzi, sp. nov. (a) head and pronotum, dorsal view; (b) head and pronotum, lateral view; (c) metathoracic scent gland, lateral view; (d) metathoracic scent gland, lateral view; (e) pygophore, lateral view; (f) pygophore, terminal view. Abbreviations: LP, left paramere; ltp, left tergal process; MtS, metathoracic spiracle; RP, right paramere.
Male genitalia of Carenotus schwartzi, sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view; (f) aedeagus, ventral view. Abbreviations: ap, apophysis; bdl, basal dorsal lobe; DS, ductus seminis; ltp, left tergal process; mp, medial process; PES(lb), proximal endosomal spicule, lateral branch; PES(mb) proximal endosomal spicule, medial branch; phg, phalloguide; sl, sensory lobe; SG, secondary gonopore.
Female genitalia of Carenotus schwartzi, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate; V(rl), vestibular right lobe.
Scanning electron micrographs of Carenotus tanami, sp. nov. (a) head and pronotum, dorsal view; (b) head and pronotum, lateral view; (c) metathoracic scent gland, lateral view; (d) metathoracic scent gland, lateral view; (e) pygophore, lateral view; (f) pygophore, terminal view. Abbreviations: LP, left paramere; ltp, left tergal process; MtS, metathoracic spiracle; RP, right paramere.
Male genitalia of Carenotus tanami, sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view; (f) aedeagus, ventral view. Abbreviations: ap, apophysis; DS, ductus seminis; ltp, left tergal process; mdm, medial dorsal margin; PES(lb), proximal endosomal spicule, lateral branch; PES(mb) proximal endosomal spicule, medial branch; phg, phalloguide; sl, sensory lobe; SG, secondary gonopore.
Female genitalia of Carenotus tanami, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate; V(rl), vestibular right lobe.
Scanning electron micrographs of Carenotus yuendumu, sp. nov. (a) head and pronotum, dorsal view; (b) head and pronotum, lateral view; (c) metathoracic scent gland, lateral view; (d) metathoracic scent gland close-up, lateral view; (e) pygophore, lateral view; (f) pygophore, ventral view. Abbreviations: LP, left paramere; ltp, left tergal process; MtS, metathoracic spiracle; pe, peritreme; RP, right paramere.
Male genitalia of Carenotus yuendumu, sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view; (f) aedeagus, ventral view. Abbreviations: ap, apophysis; DS, ductus seminis; ltp, left tergal process; mp, medial process; PES(lb), proximal endosomal spicule, lateral branch; PES(mb) proximal endosomal spicule, medial branch; phg, phalloguide; rtp, right tergal process; sl, sensory lobe; SG, secondary gonopore.
Female genitalia of Carenotus yuendumu, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate; V(rl), vestibular right lobe.
Checklist of species of Carenotus
C. arltunga McMah & Cassis, sp. nov., Northern Territory, South Australia, Western Australia.
C. louthensis McMah & Cassis, sp. nov., New South Wales, South Australia, Queensland.
C. luritja McMah & Cassis, sp. nov., Northern Territory, South Australia.
C. pullabooka McMah & Cassis, sp. nov., New South Wales, Victoria, Western Australia.
C. scaevolaphilus McMah & Cassis, sp. nov., Western Australia.
C. schwartzi McMah & Cassis, sp. nov., Northern Territory.
C. tanami McMah & Cassis, sp. nov., Northern Territory.
C. yuendumu McMah & Cassis, sp. nov., Northern Territory, South Australia, Western Australia.
| 1 | Clavus mostly cream–white, with or without brown markings (Fig. 6: C. arltunga and C. luritja); distal region of corium with broad cream–white transverse fasciae (Fig. 6: C. arltunga and C. luritja); pygophore with left and right tergal processes (e.g. Fig. 9a), never with spine on proctiger (e.g. Fig. 9a: C. arltunga) nor medial process on right paramere (e.g. Fig. 9d: C. arltunga)...2 Clavus mostly dark brown, with narrow pale stripes on anterolateral and medial margins (e.g. Fig. 6: C. louthensis); pygophore usually with left tergal process only, if both left and right tergal processes present, then either proctiger with prominent spine (e.g. Fig. 18a: C. pullabooka) or medial process on right paramere present (e.g. Fig. 30d: C. yuendumu)...3 |
| 2 | Posterior margin of pronotum without white markings; clavus uniformly cream–white, without brown markings (Fig. 6: C. luritja); left tergal process of pygophore spinelike, undivided (Fig. 15a); sensory lobe of left paramere without projections or teeth (Fig. 15b, c); right paramere medial process with 3 or 4 teeth (Fig. 15a, c); lateral branch of proximal endosomal spicule elongate, with subapical serrations, much longer than medial branch (Fig. 15e, f); Northern Territory, South Australia (Fig. 4); ex Atriplex elachophylla, Sclerolaena holtiana, Sida fibulifera...Carenotus luritja Posterior margin of pronotum with white markings; clavus mostly cream–white, with brown markings (Fig. 6: C. arltunga); left tergal process strongly arcuate, bifurcate distally (Fig. 9a); sensory lobe of left paramere with teeth (Fig. 9c); right paramere mediodorsal margin with 10–14 teeth (Fig. 9d); medial branch of proximal endosomal spicule elongate, without subapical serrations, much longer than lateral branch (Fig. 9e, f); Northern Territory, South Australia, Western Australia (Fig. 4); ex Acacia aneura and A. brachystachya...Carenotus arltunga |
| 3 | Posterior margin of pronotum with broken cream–white band (e.g. Fig. 6: C. louthensis); lateral margin of clavus with elongate cream–white stripe (e.g. Fig. 6: C. louthensis); metathoracic peritreme and evaporative area vestigial (e.g. Fig. 11c, d); left tergal process undivided (e.g. Fig. 24a: C. schwartzi); apical margin of sensory lobe rounded (e.g. Fig. 24c); sclerotised rings short, oval (e.g. Fig. 25b)...4 Posterior margin of pronotum with unbroken cream–white band (e.g. Fig. 6: C. pullabooka); metathoracic peritreme and evaporative area well developed (Fig. 17c, d); lateral margin of clavus with two small cream–white spots, remainder dark brown (Fig. 6); left tergal process large, deeply bifurcate (Fig. 18a); apical margin of sensory lobe truncate (Fig. 18c); sclerotised rings elongate, subelliptical (Fig. 19a); New South Wales, Victoria, Western Australia (Fig. 4); ex Callitris glaucophila and C. gracilis...Carenotus pullabooka |
| 4 | Left tergal process of pygophore elongate, narrow, undivided, tapered to a sharp apical point (e.g. Fig. 24a)...5 Left tergal process of pygophore elongate, broad, apically bifurcate (branches short) (e.g. Fig. 27a)...6 |
| 5 | Apophysis of left paramere narrow, outer margin in continuous arcuate line with sensory lobe (Fig. 29f, 30c); right tergal process present (Fig. 29e, 30a); phalloguide apically acute (Fig. 30b); length of mitt-shaped inter-ramal lobes subequal to inter-ramal sclerite (Fig. 31b); Northern Territory, South Australia, Western Australia (Fig. 5); ex Eremophila spp. (see Table 3 for seven host species records)...Carenotus yuendumu Apophysis of left paramere broad, outer margin sinuate relative to sensory lobe (Fig. 24c); right tergal process absent (Fig. 24a); phalloguide apically truncate (Fig. 24b); length of mitt-shaped inter-ramal lobes longer than inter-ramal sclerite (Fig. 25b); Northern Territory (Fig. 5); ex Solanum orbiculatum...Carenotus schwartzi |
| 6 | Cream–white transverse fasciae on apex of corium narrow, not expanding laterally (Fig. 7); genae enlarged, height subequal to length of first antennal segment (Fig. 20b); apophysis of left paramere short, subequal to length phalloguide (Fig. 20f, 21b); right paramere with arcuate medial process (Fig. 21a, d); medial branch of proximal endosomal spicule undivided (Fig. 21e, f); Western Australia (Fig. 5); ex Scaevola spiniscens...Carenotus scaevolaphilus Cream–white transverse fasciae on apex of corium broader, weakly expanding laterally (Fig. 7); genae not enlarged, height less than length of first antennal segment (e.g. Fig. 26b); apophysis of left paramere elongate, longer than length of phalloguide (e.g. Fig. 26f, 27b); right paramere without medial process (e.g. Fig. 27d); medial branch of proximal endosomal spicule apically bifurcate (e.g. Fig. 27e, f)...7 |
| 7 | Apical bifurcation of left tergal process asymmetrical (Fig. 27a); left paramere without basal dorsal lobe (Fig. 27c); sensory lobe of left paramere elongate, longer than apophysis (Fig. 27b); right paramere mediodorsal margin with one tooth (Fig. 27d); medial branch of proximal endosomal spicule densely serrate (Fig. 27e, f); inter-ramal lobes subequal in length to inter-ramal sclerite (Fig. 28b); Northern Territory (Fig. 5); ex Eremophila duttonii, E. gilesi and E. latrobei...Carenotus tanami Apical bifurcation of left tergal process symmetrical (Fig. 12a); left paramere with basal dorsal lobe (Fig. 12c); sensory lobe of left paramere subequal in length to apophysis (Fig. 12b, c); right paramere mediodorsal margin with three teeth (Fig. 12d); medial branch of proximal endosomal spicule smooth (Fig. 12e); inter-ramal sclerite much longer than inter-ramal lobes (Fig. 13b); New South Wales, South Australia, Queensland (Fig. 4); ex Eremophila sturtii...Carenotus louthensis |
Carenotus arltunga McMah & Cassis, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:305BCA6C-3281-45C6-A01F-7B24D5B3C5E4
C. arltunga is named after the type locality, near Arltunga, Northern Territory. Noun in apposition.
Holotype: AUSTRALIA: Western Australia: Newman Rocks, 136.5 km E of Norseman, 32.11084°S, 123.1704°E, 250 m, 22 Oct 1996, Schuh and Cassis, ♂ (UNSW_ENT 00015815) (WAM).
Paratypes: AUSTRALIA: Northern Territory: 1 km S of Henbury Craters Nature Reserve, 24.56668°S, 133.1234°E, 457 m, 29 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Acacia aneura F.Muell. ex Benth. (Fabaceae), det. NSW Herbarium staff NSW658409, 2♀♀ (UNSW_ENT 00016076, UNSW_ENT 00016077) (AM), Acacia aneura F.Muell. ex Benth. (Fabaceae), det. NSW Herbarium staff NSW658409, 7♀♀ (UNSW_ENT 00015752–UNSW_ENT 00015758) (AMNH). 7.3 km E of Stuart Highway on Horseshoe Bend Road, 25.11667°S, 133.2517°E, 437 m, 28 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Acacia aneura F.Muell. ex Benth. (Fabaceae), det. NSW Herbarium staff NSW658387, 2♂♂ (UNSW_ENT 00015837, UNSW_ENT 00015988) (AM). 26.8 km W of Tanami Road on Mount Wedge Station Road, 22.50001°S, 132.179°E, 589 m, 23 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, 4♀♀ (AMNH_PBI 00274372-AMNH_PBI 00274375) (AM). 67 km E of Stuart Highway on Arltunga Station Road, 23.28027°S, 134.37°E, 714 m, 27 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Acacia aneura F.Muell. ex Benth. (Fabaceae), det. NSW Herbarium staff NSW658368, 2♂♂ (UNSW_ENT 00014133, UNSW_ENT 00014134), 3♀♀ (UNSW_ENT 00014135–UNSW_ENT 00014137) (AM). 111.2 km NW of Bond Springs on Tanami Road, 23.26667°S, 132.9167°E, 628 m, 22 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Acacia paraneura Randell (Fabaceae), det. NSW Herbarium staff NSW658301, 2♀♀ (UNSW_ENT 00014139, UNSW_ENT 00014140) (AM). 184 km W of Stuart Highway on Lasseter Highway, 25.24417°S, 131.57028°E, 510 m, 31 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Acacia brachystachya Benth. (Fabaceae), det. NSW Herbarium staff NSW 666270, 5♂♂ (UNSW_ENT 00013746–UNSW_ENT 00013750), 9♀♀ (UNSW_ENT 00013751–UNSW_ENT 00013759) (AM, AMNH, NTM). 193 km W of Stuart Highway on Lasseter Highway, 25.22806°S, 131.4775°E, 519 m, 31 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Acacia brachystachya Benth. (Fabaceae), det. NSW Herbarium staff NSW666270, 1♂ (UNSW_ENT 00013760), 3♀♀ (UNSW_ENT 00013761–UNSW_ENT 00013763) (AM). East of Arltunga Station, 23.36667°S, 134.5971°E, 660 m, 26 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, 5♀♀ (AMNH_PBI 00274379, AMNH_PBI 00274380, AMNH_PBI 00274385, AMNH_PBI 00274386, AMNH_PBI 00274391) (AM). West MacDonnell National Park, 2.5 km W of Ochre Pits on Namatjira Drive, 23.73335°S, 132.8503°E, 755 m, 5 Nov 2001, R.T. Schuh and Schwartz, Acacia aneura F.Muell. ex Benth. (Fabaceae), det. NSW Herbarium staff NSW666336, 1♀ (UNSW_ENT 00014138) (AM). ∼38 km N of Lasseter Highway on Luritja Road, 24.90002°S, 132.2809°E, 593 m, 2 Nov 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Acacia aneura F.Muell. ex Benth. (Fabaceae), det. NSW Herbarium staff NSW666295, 2♂♂ (UNSW_ENT 00015841, UNSW_ENT 00015842) (AM, AMNH). South Australia: 35.3 km SE of Pimba, on Stuart Highway, 31.47264°S, 137.05962°E, 102 m, 3 Dec 2018, A. McMah, M. Cheng, & B. Buirchell, Acacia sp. (Fabaceae), 1♀ (UNSW_ENT 00016921), 1 sex unknown; (UNSW_ENT 00016922) (UNSW). 131 km S of Coober Pedy, on Stuart Highway, 30.05351°S, 135.19531°E, 184 m, 2 Dec 2018, A. McMah, M. Cheng, & B. Buirchell, Acacia sp. (Fabaceae), 1♂ (UNSW_ENT 00016428), 2♀♀ (UNSW_ENT 00016429, UNSW_ENT 00016430) (UNSW). 139.2 km SE of William Creek, Finnis Springs (63 km NW of Maree), 29.60001°S, 137.4175°E, 21 m, 7 Nov 2001, Cassis, Schuh, and Schwartz, Acacia brachystachya Benth. (Fabaceae), det. NSW Herbarium staff NSW666339, 1♀ (UNSW_ENT 00014141) (AM). Owieandana, Flinders Ranges, 30.446°S, 138.948°E, no date provided, Hale & Tindale, 3♂♂ (UNSW_ENT 00016151) (QM ex UQIC). Western Australia: Pilbara region: just north of Auski Village, 22.20652°S, 118.78166°E, 427 m, 23 Aug 2005, G. Cassis, S. Lassau, S. and G. Carter, Acacia aneura F.Muell. ex Benth. var. tenuis (flat) (Fabaceae), det. Perth staff PERTH 7250797, 6♂♂ (UNSW_ENT 00014095–UNSW_ENT 00014100), 2♀♀ (UNSW_ENT 00014101, UNSW_ENT 00014102) (AM, WAM), Acacia aneura F.Muell. ex Benth. var. tenuis (flat) (Fabaceae), det. Perth staff PERTH 7250797, 1♂ (UNSW_ENT 00016120), 4♀♀ (UNSW_ENT 00016121–UNSW_ENT 00016124), 12 specimens sex unknown (UNSW_ENT 00016125–UNSW_ENT 00016136) (AMNH). 4.9 km E of Karrinarri Soak, Little Sandy Desert, 24.25017°S, 124.37781°E, 401 m, 27 Jul 2018, A. McMah & B. Buirchell, 1♂ (UNSW_ENT 00016442) (UNSW). 20 km N Edjudina Station, 29.65847°S, 121.07361°E, 400 m, 20 Sep 2017, B. Buirchell, 1♀ (UNSW_ENT 00016924) (UNSW). 79 km W of Sandstone, 28.03737°S, 118.4983°E, 650 m, 26 Oct 1996, Schuh and Cassis, 2♂♂ (UNSW_ENT 00014144, UNSW_ENT 00014145) (AM). Mooloogooloo Road, N Meekatharra, 26.17356°S, 118.75469°E, 474 m, 10 Aug 2018, B. Buirchell, Acacia sp. (Fabaceae), 1♂ (UNSW_ENT 00016879), 1♀ (UNSW_ENT 00016878), 2 sex unknown; (UNSW_ENT 00016876, UNSW_ENT 00016877) (UNSW). Newman Rocks, 136.5 km E of Norseman, 32.11084°S, 123.1704°E, 250 m, 22 Oct 1996, Schuh and Cassis, 1 specimen sex unknown (UNSW_ENT 00015816) (AMNH). Junction of Great Northern Hwy and Fortescue River, 23.39922°S, 119.12511°E, 524 m, 25 May 2017, Tatarnic, Buirchell, McMah, Cassis, Acacia sp. (Fabaceae), 1♀ (UNSW_ENT 00016435) (UNSW).
South Australia: 139.2 km SE of William Creek, Finnis Springs (63 km NW of Maree), 29.60001°S, 137.4175°E, 21 m, 7 Nov 2001, Cassis, Schuh, and Schwartz, Acacia brachystachya Benth. (Fabaceae), det. NSW Herbarium staff NSW666339, 1 nymph (UNSW_ENT 00014142) (AM).
Western Australia: Pilbara region: just north of Auski Village, 22.20652°S, 118.78166°E, 427 m, 23 Aug 2005, G. Cassis, S. Lassau, S. and G. Carter, Acacia aneura F.Muell. ex Benth. var. tenuis (flat) (Fabaceae), det. Perth staff PERTH 7250797, 14 nymphs (UNSW_ENT 00014103–UNSW_ENT 00014116) (AM).
Carenotus arltunga is recognised by the following combination of characters; females submacropterous (Fig. 6); clavus and proximal half of corium mostly cream–white with transverse dark brown fasciae on medial region of corium (Fig. 6); broad transverse subtriangular cream–white fasciae apically on corium (Fig. 6); head strongly transverse, with eyes extending well beyond anterolateral margins of pronotum (Fig. 6, 8a, b); metathoracic gland, peritreme and evaporative area well developed (Fig. 8c, d); left paramere with elongate and broad sensory lobe (Fig. 9a–c); pygophore with large, hooked left and flap-like rtp (Fig. 9a); right paramere mediodorsal margin with 10–14 teeth (Fig. 9d); phallotheca with large apical flap, and short tuberculate left process (Fig. 9e, f); proximal endosomal spicule margins smooth (Fig. 9e, f).
Pronotum: mostly dark brown, with narrow cream–white narrow broken band on posterior margin. Hemelytra: clavus either mostly cream–white, with apices brown, or often with light brown stripes, with cream–white spots on upper margin subapically, mid brown at apex; corium with proximal half cream–white, distal half with broad subquadrate brown transverse fasciae, distal region with broad subtriangular cream–white transverse fasciae. Legs: femora, tibiae mostly dark brown, sometimes with light brown or orange colouration apically.
Body: length 2.99–4.07 mm (see Table 4). Eyes: large, extending well beyond anterolateral angles of pronotum (Fig. 6, 8a, b). Labium: reaching mesocoxae (Fig. 6). Pronotum: subtrapezoidal (Fig. 8a, b). Metathoracic glands: peritreme short, tongue-shaped, flattened, with dense distribution of elongate microtrichia (Fig. 8c, d); evaporative area well developed, extending beyond peritreme, dorsal margin in line with apex of mesepimeron (Fig. 8c, d).
Pygophore: moderately elongate branched ltp, basally broad and short flap-like rtp (Fig. 8e, f, 9a, b). Left paramere: with large elongate oval sensory lobe (Fig. 9b, c). Right paramere: with shelf-like medial dorsal margin, with 10–14 teeth (Fig. 9a, d). Phallotheca: large, greatly expanded apically (Fig. 9e, f). Endosoma: large PES, basally bifurcate, medial branch longest (PES(mb)), reaching length of phallotheca, weakly bifurcate broad base, margins smooth, left branch (PES(lb)), undivided, apex acute, margins smooth and subdistally branching, with two short to moderate length branches, ventral branch extending beyond dorsal branch, apically serrate with two apices, dorsal branch tapered; secondary gonopore short and narrow (Fig. 9e, f).
Mostly as in males. Submacropterous, costal margins weakly convex (Fig. 6).
Vestibulum: with large V(rl), overlapping vestibulum strongly to left side, well beyond midline; vestibular channel present (see Fig. 10a). Sclerotised rings: small, with wide base, margins thin, and broad basal fold (Fig. 10a). Posterior wall of bursa copulatrix: IRL large, mitt-shaped, weakly attenuated and rounded apically, subequal in length to IRS (Fig. 10b).
See Table 4.
Carenotus arltunga was collected from three species of Acacia and a single species of Eremophila. Acacia aneura F.Muell. ex Benth. (Fig. 3c) and A. brachystachya F.Muell. ex Benth. are the primary hosts, with ≥5 specimens (Table 3). Most specimens were collected on A. aneura, which is found in semi-arid and arid Australia, excluding Tasmania; C. arltunga was found on the latter host plant in Western Australia and the Northern Territory. Carenotus arltunga was collected on A. brachystachya in the Northern Territory and South Australia.
Carenotus arltunga is a widespread species, known from the Northern Territory, South Australia and Western Australia, and three phytogeographic subregions (Western Desert, Pilbara and Eastern Desert) (Fig. 4).
Carenotus arltunga is most like Carenotus luritja in the following characters: corium with extensive cream–white region proximally, dark brown transverse band medially, broad subtriangular cream–white fasciae apically (cf. Fig. 6), extensive evaporative area on metepisternum (cf. Fig. 8c, d and 14c, d), elongate and arcuate ltp (cf. Fig. 9a and 15a), presence of rtp (cf. Fig. 9a and 15a), right paramere with denticulate median dorsal margin (cf. Fig. 9d and 15d), and IRL and IRS subequal in length (cf. Fig. 10b and 16b).
Carenotus arltunga is differentiated from Carenotus luritja by the basally broad and bifurcate pygophoral ltp (cf. undivided and narrow; cf. Fig. 9a and 15a), left paramere with serrate basal dorsal lobe (bdl) (cf. flaplike; cf. Fig. 9c and 15c), right paramere median dorsal margin with 10–14 teeth (cf. three teeth; cf. Fig. 9d and 15d), proximal endosomal spicule smooth (cf. serrate; cf. Fig. 9e, f and 15e, f), medial branch of the proximal endosomal spicule longest branch (cf. lateral branch longest; cf. Fig. 9e, f and 15e, f), and broader apex of IRL (cf. narrow apically; cf. Fig. 10b and 16b).
Also see Remarks section for Carenotus luritja and Carenotus pullabooka.
Carenotus louthensis McMah & Cassis, sp. nov.
(Fig. 2, 3, 4, 6, 11, 12, 13.)
ZooBank: urn:lsid:zoobank.org:act:92D12906-63F4-4F55-96C0-B3246BC90A15
Carenotus louthensis is named after Louth, New South Wales, near where specimens of this species were collected. The species epithet is the genitive case of a place name.
Holotype: AUSTRALIA: Queensland: 91 km N of Quilpie, 25.99847°S, 144.4098°E, 300 m, 2 Nov 1998, Schuh, Cassis, Silveira, Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW NSW427514, ♂ (UNSW_ENT 00012223) (QM).
Paratypes: AUSTRALIA: New South Wales: 34 km SW Bourke toward Louth, 30.30001°S, 145.6667°E, 100 m, 27 Oct 1995, Schuh and Cassis, Eremophila sturtii R.Br. (Scrophulariaceae), det. J. Everett 1996 NSW 395946, 16♂♂ (UNSW_ENT 00013067–UNSW_ENT 00013073, UNSW_ENT 00013075–UNSW_ENT 00013083, UNSW_ENT 00013127), 12♀♀ (UNSW_ENT 00013084–UNSW_ENT 00013094, UNSW_ENT 00013129) (AMNH). Queensland: 9 km WSW of Adavale, 25.91094°S, 144.5143°E, 270 m, 2 Nov 1998, Schuh, Cassis, Silveira, Eremophila mitchellii Benth. (Scrophulariaceae), det. NSW Herbarium staff NSW427513, 3♂♂ (UNSW_ENT 00012425–UNSW_ENT 00012427), 6♀♀ (UNSW_ENT 00012428–UNSW_ENT 00012433) (AM, QM). 11 km E of Adavale, 25.9545°S, 144.6848°E, 300 m, 2 Nov 1998, Schuh, Cassis, Silveira, Eremophila mitchellii Benth. (Scrophulariaceae), det. NSW Herbarium staff NSW427513, 2♀♀ (UNSW_ENT 00012239, UNSW_ENT 00012240) (AM). 14.2 km E of Charleville, 26.42171°S, 146.3756°E, 375 m, 31 Oct 1998, Schuh, Cassis, Silveira, Light Trap, 1♀ (UNSW_ENT 00012090) (AM). 48.6 km NW of Charleville, 26.14706°S, 145.8637°E, 365 m, 1 Nov 1998, Schuh, Cassis, Silveira, Eremophila mitchellii Benth. (Scrophulariaceae), det. NSW Herbarium staff NSW427510, 3♀♀ (UNSW_ENT 00012089, UNSW_ENT 00012435, UNSW_ENT 00012436), 1♂ (UNSW_ENT 00012434) (AM). 91 km N of Quilpie, 25.99847°S, 144.4098°E, 300 m, 2 Nov 1998, Schuh, Cassis, Silveira, Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW427514, 19♂♂ (UNSW_ENT 00011251–UNSW_ENT 00011258, UNSW_ENT 00012220–UNSW_ENT 00012222, UNSW_ENT 00012224–UNSW_ENT 00012231), 18♀♀ (UNSW_ENT 00012078–UNSW_ENT 00012085, UNSW_ENT 00012232–UNSW_ENT 00012238, UNSW_ENT 00015930–UNSW_ENT 00015932), Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW427514, 10♀♀ (AMNH_PBI 00009198-AMNH_PBI 00009207), 10♂♂ (AMNH_PBI 00009208-AMNH_PBI 00009217) (AM, QM), Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW427514, 27♂♂ (UNSW_ENT 00012122–UNSW_ENT 00012137, UNSW_ENT 00013095–UNSW_ENT 00013105), 43♀♀ (UNSW_ENT 00012138–UNSW_ENT 00012157, UNSW_ENT 00013106–UNSW_ENT 00013125, UNSW_ENT 00013130–UNSW_ENT 00013132), 1 sex unknown (UNSW_ENT 00013126) (AMNH). South Australia: 14.3 km S of Erudina Woolshed, 31.53334°S, 139.5506°E, 86 m, 9 Nov 2001, Cassis, Schuh, Schwartz, Silveira, Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW666375, 6♂♂ (UNSW_ENT 00012874–UNSW_ENT 00012878, UNSW_ENT 00013394), 5♀♀ (UNSW_ENT 00012879, UNSW_ENT 00012880, UNSW_ENT 00013395, UNSW_ENT 00013396, UNSW_ENT 00015850) Eremophila longifolia (R.Br.) F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW666363, 2♂♂ (UNSW_ENT 00013397, UNSW_ENT 00013398), 2♀♀ (UNSW_ENT 00013399, UNSW_ENT 00013400) (AM); 9 Nov 2001, Cassis, Schuh, Schwartz, Silveira, Aristida holathera Domin var. holathera (Poaceae), det. NSW Herbarium staff NSW 666308, 1♂ (UNSW_ENT 00014192), 2♀♀ (UNSW_ENT 00014193, UNSW_ENT 00014194) (AM). Koonamore Stn., 32.06355°S, 139.38365°E, 4 Apr 1979, J. A. Forrest, 1♂ (UNSW_ENT 00016150) (SAMA), 2♀♀ (UNSW_ENT 00016148, UNSW_ENT 00016149) (QM ex UQIC). 72 km N of Yunta, Nillinghoo Creek, 32.00924°S, 139.4523°E, 194 m, 9 Nov 2001, Cassis, Schuh, and Schwartz, ex Eremophila sturtii R.Br., 12♂♂ (AMNH_PBI 00274704, AMNH_PBI 00274707-AMNH_PBI 00274711, AMNH_PBI 00274718-AMNH_PBI 00274723), 7♀♀ (AMNH_PBI 00274705, AMNH_PBI 00274706, AMNH_PBI 00274712, AMNH_PBI 00274714-AMNH_PBI 00274717), 1 sex undetermined (AMNH_PBI 00274713) (AM).
AUSTRALIA: Queensland: 91 km N of Quilpie, 25.99847°S, 144.4098°E, 300 m, 2 Nov 1998, Schuh, Cassis, Silveira, Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW427514, 3 nymphs (UNSW_ENT 00012086–UNSW_ENT 00012088) (AM).
Carenotus louthensis is recognised by the following combination of characters: posterior margin of pronotum with broken white band on posterior margin (Fig. 6); pygophore with broad and arcuate ltp with paired apical processes (Fig. 12a); rtp absent (Fig. 12a); left paramere with small digitiform basal process (Fig. 12c); right paramere mediodorsal margin with three teeth (Fig. 12a, d); phallotheca without folds or processes (Fig. 12e, f); proximal endosomal medial branch apically bifurcate, left branch longer than medial branch (Fig. 12e, f).
Pronotum: mostly dark brown, red–brown anteriorly, with broken cream–white narrow band on posterior margin (Fig. 6). Hemelytra: lateral and anteromedial margins cream–white (anteromedial stripe wider, lateral margin longer), bounding medial brown stripe, latter expanding in width from humeral angle to tip of claval commissure; corium mostly brown, with base of exocorium and narrow apical transverse fasciae cream–white. Legs: femora and tibiae dark brown, sometimes orange or pale colouration apically (Fig. 6).
Body: length 2.99–3.14 mm (See Table 4). Head: interocular distance less than 1/2 width of anterior margin of pronotum (Fig. 11a). Labium: reaching metacoxae (Fig. 6). Pronotum: subtrapezoidal to weakly campanulate (Fig. 6, 11a); callosite region obsolete (Fig. 11a, b). Thoracic pleura: metathoracic spiracular vestibule without evaporative bodies (Fig. 11c, d). Metathoracic glands: greatly reduced, with slitlike ostiolar canal (Fig. 11c, d); peritreme and evaporative area obsolete (Fig. 11c, d). Hemelytra: costal margins straight to weakly convex (Fig. 6). Abdominal venter: pregenital sterna strongly concertinaed (Fig. 6).
Pygophore: with broad and arcuate ltp, with paired apical short recurved hornlike processes (Fig. 11e, 12a); lacking a rtp (Fig. 12a). Left paramere: with subtriangular sensory lobe and small digitiform bdl, opposed to apophysis (Fig. 11f, 12b, c). Right paramere: club-shaped, mediodorsal margin (mdm) with three teeth (Fig. 12d). Phallotheca: arcuate, without folds or processes (Fig. 12e, f). Endosoma: PES with two smooth branches, medial branch (PES(mb)) apically bifurcate, left branch elongate (PES(lb)), arcuate, longer than medial branch (Fig. 12e, f).
Similar to males in colouration, vestiture and non-genitalic morphology (Fig. 6). Submacropterous (Fig. 6).
Vestibulum: V(rl) overlapping vestibulum strongly to left side, well beyond midline; VC present (Fig. 13a). Sclerotised rings: small, with thin margins, and broad basal fold (Fig. 13a). Posterior wall of bursa copulatrix: posterior wall with broad IRS, medially excavate, weakly sclerotised; IRL spinose and mitt-shaped, shorter than IRS (Fig. 13b).
See Table 4.
There are two host plant species of Carenotus louthensis: Eremophila mitchellii Benth. and E. sturtii R.Br. (Fig. 3d) (Scrophulariaceae), each with >5 specimens (Table 3). Aristida holathera and Eremophila longifolia are considered sitting records, based on <5 specimens for each plant species (Table 3). Eremophila longifolia is possibly an additional host plant, with four C. louthensis specimens recorded from this host, which included both males and females, but further sampling is required.
The species is known from Queensland, New South Wales and South Australia, at nine locations within the Eastern Desert phytogeographic subregion (Fig. 4).
Carenotus louthensis is most like Carenotus schwartzi in the following characters: corium medially dark brown with thin cream–white transverse fasciae apically (cf. Fig. 6 and 7); reduced peritreme and evaporative area on metepisternum (cf. Fig. 11c, d and 23c, d); and the absence of a pygophoral rtp (cf. Fig. 12a and 24a).
Carenotus louthensis is differentiated from Carenotus schwartzi by the bifurcate pygophoral ltp (cf. undivided; cf. Fig. 12a and 24a), the presence of a bdl on the left paramere (cf. absence; cf. Fig. 12c and 24c), the presence of a medial dorsal margin on the right paramere (cf. absence; cf. Fig. 12d and 24d), and the proximal endosomal spicule with smooth margins (cf. serrate; cf. Fig. 12e, f and 24e, f).
Carenotus luritja McMah & Cassis, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:74868077-679C-4118-B192-FBA36353A3AD
Carenotus luritja is named after Luritja Road, Northern Territory, where specimens of this species were collected. Noun in apposition.
Holotype: AUSTRALIA: South Australia: 20 km NW of Innamincka, Innamincka Reg. Res., 27.60897°S, 140.6333°E, 170 m, 5 Nov 1998, Schuh, Cassis, Silveira, Salsola kali L. (Amaranthaceae), det. NSW Herbarium staff NSW, 1♂ (UNSW_ENT 00014165) (SAMA).
Paratypes: AUSTRALIA: Northern Territory: 1 km S of Henbury Craters Nature Reserve, 24.56668°S, 133.1234°E, 457 m, 29 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Sclerolaena holtiana W.T. Aiton (Amaranthaceae), det. NSW Herbarium staff NSW658407, 11♀ (UNSW_ENT 00013796–UNSW_ENT 00013806) (AM, SAMA). 7.3 km E of Stuart Highway on Horseshoe Bend Road, 25.11667°S, 133.2517°E, 437 m, 28 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Acacia aneura F.Muell. ex Benth. (Fabaceae), det. NSW Herbarium staff NSW658387, 1♀ (UNSW_ENT 00015838) (AMNH). 17.5 km E of Stuart Highway on Horseshoe Bend Road, 25.16667°S, 133.3223°E, 412 m, 29 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, 1♀ (AMNH_PBI 00274395) (AM). Junction of Stuart Highway and Horseshoe Bend Road, 25.06667°S, 133.2006°E, 474 m, 28 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Sida fibulifera Lindl. (Malvaceae), det. NSW Herbarium staff NSW658383, 2♂♂ (UNSW_ENT 00014125, UNSW_ENT 00014129), 5♀♀ (UNSW_ENT 00014127, UNSW_ENT 00014128, UNSW_ENT 00014130–UNSW_ENT 00014132) (AM), Sida fibulifera Lindl. (Malvaceae), det. NSW Herbarium staff NSW658383, 3♀♀ (UNSW_ENT 00016432–UNSW_ENT 00016434) (AMNH). ~8 km N of Lasseter Highway on Luritja Road, 24.90002°S, 132.2809°E, 593 m, 2 Nov 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Atriplex elachophylla F.Muell. (Amaranthaceae), det. NSW Herbarium staff NSW666293, 5♂♂ (UNSW_ENT 00013764, UNSW_ENT 00013765, UNSW_ENT 00013767, UNSW_ENT 00013768, UNSW_ENT 00013792), 22♀♀ (UNSW_ENT 00013769–UNSW_ENT 00013788, UNSW_ENT 00013793, UNSW_ENT 00013795), 3♂♂ (UNSW_ENT 00013789–UNSW_ENT 00013791) (AM, NTM). South Australia: 25 km E of Copely, Flinders Range, 30.51668°S, 138.631°E, 450 m, 8 Nov 2001, Cassis, Schuh, and Schwartz, Atriplex elachophylla F.Muell. (Amaranthaceae), det. NSW Herbarium staff NSW666355, 1♂ (UNSW_ENT 00014143) (AM).
Carenotus luritja is recognised by the following combination of characters: females submacropterous or brachypterous (Fig. 6); posterior margin of pronotum without cream–white markings (Fig. 6); most of clavus and proximal half of corium cream–white, with apex of clavus brown (Fig. 6); distal region of corium with broad subtriangular cream–white transverse fasciae (Fig. 6); metathoracic gland, peritreme and evaporative area well developed (Fig. 14c, d); pygophore with undivided ltp (Fig. 15a); pygophore with rtp (Fig. 15a); left paramere without teeth (Fig. 15c); right paramere medial dorsal margin with 3 teeth (Fig. 15d); lateral branch of proximal endosomal spicule elongate, longer than medial branch, with subapical serrations (Fig. 15e, f); medial branch apically bifurcate, smooth (Fig. 15e, f); sclerotised rings small, oval (Fig. 16a); IRL subequal in length to IRS (Fig. 16b).
Pronotum: uniformly dark brown, without cream markings on posterior margin. Hemelytra: clavus mostly pale cream, proximally and distally whitish; corium with proximal half cream–white, distal half with subtriangular broad brown transverse fasciae, distally with broad subtriangular cream–white transverse fasciae; cuneus uniformly dark brown; membrane translucent. Legs: femora mostly reddish-brown, profemora sometimes paler apically; tibiae mid reddish-brown (Fig. 6).
Body: length 2.99–3.76 mm (Table 4). Eyes: large, extending well beyond anterolateral angles of pronotum (Fig. 14a, b). Pronotum: subtrapezoidal to weakly campanulate (Fig. 14a). Metathoracic glands: peritreme and evaporative area well developed; close proximity to metathoracic spiracle, variation within species (Fig. 14c, d).
Pygophore: elongate and undivided ltp; short thorn-like rtp (Fig. 15a). Left paramere: without teeth, apophysis with weak apical hook (Fig. 14e, 15b, c). Right paramere: shelf-like medial process, with 3 teeth (Fig. 15a, d). Phallotheca: without flaps or processes (Fig. 15e, f). Endosoma: lateral branch of proximal endosomal spicule (PES(lb)) greatly elongate and narrow, extending well beyond medial branch (PES(mb)), subapically serrate, branch bifurcate (Fig. 15e, f).
Mostly as in males. Hemelytra submacropterous, subequal in length to abdomen (Fig. 6); hemelytral costal margins weakly rounded (Fig. 6).
Vestibulum: with large V(rl), overlapping vestibulum to left side, beyond midline; vestibular channel well developed (Fig. 16a). Sclerotised rings: small, with thin margins, and broad basal fold (Fig. 16a). Posterior wall of bursa copulatrix: with broad IRS, medially excavate, weakly sclerotised; IRL spinose and mitt-shaped, subequal in length to IRS (Fig. 16b).
See Table 4.
The species is known from south central regions of the Northern Territory and north-eastern South Australia (Fig. 4).
Most specimens were found on two species of the family Amaranthaceae (Atriplex elachophylla and Sclerolaena holtiana) which are considered host plants, with >5 specimens. Specimens were also collected on the malvaceous species Sida fibulifera, with >5 specimens. The Salosa kali Linnaeus and Acacia aneura are both singleton records, and are thus considered sitting records (Table 3).
Carenotus luritja is most similar to Carenotus arltunga in the pale cream–white corial region of the hemelytra (Fig. 6). It can be separated by: lack of cream–white markings on the posterior margin of the pronotum (cf. presence of cream–white markings) (Fig. 6); clavus entirely pale (cf. presence of medial brown stripe); serrate lateral branch of proximal endosomal spicule (cf. smooth) (Fig. 6); medial branch of proximal endosomal spicule longer than lateral branch (cf. shorter than lateral branch) (cf. Fig. 9e, f and 15e, f); left paramere without teeth (cf. with teeth) (cf. Fig. 9c and 15c); and, medial dorsal margin of right paramere with 3 teeth (cf. 10–14 teeth) (cf. Fig. 9d and 15d).
Carenotus luritja also differs from Carenotus arltunga in host plant associations (Table 3). It is found on Atriplex and Sida, whereas Carenotus arltunga is an Acacia specialist (see above). These host plant differences may explain their sympatry in the central desert of the Northern Territory and southeastern South Australia, with all locations within the Eastern Desert phytogeographic subregion (Fig. 4).
Carenotus pullabooka McMah & Cassis, sp. nov.
(Fig. 2, 3, 4, 6, 17, 18, 19.)
ZooBank: urn:lsid:zoobank.org:act:69E10554-AC80-42F3-B31C-A6A63494AEA9
Carenotus pullabooka is named after Pullabooka State Forest, New South Wales, the type locality of the species. Noun in apposition.
Holotype: AUSTRALIA: Victoria: Wyperfeld National Park, Moonah Track, 35.46302°S, 142.0464°E, 65 m, 4 Nov 2002, Cassis, Schuh, Schwartz, Silveira, Callitris gracilis R.T. Baker (Cupressaceae), det. NSW Herbarium staff NSW658101, 1♂ (UNSW_ENT 00013945) (NMV)
Paratypes: AUSTRALIA: New South Wales: Nyngan, Dec 1960, J. Armstrong, 2♀♀ (UNSW_ENT 00032196, UNSW_ENT 00032197) (QM ex UQIC). Pullabooka SF, 33.84111°S, 147.81027°E, 20 Nov 1997, Aust. Mus. Terr. Ecol. Dept., Callitris glaucophylla Joy. Thomps. & L.A.S. Joh (Cupressaceae), 1♂ (UNSW_ENT 00013539) (AM). Pullabooka SF, 33.82444°S, 147.81361°E, 20 Nov 1997, Aust. Mus. Terr. Ecol. Dept., Callitris glaucophylla Joy. Thomps. & L.A.S. Joh (Cupressaceae), 1 specimen sex unknown (UNSW_ENT 00013546) (AM); 5 Nov 1998, Aust. Mus. Terr. Ecol. Dept., Callitris glaucophylla Joy. Thomps. & L.A.S. Joh (Cupressaceae), 3♂♂ (UNSW_ENT 00013541–UNSW_ENT 00013543), 2♀♀ (UNSW_ENT 00013544, UNSW_ENT 00013545) (AM). Victoria: Wyperfeld National Park, Moonah Track, 35.46302°S, 142.0464°E, 65 m, 4 Nov 2002, Cassis, Schuh, Schwartz, Silveira, Callitris gracilis R.T. Baker (Cupressaceae), det. NSW Herbarium staff NSW658101, 2♂♂ (UNSW_ENT 00013944, UNSW_ENT 00013946), 1♀ (UNSW_ENT 00013947) (AMNH, AM, UNSW). Western Australia: Great Eastern Highway, 70.9 km E of Merredin, 31.3322°S, 118.97907°E, 391 m, 21 Nov 2018, A. McMah, M. Cheng, & B. Buirchell, Callitris glaucophylla Joy. Thomps. & L.A.S. Johnson (Cupressaceae), det. B. Buirchell – Field ID, 1♀ (UNSW_ENT 00016923) (UNSW).
Victoria: Wyperfeld National Park, Moonah Track, 35.46302°S, 142.0464°E, 65 m, 4 Nov 2002, Cassis, Schuh, Schwartz, Silveira, Callitris gracilis R.T. Baker (Cupressaceae), det. NSW Herbarium staff NSW658101, 2 nymphs (UNSW_ENT 00013948, UNSW_ENT 00013949) (AM).
Carenotus pullabooka is recognised by the following combination of characters (Fig. 6); females submacropterous or brachypterous (Fig. 6); posterior margin of pronotum with unbroken cream–white marking (Fig. 6); most of clavus and proximal half of corium brown, with two apical cream–white spots on clavus (Fig. 6); distal region of corium with thin to moderate subtriangular cream–white transverse fasciae (Fig. 6); metathoracic gland, peritreme and evaporative area well developed (Fig. 17c, d); pygophore with divided ltp (Fig. 18a); rtp present (Fig. 18a); left paramere with teeth and bdl (Fig. 18c); right paramere medial dorsal margin with 7–8 teeth (Fig. 18d); proximal endosomal spicule without serration (Fig. 18e, f); sclerotised rings subelliptical, elongate (Fig. 19a); IRL longer than IRS (Fig. 19c).
Pronotum: narrow cream–white band on posterior margin (Fig. 6). Hemelytra: clavus dark brown with cream–white stripe on medial margin and two cream–white apical spots near apex on lateral margin (Fig. 6); corium with proximal half of exocorium with cream–white marking; distal half of corium with subquadrate broad dark brown transverse fasciae, apically with moderate subtriangular cream–white transverse fasciae; cuneus uniformly dark brown (Fig. 6). Legs: femora and tibiae mostly reddish-brown (Fig. 6).
Body: length 2.73–3.33 mm (see Table 4). Labium: reaching mesocoxae (Fig. 6). Pronotum: subtrapezoidal (Fig. 6, 17a). Metathoracic glands: peritreme short, tongue-shaped, flattened, with dense distribution of elongate microtrichia (Fig. 17c, d); evaporative area well developed, extending beyond peritreme, in line with dorsal margin in line with apex of mesepimeron (Fig. 17c, d).
Proctiger: with large spine on right-side of midline (Fig. 18a). Pygophore: greatly elongate bifurcate ltp (Fig. 17e, 18a); short ledge-like rtp (Fig. 18a). Left paramere: greatly elongate, with exaggerated and subquadrate sensory lobe, apex of apophysis weakly hooked (Fig. 17e, 18b, c). Right paramere: medial dorsal margin with 7–8 continuous teeth (Fig. 17f, 18a, d). Phallotheca: with ventral flange (PH(vf)) and left ventral flange (PH(lvf)) (Fig. 18e, f). Endosoma: single proximal endosomal spicule, deeply bifurcate, lateral branch (PES(lb)) greatly elongate and narrow, extending well beyond medial branch (PES(mb)); PES(mb) margins smooth with tapered apex (Fig. 18e, f).
Mostly as in males. Hemelytra brachypterous or submacropterous, shorter or subequal in length to abdomen, lateral margins rounded (Fig. 6).
Vestibulum: with large V(rl), overlapping vestibulum to left side, beyond midline (Fig. 19a); vestibular channel well developed (Fig. 19a). Sclerotised rings: elongate, subelliptical with thin margins (Fig. 19a). Posterior wall of bursa copulatrix: broad IRS, medially excavate, weakly sclerotised; IRL spinose and mitt-shaped, longer than IRS (Fig. 19b).
See Table 4.
Carenotus pullabooka was collected on two species of the family Cupressaceae; Callitris glaucophylla Joy. Thomps. & L.A.S. Joh (Fig. 3e) and C. gracilis R.T. Baker, with both represented by >5 specimens, including both sexes (Table 3). The majority of specimens were collected at two sites in the Pullabooka State Forest, both in an ecological study of insects associated with C. glaucophylla in western New South Wales (Major et al. 2003).
This species is known from New South Wales, Victoria and Western Australia, in the Eastern Desert and Southwest Interzone phytogeographic subregions (Fig. 4). It has a major east-west biogeographic disjunction, with four locations in eastern Australia (New South Wales and Victoria) and one in Western Australia.
Carenotus pullabooka is most similar to Carenotus arltunga in the following characters: two cream–white apical spots on clavus (Fig. 6), well-developed peritreme and evaporative area on the metepisternum (cf. Fig. 8c, d and 17c, d), short apophysis of the left paramere (cf. Fig. 9c and 18c), the comblike mediodorsal margin of the right paramere (cf. Fig. 9d and 18d) and the presence of a ledgelike pygophoral rtp (cf. Fig. 9a and 18a). It is differentiated from Carenotus arltunga by the more extensive brown colour on the dorsum, with thinner corial apical transverse fasciae (Fig. 6), the proctiger with a right spinose process (cf. absent; cf. Fig. 9a and 18a), the deeply bifurcate pygophoral ltp (cf. distally bifurcate; cf. Fig. 9a and 18a), the absence of a phallothecal lateral process (cf. present; cf. Fig. 9e and 18e), the right paramere medial dorsal margin with 7–8 teeth (cf. 10–14 teeth; cf. Fig. 9d and 18d), and the elongate and subelliptical sclerotised rings (cf. short and elongate; cf. Fig. 10a and 19a).
In addition, Carenotus pullabooka is more southern in distribution than Carenotus arltunga, and these two species do not overlap spatially (Fig. 4). Lastly, these species do not share hosts, not even at the genus-group rank and above, with Carenotus pullabooka a Callitris specialist and Carenotus arltunga hosted on two species of Acacia (Table 3).
Carenotus scaevolaphilus McMah & Cassis, sp. nov.
(Fig. 2, 3, 5, 7, 20, 21, 22.)
ZooBank: urn:lsid:zoobank.org:act:F9F8F039-5FD7-4D10-AA01-987B4D1481B3
Carenotus scaevolaphilus is named after the host genus, Scaevola Linnaeus, and suffix philus from the Greek. Adjectival form of host plant genus Scaveola.
Holotype: AUSTRALIA: Western Australia: North West Coastal Highway 45.8 km NE of jct with Blowholes Road, 24.40709°S, 114.0057°E, 50 m, 28 Oct 2004, Cassis, Wall, Weirauch, Tatarnic, Symonds, Light Trap, ♂ (UNSW_ENT 00013140) (WAM).
Paratypes: AUSTRALIA: Western Australia: North West Coastal Highway 45.8 km NE of jct with Blowholes Road, 24.40709°S, 114.0057°E, 50 m, 28 Oct 2004, Cassis, Wall, Weirauch, Tatarnic, Symonds, Scaevola spinescens R.Br. (Goodeniaceae), det. WA Herbarium PERTH6987524, 8♂♂ (UNSW_ENT 00013134–UNSW_ENT 00013139, UNSW_ENT 00013141), 8♀♀ (UNSW_ENT 00013142–UNSW_ENT 00013147, UNSW_ENT 00014018 (AM, AMNH, UNSW).
This species is recognised by the following combination of characters; posterior margin of pronotum with broken cream–white colouring (Fig. 7); clavus mostly mid brown with whitish-cream markings (Fig. 7); peritreme and evaporative area reduced (Fig. 20c, d); pygophore with bifurcate ltp (Fig. 21a); right paramere with hooked medial process (Fig. 21a, d); proximal endosomal spicule bifurcate at base, lateral branch extends beyond medial branch (Fig. 21e, f) and medial branch serrate (Fig. 21e, f); IRL shorter than IRS (Fig. 22b).
Pronotum: narrow, divided cream–white band on posterior margin (Fig. 7). Hemelytra: clavus dark orange–brown with cream–white stripes on medial and anterolateral margins; corium with proximal half of exocorium with cream–white marking; distal half of corium with subquadrate broad dark brown transverse fasciae, apically with thin subtriangular cream–white transverse fasciae (Fig. 7); cuneus uniformly dark brown (Fig. 7). Legs: femora mostly brown; tibiae dark brown, sometimes pale orange apically (Fig. 7).
Body: length 2.99–3.11 mm (see Table 4). Labium: reaching mesocoxae (Fig. 7). Pronotum: subtrapezoidal (Fig. 7, 20a). Metathoracic glands: Metathoracic spiracle large; peritreme and evaporative area greatly reduced (Fig. 20c, d).
Pygophore: with large and wide ltp with two prongs, no rtp (Fig. 21a). Left paramere: triangular sensory lobe, extended hooked apophysis (ap), bdl present (Fig. 20f, 21a–c). Right paramere: club-shaped, with hook-like medial process, with 3 teeth (Fig. 20e, f, 21a, b, d). Endosoma: single proximal endosomal spicule with two branches with smooth margins (Fig. 21e, f); lateral branch (PES(lb)) greatly elongate and narrow, extending beyond medial branch (PES(mb)) (Fig. 21e, f), medial branch with apical serration (Fig. 21e, f).
Mostly as in males. Hemelytra submacropterous, shorter or subequal in length to end of abdomen, lateral margins rounded (Fig. 7).
Vestibulum: with large V(rl), overlapping vestibulum at midline; vestibular channel well developed (Fig. 22a). Sclerotised rings: short, oval with thin margins (Fig. 22a). Posterior wall of bursa copulatrix: IRS broad, medially excavate, weakly sclerotised; IRL spinose and mitt-shaped, shorter than IRS (Fig. 22b).
See Table 4.
The species is known from the Northwest Cape of Western Australia, in the Western Desert phytogeographic subregion (Fig. 5).
Carenotus scaevolaphilus is most similar to Carenotus schwartzi and Carenotus tanami on the basis of the following characters: corium medially with dark brown transverse band, thin to medium subtriangular cream–white fasciae apically (cf. Fig. 7), reduced peritreme and evaporative area on metepisternum (cf. Fig. 20c, d, 23c, d and 26c, d) and the absence of a pygophoral rtp (cf. Fig. 21a, 24a and 27a).
Carenotus scaevolaphilus is differentiated from Carenotus schwartzi and Carenotus tanami by the lighter brown colouration on the dorsum (cf. Fig. 7), the bifurcate pygophoral ltp (cf. Fig. 21a, 24a and 27a) and the presence of a right paramere medial process (cf. Fig. 21d, 24d and 27d).
Carenotus schwartzi McMah & Cassis, sp. nov.
(Fig. 1, 2, 5, 7, 23, 24, 25.)
ZooBank: urn:lsid:zoobank.org:act:738255D0-70DB-4382-AE7F-74D60E03905A
Carenotus schwartzi is named after Dr Michael Schwartz, one of the collectors of this species. Species epithet is the genitive case of a male person.
Holotype: AUSTRALIA: Northern Territory: 8.8 km N of Mount Wedge Stn Road junction on Tanami Road, 22.63335°S, 132.3525°E, 598 m, 23 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, ex Solanum orbiculatum Dunal ex Poir. subsp. orbiculatum (Solanaceae), det. NSW Herbarium staff NSW658318, ♂ (AMNH_PBI 00274552) (NTM).
Paratypes: AUSTRALIA: Northern Territory: 8.8 km N of Mount Wedge Stationn Road junction on Tanami Road, 22.63335°S, 132.3525°E, 598 m, 23 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, ex Solanum orbiculatum Dunal ex Poir. subsp. orbiculatum (Solanaceae), det. NSW Herbarium staff NSW658318, 28♂♂ (AMNH_PBI 00274524-AMNH_PBI 00274526, AMNH_PBI 00274530, AMNH_PBI 00274531, AMNH_PBI 00274533-AMNH_PBI 00274535, AMNH_PBI 00274537, AMNH_PBI 00274539-AMNH_PBI 00274543, AMNH_PBI 00274545, AMNH_PBI 00274546, AMNH_PBI 00274548, AMNH_PBI 00274549, AMNH_PBI 00274553, AMNH_PBI 00274555, AMNH_PBI 00274557-AMNH_PBI 00274561, AMNH_PBI 00274563, AMNH_PBI 00274564, AMNH_PBI 00274567), 16♀♀ (AMNH_PBI 00274526-AMNH_PBI 00274529, AMNH_PBI 00274532, AMNH_PBI 00274536, AMNH_PBI 00274538, AMNH_PBI 00274547, AMNH_PBI 00274550, AMNH_PBI 00274551, AMNH_PBI 00274554, AMNH_PBI 00274556, AMNH_PBI 00274562, AMNH_PBI 00274565, AMNH_PBI 00274566, AMNH_PBI 00274568-AMNH_PBI 00274570), 1♀ (UNSW_ENT 00015843) (AMNH). 1 sex undetermined (AMNH_PBI 00274544) (AM). 74.2 km NW of Bond Springs on Tanami Road, 23.41668°S, 133.2307°E, 671 m, 22 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, 1♀ (AMNH_PBI 00274504) (AM).
Carenotus schwartzi is recognised by the following combination of characters; hemelytra predominantly brown or orange–brown (Fig. 7); with thin cream–white transverse fasciae (Fig. 7); metathoracic gland, peritreme and evaporative areas reduced (Fig. 23c, d); long strap-like ltp (Fig. 23e, 24a); and, endosomal spicule serrate (Fig. 24e, f).
Pronotum: cream–white broken band on posterior margin (Fig. 7). Hemelytra: distal half of corium with subquadrate broad dark brown transverse fasciae, apically with moderate subtriangular cream–white transverse fasciae (Fig. 7); cuneus uniformly orange–brown (Fig. 7). Legs: femora mostly orange–brown and tibiae reddish-brown, sometimes cream apically (Fig. 7).
Body: length 2.99–3.18 mm (see Table 4). Labium: reaching mesocoxae (Fig. 7). Pronotum: subtrapezoidal, with reduced callosite region (Fig. 7, 23a, b). Metathoracic glands: peritreme and evaporative area significantly reduced (Fig. 23c, d); metathoracic spiracle large, variation within the species (Fig. 23c, d).
Pygophore: greatly elongate, undivided ltp (Fig. 23e, 24a). Left paramere: greatly enlarged subquadrate sensory lobe, apex of apophysis weakly hooked (Fig. 23e, f, 24b, c). Right paramere: club-shaped, with denticulate medial process, with 3–4 teeth (Fig. 24b, d). Endosoma: single proximal endosomal spicule lateral branch greatly elongate, both branches serrate (Fig. 24e, f).
Mostly as in males. Hemelytra submacropterous, lateral margins rounded (Fig. 7).
Vestibulum: with large multilobed V(rl), overlapping vestibulum to left side, beyond midline; vestibular channel well developed (Fig. 25a). Sclerotised rings: short, oval with thin margins (Fig. 25a). Posterior wall of bursa copulatrix: broad IRS, medially excavate, weakly sclerotised; IRL spinose and mitt-shaped, subequal in length to IRS (Fig. 25b).
See Table 4.
All specimens of Carenotus schwartzi were collected on Solanum orbiculatum Dunal ex Poir. (Solanaceae) (Table 3).
The species is known from the Northern Territory, with all locations within the Eastern Desert phytogeographic subregion (Fig. 5).
Carenotus schwartzi is most similar to Carenotus yuendumu in the following characters: colour patterning of the corium (cf. Fig. 7), reduced evaporative area on the metepisternum (cf. Fig. 23c, d and 29c, d), elongate, undivided ltp (cf. Fig. 24a and 30a), left paramere sensory lobe structure (cf. Fig. 24c and 30c), and endosomal spicule structure (cf. Fig. 24e, f and 30e, f).
Carenotus schwartzi is differentiated from Carenotus yuendumu by the lighter brown dorsum (cf. Fig. 7), thicker cream–white colouration on the clavus and corial transverse fasciae (cf. Fig. 7), the absence of a rtp (cf. Fig. 24a and 30a), and differences in the shape of the right paramere (cf. Fig. 24d and 30d).
Carenotus tanami, sp. nov.
ZooBank: urn:lsid:zoobank.org:act:C94724D9-3D4E-4CA0-85F5-BBE36EAB8884
Holotype: Northern Territory: 87.3 km E of Yuendumu Mount Denison–Coniston Road, 22.13334°S, 132.5387°E, 634 m, 24 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW658329, ♂ (UNSW_ENT 00014185) (NTM).
Paratypes: AUSTRALIA: Northern Territory: 25.3 km NW of Bond Springs on Tanami Road, 23.51668°S, 133.6212°E, 746 m, 21 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila gilesii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658289, 2♂♂ (UNSW_ENT 00012844, UNSW_ENT 00014166), 4♀♀ (UNSW_ENT 00012845, UNSW_ENT 00012846, UNSW_ENT 00014167, UNSW_ENT 00014168) (AM). 26.8 km W of Tanami Road on Mount Wedge Station Road, 22.50001°S, 132.179°E, 589 m, 23 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila sp. (Scrophulariaceae), det. Field ID NSW658314, 4♂♂ (UNSW_ENT 00013905–UNSW_ENT 00013908), 9♀♀ (UNSW_ENT 00013909–UNSW_ENT 00013917) (AM). 54.5 km E of Yulara, on Lasseter Highway, 25.22734°S, 131.48648°E, 494 m, 28 Nov 2018, A. McMah, M. Cheng, & B. Buirchell, Eremophila latrobei F.Muell. (Scrophulariaceae), det. B. Buirchell – Field ID, 1♀ (UNSW_ENT 00016426), 1♂ (UNSW_ENT 00016427) (UNSW). 74.8 km E of Yuendumu on Mount Denison–Coniston Road, 22.1°S, 132.4231°E, 646 m, 24 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658323, 1♂ (UNSW_ENT 00012847), 7♀♀ (UNSW_ENT 00012848–UNSW_ENT 00012854). East of Arltunga Station, 23.36667°S, 134.5971°E, 660 m, 26 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila sp. (Scrophulariaceae), det. Field ID NSW658360, 4♂♂ (UNSW_ENT 00016046, UNSW_ENT 00016047, AMNH_PBI 00274650, AMNH_PBI 00274651), 4♀♀ (UNSW_ENT 00016060–UNSW_ENT 00016062, UNSW_ENT 00016066, 2 specimens sex unknown (UNSW_ENT 00016063, UNSW_ENT 00016064) (AM), Eremophila sp. (Scrophulariaceae), det. Field ID NSW658360, 3♂♂ (UNSW_ENT 00016048, UNSW_ENT 00016050, UNSW_ENT 00016053), 8♀♀ (UNSW_ENT 00016055–UNSW_ENT 00016057, UNSW_ENT 00016065, UNSW_ENT 00016067–UNSW_ENT 00016070) (AMNH).
Carenotus tanami is recognised by the following combination of characters; hemelytra predominantly mid brown (Fig. 7); with thin or medium white or cream transverse fasciae (Fig. 7); broad, two-pronged ltp (Fig. 27a); and endosomal spicule with serrated margins (Fig. 27e, f).
Pronotum: cream–white broken band on posterior margin, sometimes with orange markings anteriorly (Fig. 7). Hemelytra: clavus mostly mid brown with medial and anterolateral cream–white stripes (Fig. 7); corium with proximal half of exocorium with cream–white (Fig. 7); distal half of corium with broad subquadrate dark brown transverse fasciae, apically with moderate subtriangular cream–white transverse fasciae (Fig. 7); cuneus uniformly brown (Fig. 7). Legs: femora and tibiae mostly orange–brown, sometimes light brown or cream apically (Fig. 7).
Body: length 2.82–3.11 mm (see Table 4). Labium: reaching mesocoxae (Fig. 7). Pronotum: subtrapezoidal, narrow (Fig. 7, 26a). Metathoracic glands: peritreme and evaporative area obsolete (Fig. 26c, d); metathoracic spiracle large (Fig. 26c, d).
Pygophore: broad, ltp apically bifurcate (Fig. 26e, 27a, b). Left paramere: greatly enlarged triangular sensory lobe with bdl, apex of apophysis hooked (Fig. 26e, f, 27b, c). Right paramere: club-shaped with multiple teeth (Fig. 26e, f, 27a, b, d). Endosoma: proximal endosomal spicule mesially bifurcate, lateral branch greatly elongate, undivided, apically serrate, medial branch bifurcate, apically serrate (Fig. 27e, f).
Mostly as in males. Hemelytra submacropterous, shorter or subequal in length to abdomen, lateral margins rounded (Fig. 7).
Vestibulum: with large V(rl), mostly positioned on midline (Fig. 28a); VC well developed (Fig. 28a). Sclerotised rings: short, oval with thin margins (Fig. 28a). Posterior wall of bursa copulatrix: IRS broad, medially excavate, weakly sclerotised; IRL spinose and mitt-shaped, subequal in length to IRS (Fig. 28b).
See Table 4.
The species is known only from the Northern Territory, in the Eastern and Western phytogeographic subregions (Fig. 5).
This species is found on three Eremophila species (E. duttonii F.Muell., E. gilesi F.Muell. and E. latrobei F.Muell.) that are rated as host species, with >5 specimens (Table 3). Eremophila sturtii is considered as a sitting record based on a singleton record.
Carenotus tanami is most similar to Carenotus scaevolaphilus in the following characters: colour patterning of the corium (cf. Fig. 7); absence of a rtp (cf. Fig. 21a and 27a); reduced peritreme and obsolete evaporative area on metepisternum (cf. Fig. 20c, d and 26c, d); and, left paramere sensory lobe shape (cf. Fig. 20e, f, 21a–c, 26e, f and 27a–c).
Carenotus tanami is differentiated from Carenotus scaevolaphilus by darker brown colouration on dorsum (cf. Fig. 7); broader cream–white colouration on clavus and corial transverse fasciae (cf. Fig. 7); the absence of a right paramere medial process (cf. Fig. 21d, 27d); and differences in the proximal endosomal spicule shape (cf. Fig. 21e, f, 27e, f).
Carenotus yuendumu, sp. nov.
(Fig. 2, 3, 5, 7, 29, 30, 31.)
ZooBank: urn:lsid:zoobank.org:act:11F579FD-FAA8-489A-AFFB-528593605DE4
Carenotus yuendumu is named after Yuendumu, Northern Territory, where some specimens were collected.
Holotype: AUSTRALIA: South Australia: Henbury Station: 10 km W of Henbury Homestead, 24.605°S, 133.16528°E, 435 m, 15 May 2013, M. Cheng, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NT Herbarium Staff – Alice Springs, ♂ (UNSW_ENT 00052426) (SAMA).
Paratypes: AUSTRALIA: Northern Territory: 1 km S of Henbury Craters Nature Reserve, 24.56668°S, 133.1234°E, 457 m, 29 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658411, 5♂♂ (UNSW_ENT 00012676–UNSW_ENT 00012680), 23♀♀ (UNSW_ENT 00012681–UNSW_ENT 00012703) Eremophila freelingii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658410, 3♂♂ (UNSW_ENT 00016072–UNSW_ENT 00016074), 1♀♀ (UNSW_ENT 00016075 (AM). 3.7 km SE of Kings Canyon Resort, Watarrka National Park, 24.26667°S, 131.5375°E, 623 m, 3 Nov 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila longifolia (R.Br.) F.Muell. (Scrophulariaceae), 1♀ (UNSW_ENT 00011212) (AM). 25.3 km NW of Bond Springs on Tanami Road, 23.51668°S, 133.6212°E, 746 m, 21 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila gilesii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658289, 2♀♀ (UNSW_ENT 00013047, UNSW_ENT 00013048) (AM). 45.3 km NW of Bond Springs on Tanami Road, 23.51668°S, 133.4626°E, 695 m, 21 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658298, 29♂♂ (UNSW_ENT 00012492–UNSW_ENT 00012496, UNSW_ENT 00013838–UNSW_ENT 00013860, UNSW_ENT 00014169) (AMNH), 54♀♀ (UNSW_ENT 00012497–UNSW_ENT 00012507, UNSW_ENT 00013861–UNSW_ENT 00013989, UNSW_ENT 00014170, UNSW_ENT 00014172) (AMNH), Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW658297, 9♀♀ (UNSW_ENT 00011198–UNSW_ENT 00011205, 3♂♂ (UNSW_ENT 00011195–UNSW_ENT 00011197) (AM). 51.6 km W of Stuart Highway on Mount Denison–Coniston Road, 22.30001°S, 132.8951°E, 722 m, 24 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658333, 8♂♂ (UNSW_ENT 00013010–UNSW_ENT 00013017), 15♀♀ (UNSW_ENT 00013018, UNSW_ENT 00013020–UNSW_ENT 00013033) (AM). 67 km E of Stuart Highway on Arltunga Station Road, 23.28027°S, 134.37°E, 714 m, 27 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658367, 1♂ (UNSW_ENT 00013046) Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW658366, 11♂ (UNSW_ENT 00015840, UNSW_ENT 00016028–UNSW_ENT 00016037), 8♀♀ (UNSW_ENT 00016038–UNSW_ENT 00016045) (AMNH). 71.6 km NE of Kings Canyon Resort, 23.80002°S, 131.6635°E, 743 m, 3 Nov 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila latrobei F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW666316, 3♂♂ (UNSW_ENT 00012508–UNSW_ENT 00012510), 4♀♀ (UNSW_ENT 00012511, UNSW_ENT 00013941–UNSW_ENT 00013943) Eremophila gilesii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW666317, 3♂♂ (UNSW_ENT 00013041–UNSW_ENT 00013043), 2♀♀ (UNSW_ENT 00013044, UNSW_ENT 00013045) (AM, AMNH). 74.8 km E of Yuendumu on Mount Denison–Coniston Road, 22.1°S, 132.4231°E, 646 m, 24 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658323, 89♂♂ UNSW_ENT 00010970–UNSW_ENT 00010972, UNSW_ENT 00011133–UNSW_ENT 00011157, UNSW_ENT 00012906–UNSW_ENT 00012921, UNSW_ENT 00012936–UNSW_ENT 00012967, UNSW_ENT 00013923–UNSW_ENT 00013931, UNSW_ENT 00014173–UNSW_ENT 00014176), 86♀♀ (UNSW_ENT 00010939, UNSW_ENT 00010441–UNSW_ENT 00010950, UNSW_ENT 00011158–UNSW_ENT 00011184, UNSW_ENT 00012922–UNSW_ENT 00012935, UNSW_ENT 00012968–UNSW_ENT 00012990, UNSW_ENT 00013933–UNSW_ENT 00013940, UNSW_ENT 00014177, UNSW_ENT 00014178), 1 sex unknown (UNSW_ENT 00014179) (AM, AMNH, WAM), Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658323, 3♂♂ (UNSW_ENT 00010936–UNSW_ENT 00010938), 3♀♀ (UNSW_ENT 00010951–UNSW_ENT 00010953) (UNSW). 80.3 km E of Yuendumu on Mount Denison–Coniston Road, 22.11667°S, 132.4298°E, 633 m, 24 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658327, 16♂♂ (UNSW_ENT 00010954–UNSW_ENT 00010960, UNSW_ENT 00013807–UNSW_ENT 00013814, UNSW_ENT 00014180), 34♀♀ (UNSW_ENT 00010962–UNSW_ENT 00010968, UNSW_ENT 00013815–UNSW_ENT 00013836, UNSW_ENT 00013920, UNSW_ENT 00013921, UNSW_ENT 00014181–UNSW_ENT 00014183) (AMNH), Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658327, 1♂ (UNSW_ENT 00010961), 1♀ (UNSW_ENT 00010969) (UNSW). 86 km E of Yulara, on Lasseter Highway, 25.31576°S, 131.76932°E, 489 m, 28 Nov 2018, A. McMah, M. Cheng, & B. Buirchell, Eremophila J. Black (Scrophulariaceae), det. B. Buirchell – Field ID, 3♀♀ (UNSW_ENT 00016404–UNSW_ENT 00016406), 2♂♂ (UNSW_ENT 00016407, UNSW_ENT 00016408) (UNSW). 87.3 km E of Yuendumu Mount Denison–Coniston Road, 22.13334°S, 132.5387°E, 634 m, 24 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW658329, 3♂♂ (UNSW_ENT 00011207, UNSW_ENT 00014186, UNSW_ENT 00014187), 6♀♀ (UNSW_ENT 00011208, UNSW_ENT 00011209, UNSW_ENT 00014188–UNSW_ENT 00014191) (AM). 105 km E of Docker River, on Tjukaruru Road, 25.08021°S, 130.00616°E, 597 m, 25 Nov 2018, A. McMah, M. Cheng, & B. Buirchell, 1♂ (UNSW_ENT 00016942) Acacia sp. (Fabaceae), 1♂ (UNSW_ENT 00016431) (UNSW). 133 km E of Yulara, on Lasseter Highway, 25.16521°S, 132.13977°E, 486 m, 28 Nov 2018, A. McMah, M. Cheng, & B. Buirchell, Eremophila neglecta J. Black (Scrophulariaceae), det. B. Buirchell – Field ID, 8♀♀ (UNSW_ENT 00016411–UNSW_ENT 00016418), 4♂♂ (UNSW_ENT 00016419–UNSW_ENT 00016422) (UNSW). East of Arltunga Station, 23.36667°S, 134.5971°E, 660 m, 26 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila sp. (Scrophulariaceae), det. Field ID NSW658360, 2♂♂ (UNSW_ENT 00016049, UNSW_ENT 00016054) (AMNH). Henbury Station, 14 km NE from Henbury Homestead, North of Chandler Range approx. 2.3 km from Stuart Highway, 24.46556°S, 133.35194°E, 549 m, 17 May 2013, M. Cheng & C. Duykers, Eremophila sturtii R.Br. (Scrophulariaceae), det. NT Herbarium Staff – Alice Springs, 1♂ (UNSW_ENT 00052427) Eremophila sturtii R.Br. (Scrophulariaceae), det. NT Herbarium Staff – Alice Springs, 1♀ (UNSW_ENT 00052428), 1♀ (UNSW_ENT 00052429) (UNSW). Henbury Station: 10 km W of Henbury Homestead, 24.605°S, 133.16528°E, 435 m, 15 May 2013, M. Cheng, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NT Herbarium Staff – Alice Springs, 6♂♂ (UNSW_ENT 00015759–UNSW_ENT 00015764) (AMNH), Eremophila duttonii F.Muell. (Scrophulariaceae), det. NT Herbarium Staff – Alice Springs, 17♀♀ (UNSW_ENT 00015765–UNSW_ENT 00015781) (UNSW). Yuendumu, 22.258°S, 131.797°E, Feb 1968, Unknown collector, 1♂ (UNSW_ENT 00015852) (SAMA). South Australia: 14.3 km S of Erudina Woolshed, 31.53334°S, 139.5506°E, 86 m, 9 Nov 2001, Cassis, Schuh, Schwartz, Silveira, Eremophila sturtii R.Br. (Scrophulariaceae), det. NSW Herbarium staff NSW666375, 1♀ (UNSW_ENT 00011210) Aristida holathera Domin var. holathera (Poaceae), det. NSW Herbarium staff NSW666308, 1♀ (UNSW_ENT 00011211) (AMNH). Great Victoria Desert, BB Standard Survey Site 2, 28.81504°S, 129.53345°E, 360 m, 20 Sep 2017, N. Tatarnic & M. Cheng, Eremophila gilesii F.Muell. (Scrophulariaceae), det. SA State Herbarium, 1♀ (UNSW_ENT 00016394) (UNSW). Great Victoria Desert, Middle Road, 1.75 km E of Rodinia Road, 29.10931°S, 129.55907°E, 270 m, 22 Sep 2017, N. Tatarnic & M. Cheng, Eremophila glabra (R.Br.) Ostenf. (Scrophulariaceae), det. SA State Herbarium, 2♂♂ (UNSW_ENT 00016236, UNSW_ENT 00016381), 2♀♀ (UNSW_ENT 00016382, UNSW_ENT 00016383) (UNSW). Great Victoria Desert, Rodinia Road, S face of sand dune, 28.88136°S, 129.53486°E, 317 m, 19 Sep 2017, N. Tatarnic & M. Cheng, Eremophila gilesii F.Muell. (Scrophulariaceae), det. SA State Herbarium, 3♂♂ (UNSW_ENT 00046693, UNSW_ENT 00016391, UNSW_ENT 00016392), 1♀ (UNSW_ENT 00016393) (UNSW). Great Victoria Desert, Rodinia Road, S of airstrip at Middle Road intersection, 29.11362°S, 129.54127°E, 260 m, 23 Sep 2017–26 Sep 2017, N. Tatarnic & M. Cheng, Eremophila gilesii F.Muell. (Scrophulariaceae), det. SA State Herbarium, 1♂ (UNSW_ENT 00016235) Eremophila scoparia (R.Br.) F.Muell. (Scrophulariaceae), det. SA State Herbarium, 2♂♂ (UNSW_ENT 00016386, UNSW_ENT 00016387), 2♀♀ (UNSW_ENT 00016388, UNSW_ENT 00016389) (SAMA, UNSW); 25 Sep 2017, N. Tatarnic & M. Cheng, Eremophila gilesii F.Muell. (Scrophulariaceae), det. SA State Herbarium, 1♂ (UNSW_ENT 00016395), 1♀ (UNSW_ENT 00016396) (UNSW). Western Australia: 53.3 km E of North West Coastal Highway on Mardathuna Road, 24.41311°S, 114.4254°E, 90 m, 31 Oct 2004, Cassis, Wall, Weirauch, Tatarnic, Symonds, Eremophila forrestii F.Muell. (Scrophulariaceae), det. PERTH staff PERTH6990045, 1♂ (UNSW_ENT 00015949), 2♀♀ (UNSW_ENT 00015950, UNSW_ENT 00015951) (AMNH).
Northern Territory: 1 km S of Henbury Craters Nature Reserve, 24.56668°S, 133.1234°E, 457 m, 29 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658411, 2 nymphs (UNSW_ENT 00012674, UNSW_ENT 00012675) (AM). 3.7 km SE of Kings Canyon Resort, Watarrka National Park, 24.26667°S, 131.5375°E, 623 m, 03 Nov 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila longifolia (R.Br.) F.Muell. (Scrophulariaceae), 2 nymphs (UNSW_ENT 00011213, UNSW_ENT 00011214) (AM). 45.3 km NW of Bond Springs on Tanami Road, 23.51668°S, 133.4626°E, 695 m, 21 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658298, 12 nymphs (UNSW_ENT 00013901–UNSW_ENT 00013904, UNSW_ENT 00014117–UNSW_ENT 00014124) (AM). 80.3 km E of Yuendumu on Mount Denison–Coniston Road, 22.11667°S, 132.4298°E, 633 m, 24 Oct 2001, Cassis, Schuh, Schwartz, Silveira, Wall, Eremophila duttonii F.Muell. (Scrophulariaceae), det. NSW Herbarium staff NSW658327, 1 nymph (UNSW_ENT 00014184) (AM). 86 km E of Yulara, on Lasseter Highway, 25.31576°S, 131.76932°E, 489 m, 28 Nov 2018, A. McMah, M. Cheng, & B. Buirchell, Eremophila J. Black (Scrophulariaceae), det. B. Buirchell – Field ID, 2 nymphs (UNSW_ENT 00016409, UNSW_ENT 00016410). 133 km E of Yulara, on Lasseter Highway, 25.16521°S, 132.13977°E, 486 m, 28 Nov 2018, A. McMah, M. Cheng, & B. Buirchell, Eremophila neglecta J. Black (Scrophulariaceae), det. B. Buirchell – Field ID, 3 nymphs (UNSW_ENT 00016423–UNSW_ENT 00016425) (UNSW).
South Australia: Great Victoria Desert, Middle Road, 1.75 km E of Rodinia Road, 29.10931°S, 129.55907°E, 270 m, 22 Sep 2017, N. Tatarnic & M. Cheng, Eremophila glabra (R.Br.) Ostenf. (Scrophulariaceae), det. SA State Herbarium, 7 nymphs (UNSW_ENT 00016373–UNSW_ENT 00016379). Great Victoria Desert, Rodinia Road, S face of sand dune, 28.88136°S, 129.53486°E, 317 m, 19 Sep 2017, N. Tatarnic & M. Cheng, Eremophila gilesii F.Muell. (Scrophulariaceae), det. SA State Herbarium, 1 nymph (UNSW_ENT 00016390). Great Victoria Desert, Rodinia Road, S of airstrip at Middle Road intersection, 29.11362°S, 129.54127°E, 260 m, 23 Sep 2017–26 Sep 2017, N. Tatarnic & M. Cheng, Eremophila gilesii F.Muell. (Scrophulariaceae), det. SA State Herbarium, Eremophila scoparia (R.Br.) F.Muell. (Scrophulariaceae), det. SA State Herbarium, 2 nymphs (UNSW_ENT 00016384, UNSW_ENT 00016385).
Carenotus yuendumu is characterised by the following combination of characters: strap-like ltp (Fig. 29e, 30a); serration of the margins of the endosomal spicule (Fig. 30e, f); and teeth on both left and right parameres (Fig. 30c, d).
Pronotum: cream–white broken band on posterior margin, sometimes orange–brown anteriorly (Fig. 7). Hemelytra: clavus dark brown, with cream–white medial and anterolateral stripes, apex dark brown; corium with proximal half of exocorium with yellowish-cream to white (Fig. 7); distal half of corium with broad subquadrate broad dark brown transverse fasciae, apically with moderate subtriangular cream–white transverse fasciae (Fig. 7); cuneus uniformly dark brown (Fig. 7). Legs: femora and tibiae mostly dark brown, sometimes with light brown or orange apically (Fig. 7).
Body: length 2.99–3.21 mm (see Table 4). Labium: reaches mesocoxae (Fig. 7). Pronotum: subtrapezoidal, without collar or callosite region (Fig. 7, 29a, b); posterior margin concave (Fig. 29a, b). Metathoracic glands: peritreme large, tongue-like and flattened, evaporative area significantly reduced (Fig. 29c, d).
Pygophore: elongate, undivided ltp, rtp flap-like (Fig. 29e, 30a). Left paramere: with exaggerated oval sensory lobe, without basal dorsal process, apophysis short (Fig. 29e, f, 30a–c). Right paramere: club-shaped, apically attenuated, medial process present, with 3 or 4 teeth (Fig. 29f, 30a, b, d). Endosoma: single proximal endosomal spicule present, with lateral branch greatly elongate and narrow, weak serration subapically (Fig. 30e, f), and medial branch with subdistal serration (Fig. 30e, f).
Mostly as in males. Hemelytra submacropterous, subequal in length to abdomen, lateral margins rounded (Fig. 7).
Vestibulum: with large multilobed V(rl), positioned from right to left of midline (Fig. 31a); vestibular channel prominent (Fig. 31a). Sclerotised rings: short, oval with thin margins (Fig. 31a). Posterior wall of bursa copulatrix: IRS broad, medially excavate, weakly sclerotised; IRL spinose and mitt-shaped, subequal in length to IRS (Fig. 31b).
See Table 4.
Carenotus yuendumu was collected on 14 plant species (Table 3) from the families Fabaceae, Goodeniaceae, Poaceae and Scrophulariaceae. The following Eremophila species are recognised as host plants: E. duttonii (Fig. 3f), E. gilesii, E. glabra, E. latrobei, E. neglecta, E. scoparia and E. sturtii (Fig. 3d); and E. freelingii (Fig. 3b) is recognised as a possible host plant of the species.
The species is known from the Northern Territory, Western Australia and South Australia, in the Eastern Desert and Western Desert phytogeographic subregions (Fig. 5).
Carenotus yuendumu is most similar to Carenotus schwartzi in the following characters: colour patterning of the corium (cf. Fig. 7), reduced evaporative area on metepisternum (cf. Fig. 23c, d and 29c, d) and left paramere sensory lobe shape and size (cf. Fig. 23a–c, e, f, 29e, f, 30a–c).
Carenotus yuendumu is differentiated from Carenotus schwartzi by darker brown colouration on the dorsum (cf. Fig. 7); the presence of a rtp on the pygophore (cf. Fig. 24a and 30a,); the absence of a right paramere medial process (cf. Fig. 24a, 30a); and the right paramere shape (cf. Fig. 24b, c and 30b, c).
Kirkaldyella Poppius, 1921
Kirkaldyella Poppius, 1921, p. 54 (original description). Cassis and Gross (1995), p. 191 (catalogue); Cassis and Moulds (2002), p. 55 (diagnosis, new species).
Kirkaldyella was revised by Cassis and Moulds (2002), including the description of 12 new species. This genus is hypothesised as a poor ant mimic, without pale transverse fasciae on the hemelytra and abdominal venter. Its myrmecomorphy is supported by several traits, including the dark body, the strongly triangular head, the robust body with the pronotum rugopunctate, and the weakly excavated hemelytra, with the median flexion line almost contiguous with R + M vein. In addition, the nymphs are putatively myrmecomorphic, with a dark body and strongly triangular head.
Cassis and Moulds (2002) provided simple illustrations of the male aedeagus and did not examine the female genitalia. Given the similarity of the left paramere of Carenotus species (i.e. elongate apophysis lying on ventral wall of pygophore), and putative close relationship of the two genera, more detailed examinations of the male genitalia and first female genitalic observations were made of two species of Kirkaldyella (i.e. K. pilosa Cassis and Moulds, K. rugosa Poppius), to provide character information for the phylogenetic analysis above (Fig. 1).
Kirkaldyella pilosa Cassis & Moulds, 2022
(Fig. 32, male genitalia; 33, female genitalia.)
Kirkaldyella pilosa Cassis & Moulds, 2002, p. 78 (original description). Cassis and Gross (1995), p. 191 (catalogue); Cassis and Moulds (2002), p. 81 (redescription).
Victoria: Little Desert National Park, 14 km E on McDonald Highway, 36.56668°S, 141.4°E, 150 m, 4 Nov 1995, Schuh and Cassis, Pultenaea tenuifolia R.Br. ex Sims (Fabaceae), det. P.H. Weston 1996 NSW 395987, 1♂ (AMNH_PBI 00008891), 1♀ (AMNH_PBI 00008894) (AM).
Pygophore: without tergal processes (Fig. 32a). Left paramere: with large elongate oval sensory lobe (e.g. Fig. 32b) and bdl (e.g. Fig. 32b, c), elongate apophysis with small counter-clockwise apical hook, positioned on ventral wall of pygophore at rest (Fig. 32b, c). Right paramere: with shelf-like mediodorsal margin, with a single tooth (Fig. 32d). Phallotheca: large, greatly expanded apically, without flaps (Fig. 32e). Endosoma: large PES, medially bifurcate, medial branch longest (PES(mb)), reaching length of phallotheca, with capitate and serrate apex, left branch thin, tapered (PES(lb)) (Fig. 32e).
Vestibulum: without enlarged V(rl); with short VC (Fig. 33a). Sclerotised rings: small, with wide base, margins thin, and broad basal fold (Fig. 33a). Posterior wall: IRL large, shorter in length than IRS, mitt-shaped, apically attenuate and medially excavate (Fig. 33b).
Male genitalia of Kirkaldyella pilosa sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view. Abbreviations: ap, apophysis; bdl, basal dorsal lobe; DS, ductus seminis; PES(lb), proximal endosomal spicule, lateral branch; mp, medial process; PES(mb) proximal endosomal spicule, medial branch; phg, phalloguide; sl, sensory lobe; SG, secondary gonopore.
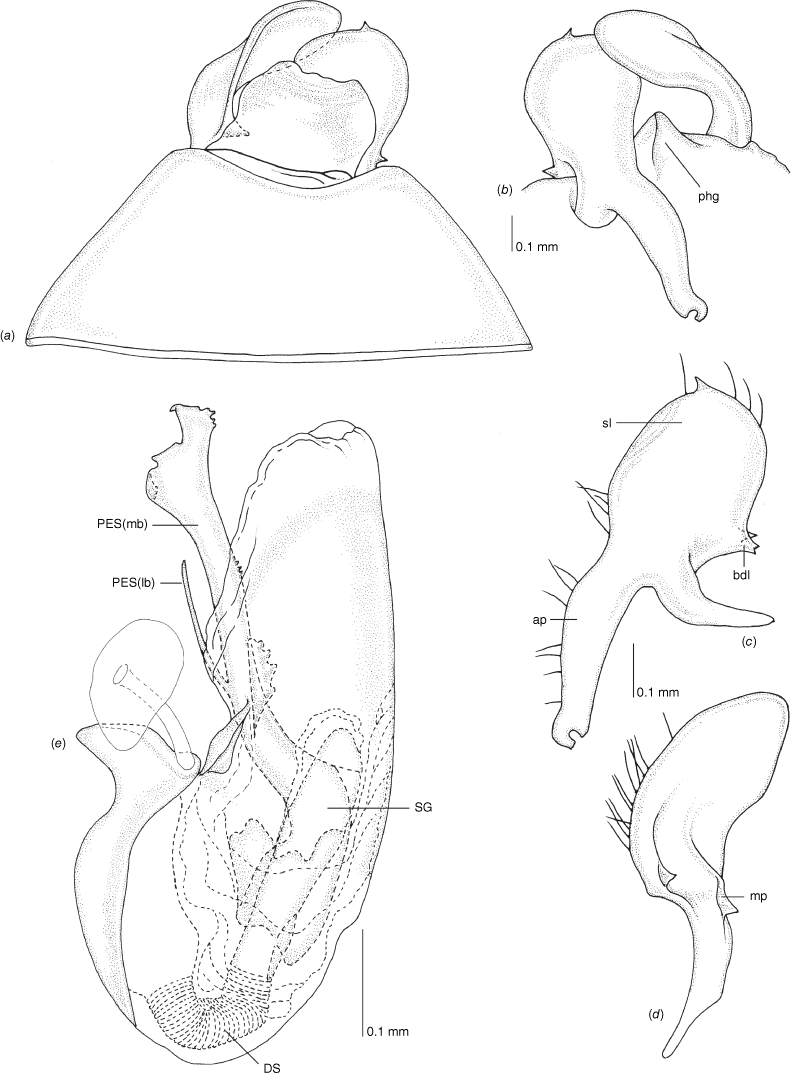
Female genitalia of Kirkalydyella pilosa, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate.
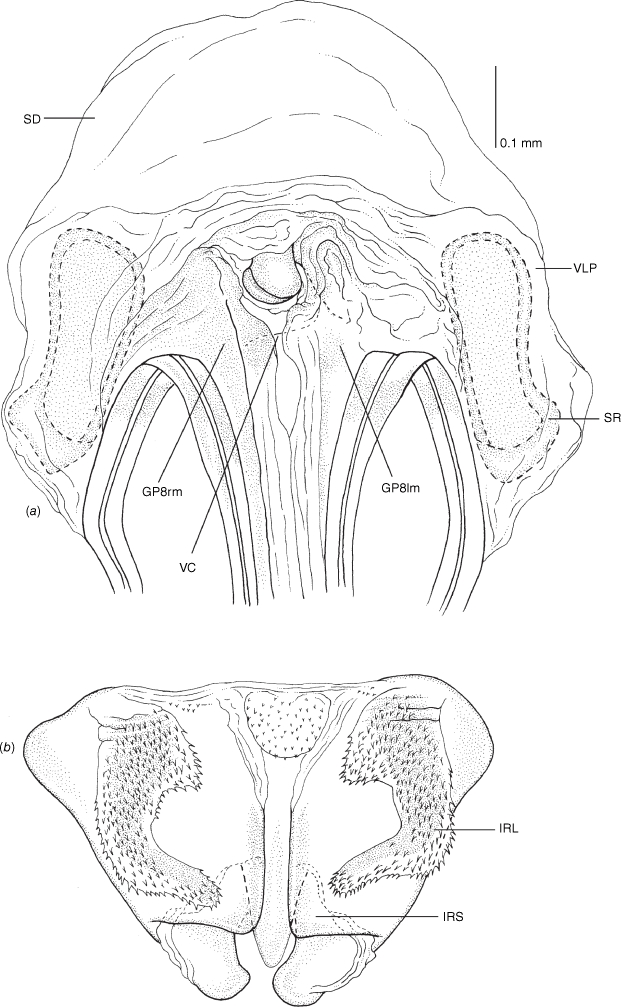
Kirkaldyella rugosa Poppius, 1921
(Fig. 34, male genitalia; 35, female genitalia.)
Kirkaldyella rugosa Poppius, 1921 (original description)
New South Wales: Brogo River crossing on Princes Hwy, 36.54°S, 149.827°E, 7 Nov 1988, G. Cassis, Lomandra sp. (Asparagaceae), det. K.L. Wilson 1997 NSW 396001, 1♂ (AMNH_PBI 00008981) (AM). Brogo River, Pacific Highway 15 km N of Bega, 36.61668°S, 149.8333°E, 50 m, 10 Nov 1995, Schuh and Cassis, Lomandra longifolia Labill. (Asparagaceae), det. K.L. Wilson 1997 NSW 396001, 1♀ (AMNH_PBI 0000892) (AM).
Pygophore: with mtp (Fig. 34a). Left paramere: with subquadrate elongate sensory lobe (e.g. Fig. 34b) and bdl (e.g. Fig. 34b, c), elongate apophysis with small–clockwise apical hook, positioned on ventral wall of pygophore at rest (Fig. 32b, 34c). Right paramere: with shelf-like mediodorsal margin, with a single tooth (Fig. 34d). Phallotheca: large, greatly expanded apically, without flaps (Fig. 34e). Endosoma: large PES, medially trifurcate, medial branch longest (PES(rb)), almost reaching length of phallotheca, apically bifurcate, lateral branch tapered apically (PES(lb)), medial branch (PES(mb)) shortest, apically bifid (Fig. 34e).
Vestibulum: without enlarged V(rl); with short VC (Fig. 35a). Sclerotised rings: small, with wide base, margins thin and broad basal fold (Fig. 35a). Posterior wall of bursa copulatrix: IRL large, subequal in length to IRS, mitt-shaped, apically attenuate, medially excavate (Fig. 35b).
Male genitalia of Kirkaldyella rugosa sp. nov. (a) pygophore, dorsal view; (b) parameres, ventral view; (c) left paramere, dorsal view; (d) right paramere, ventral view; (e) aedeagus, left lateral view; (f) secondary gonopore and base of endosomal spicules, ventral view. Abbreviations: ap, apophysis; bdl, basal dorsal lobe; DS, ductus seminis; mp, medial process; mtp, medial tergal process; PES(lb), proximal endosomal spicule, lateral branch; PES(mb) proximal endosomal spicule, medial branch; PES(rb) proximal endosomal spicule, right branch; phg, phalloguide; sl, sensory lobe; SG, secondary gonopore.
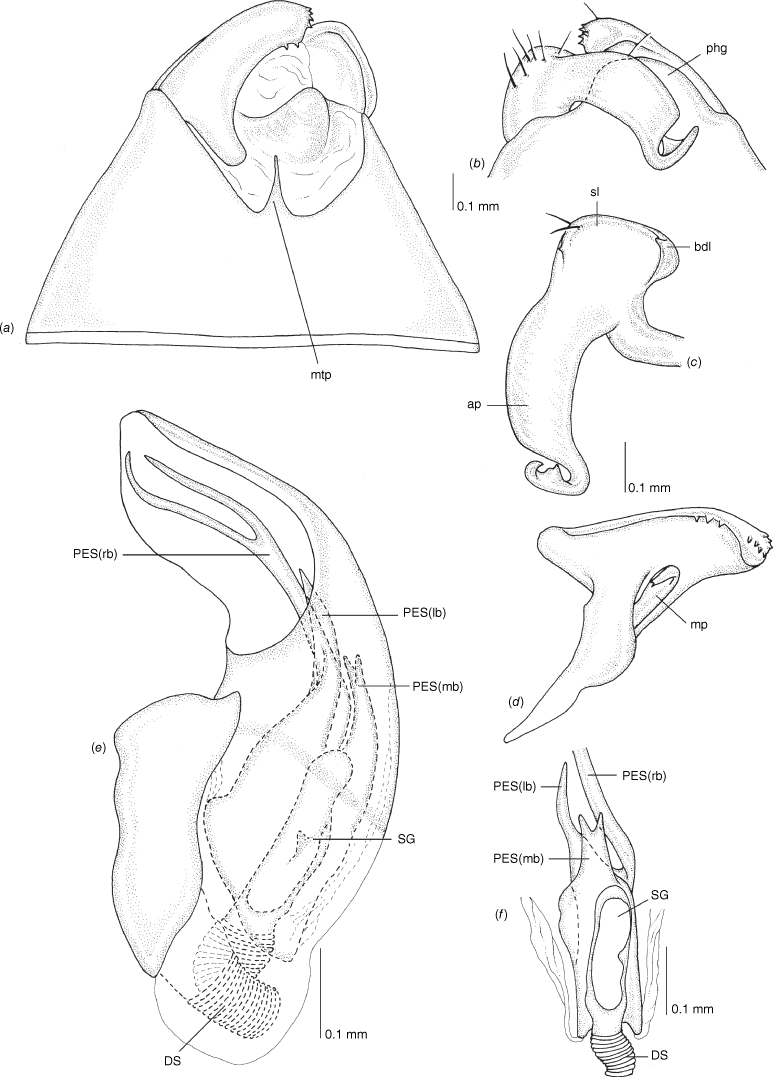
Female genitalia of Kirkalydyella rugosa, sp. nov. (a) posterior wall of bursa copulatrix, ventral view; (b) inter-ramal sclerite and lobes. Abbreviations: IRL, inter-ramal lobe; IRS, inter-ramal sclerite; GP8lm, gonapophysis 8 left membrane; GP8rm, gonapophysis 8 right membrane; SD, seminal depository; SR, sclerotised rings; VC, vestibular channel; VLP, ventral labiate plate.
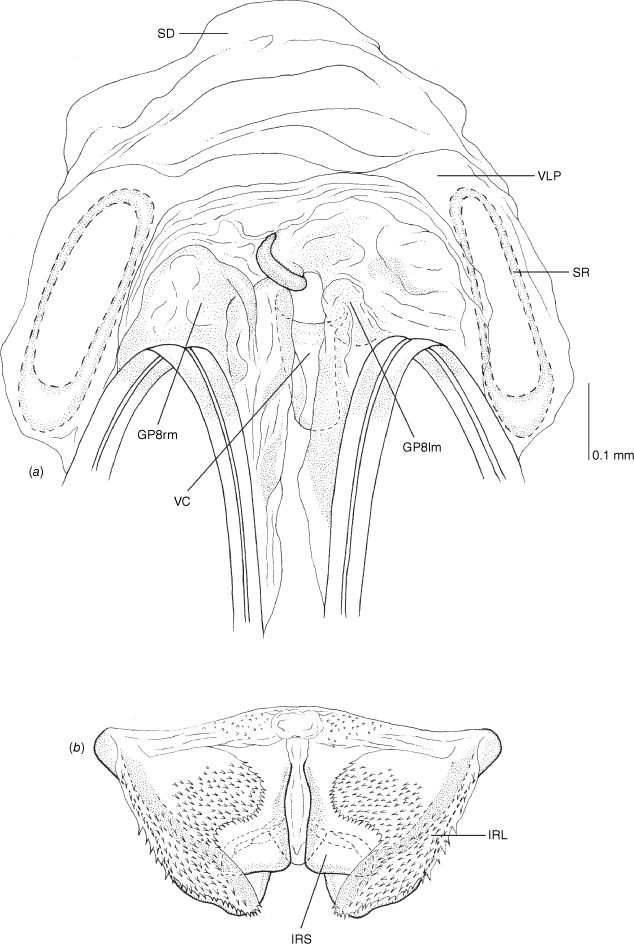
Discussion
Myrmecomorphic traits
Much of what we know about mirid myrmecomorphy is drawn from the taxonomic literature (see Schuh and Slater 1995; Schuh and Weirauch 2020 for summaries), where mostly morphological traits are hypothesised as ant like, and only a few such species interactions have been subject to behavioural experimentation or natural history observations (e.g. McIver 1987). Polemics about myrmecomorphy also concern the accuracy of such traits relative to both observed ant model(s) or generalised ant facies. The perfecting hypothesis (McLean et al. 2019) predicts that there is a gradual increase in accuracy relative to model(s). This could be operationally demonstrated as a stepwise progression of myrmecomorphic traits within a clade. This however presents a conundrum as to why there is variation in mimicry accuracy in a monophyletic group or persistence of poor or imperfect mimics (McLean et al. 2019), as reported in spiders (Cushing 1997, 2012) and hover flies (Edmunds 2000).
Declaration of funding
The taxonomic component of this study was supported by a Bush Blitz Tactical Taxonomy grant DNP-BCK-2021-007 from the Australian Biological Resources Study (ABRS) to Arlee McMah. The Australian Research Council (DP170101617 grant) funding to Gerry Cassis supported the myrmecomorphic trait component of this work.
Acknowledgements
We thank the Bush Blitz program for support in field surveys and in enabling the collection of specimens for this study. Hannah Mathews is thanked for her work on the genitalic dissections and illustrations of species. Curators of the museums involved in this work are thanked for their support. Plant identifications were provided by botanists at the Western Australian and New South Wales herbaria.
References
Carvalho JCM (1952) On the major classification of the Miridae (Hemiptera). (With keys to subfamilies and tribes and a catalogue of the world genera). Anais da Academia Brasileira de Ciências 24, 31-110.
| Google Scholar |
Carvalho JC, Ferreira P (1973) Neotropical Miridae, CLXXVIII: studies on the tribe Herdoniini Distant XVI: key to the world genera (Hemiptera). Revista Brasiliera de Biologia 33, 197-200.
| Google Scholar |
Cassis G (2008) The Lattinova complex of Austromirine plant bugs (Hemiptera: Heteroptera: Miridae: Orthotylinae). Proceedings of the Entomological Society of Washington 110(4), 845-939.
| Crossref | Google Scholar |
Cassis G, Moulds T (2002) A systematic revision of the plantbug genus Kirkaldyella Poppius (Heteroptera: Miridae: Orthotylinae: Austromirini). Insect Systematics & Evolution 33(1), 53-90.
| Google Scholar |
Cassis G, Schuh RT (2012) Systematics, biodiversity, biogeography, and host associations of the Miridae (Insecta: Hemiptera: Heteroptera: Cimicomorpha). Annual Review of Entomology 57, 377-404.
| Crossref | Google Scholar | PubMed |
Cassis G, Symonds C (2012) Systematic revision and phylogeny of the Australian myrmecomorphic seed bug genus Daerlac Signoret (Insecta: Heteroptera: Rhyparochromidae: Udeocorini). Invertebrate Systematics 26(1), 41-66.
| Crossref | Google Scholar |
Cassis G, Symonds C (2014) Systematics and host plant associations of a new genus of Acacia-inhabiting plant bugs from arid Australia (Insecta: Hemiptera: Heteroptera: Miridae: Orthotylinae). Invertebrate Systematics 28(5), 522-554.
| Crossref | Google Scholar |
Cassis G, Symonds C (2016) Plant bugs, plant interactions and the radiation of a species rich clade in south-western Australia: Naranjakotta, gen. nov. and eighteen new species (Insecta: Heteroptera: Miridae: Orthotylinae). Invertebrate Systematics 30(2), 95-186.
| Crossref | Google Scholar |
Cassis G, Wall MA (2010) Systematics and phylogeny of the hatchet head plant bug genus Myrmecoroides Gross (Insecta: Heteroptera: Miridae: Orthotylinae). Entomologica Americana 116(3), 29-49.
| Crossref | Google Scholar |
Cheng M, Cassis G (2019) Combined molecular and morphological phylogeny of Myrtlemiris, evolution of endosomal spicules, description of two new species and Neomyrtlemiris, gen. nov. (Insecta: Heteroptera: Miridae: Orthotylinae). Invertebrate Systematics 33(5), 719-756.
| Crossref | Google Scholar |
China WE, Carvalho JC (1951) XXII.—A new ant-like Mirid from Western Australia (Hemiptera, Miridae). Annals and Magazine of Natural History 4(39), 221-225.
| Crossref | Google Scholar |
Cushing PE (1997) Myrmecomorphy and myrmecophily in spiders: a review. Florida Entomologist 80, 165-193.
| Crossref | Google Scholar |
Cushing PE (2012) Spider-ant associations: an updated review of myrmecomorphy, myrmecophily, and myrmecophagy in spiders. Psyche: A Journal of Entomology 2012, 1-23.
| Crossref | Google Scholar |
Davis NT (1955) Morphology of the female organs of reproduction in the Miridae (Hemiptera). Annals of the Entomological Society of America 48(3), 132-150.
| Crossref | Google Scholar |
Ebach MC, Gonzalez-Orozco CE, Miller JT, Murphy DJ (2015) A revised area taxonomy of phytogeographical regions within the Australian Bioregionalisation Atlas. Phytotaxa 208(4), 261-277.
| Google Scholar |
Edmunds M (2000) Why are there good and poor mimics? Biological Journal of the Linnean Society of London 70, 459-466.
| Crossref | Google Scholar |
Goloboff PA, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24, 774-786.
| Crossref | Google Scholar |
González-Orozco CE, Ebach MC, Laffan S, Thornhill AH, Knerr NJ, Schmidt-Lebuhn AN, Cargill CC, Clements M, Nagalingum NS, Mishler BD, Miller JT (2014) Quantifying phytogeographical regions of Australia using geospatial turnover in species composition. PLoS ONE 9(3), e92558.
| Crossref | Google Scholar | PubMed |
Gross GF (1964) A new ant-mimicking mirid bug (Hemiptera: Heteroptera) from Victoria. Memoirs of the National Museum of Victoria 26, 7-9.
| Crossref | Google Scholar |
Henry TJ (2015) Revision of the ceratocapsine Renodaeus group: Marinonicoris, Pilophoropsis, Renodaeus, and Zanchisme, with descriptions of four new genera (Heteroptera, Miridae, Orthotylinae). ZooKeys 490, 1-156.
| Crossref | Google Scholar | PubMed |
Kim J, Cassis G, Jung S (2023) Phylogenetic analysis of the predatory plant bug subfamily Deraeocorinae (Hemiptera: Heteroptera: Miridae) based on molecular and morphological data. Zoological Journal of the Linnean Society 197(1), 246-266.
| Crossref | Google Scholar |
McIver J (1987) On the myrmecomorph Coquillettia insignis Uhler (Hemiptera: Miridae): arthropod predators as operators in an ant-mimetic system. Zoological Journal of the Linnean Society 90, 133-144.
| Crossref | Google Scholar |
McIver J, Stonedahl G (1993) Myrmecomorphy: morphological and behavioural mimicry of ants. Annual Review of Entomology 38, 351-377.
| Google Scholar |
McLean DJ, Cassis G, Kikuchi DW, Giribet G, Herberstein ME (2019) Insincere flattery? Understanding the evolution of imperfect deceptive mimicry. The Quarterly Review of Biology 94(4), 395-415.
| Crossref | Google Scholar |
Major RE, Christie FJ, Gowing G, Cassis G, Reid CA (2003) The effect of habitat configuration on arboreal insects in fragmented woodlands of south-eastern Australia. Biological Conservation 113(1), 35-48.
| Google Scholar |
Menard KL, Schuh RT (2011) Revision of Leucophoropterini: diagnoses, key to genera, redescription of the Australian fauna, and descriptions of new Indo-Pacific genera and species (Insecta: Hemiptera: Miridae). Bulletin of the American Museum of Natural History 2011(361), 1-159.
| Crossref | Google Scholar |
Menard KL, Schuh RT, Woolley JB (2014) Total‐evidence phylogenetic analysis and reclassification of the Phylinae (Insecta: Heteroptera: Miridae), with the recognition of new tribes and subtribes and a redefinition of Phylini. Cladistics. 30(4), 391-427.
| Crossref | Google Scholar | PubMed |
Nelson XJ (2011) A predator’s perspective of the accuracy of ant mimicry in spiders. Psyche: A Journal of Entomology 2012, 1-5.
| Crossref | Google Scholar |
Nelson XJ, Jackson RR, Li D, Barrion AT, Edwards GB (2006) Innate aversion to ants (Hymenoptera: Formicidae) and ant mimics: experimental findings from mantises (Mantodea). Biological Journal of the Linnean Society 88, 23-32.
| Crossref | Google Scholar |
Poppius B, Bergroth E (1921) Beiträge zur Kenntnis der myrmecoiden Heteropteren. Annales Historico-Naturales Musei Nationalis Hungarici (Zoologica) 18, 31-88 [In German].
| Google Scholar |
Preece M, Harding J, West JG (2015) Bush Blitz: journeys of discovery in the Australian outback. Australian Systematic Botany 27(6), 325-332.
| Crossref | Google Scholar |
Schuh RT (1974) The Orthotylinae and Phylinae (Hemiptera: Miridae) of South Africa with a phylogenetic analysis of the ant-mimetic tribes of the two subfamilies for the world. Entomologica Americana 47, 1-332.
| Google Scholar |
Schuh RT (1984) Revision of the Phylinae (Hemiptera: Miridae) of the Indo-Pacific. Bulletin of the American Museum of Natural History 177, 1-476.
| Google Scholar |
Schuh RT (1991) Phylogenetic, host and biogeographic analysis of the Pilophorini (Heteroptera: Miridae: Phylinae). Cladistics 7, 157-189.
| Crossref | Google Scholar | PubMed |
Schuh RT (2013). On-line systematic catalog of plant bugs (Insecta: Heteroptera: Miridae). In ‘Plant Bug: Planetary Biodiveristy Inventory’. (The American Museum of Natural History) Available at http://research.amnh.org/pbi/catalog/ [Verified 12 March 2023]
Schuh RT, Menard KL (2013) A revised classification of the Phylinae (Insecta: Heteroptera: Miridae): Arguments for the placement of genera. American Museum Novitates 2013(3785), 1-72.
| Crossref | Google Scholar |
Schwartz MD (2008) Revision of the Stenodemini with a review of the included genera (Hemiptera: Heteroptera: Miridae: Mirinae). Proceedings of the Entomological Society of Washington 110(4), 1111-1201.
| Crossref | Google Scholar |
Schwartz MD (2011) Revision and phylogenetic analysis of the North American genus Slaterocoris Wagner with new synonymy, the description of five new species and a new genus from Mexico, and a review of the genus Scalponotatus Kelton (Heteroptera: Miridae: Orthotylinae). Bulletin of the American Museum of Natural History 354, 1-290.
| Crossref | Google Scholar |
Stevens PF (2012) Angiosperm Phylogeny Website. Version 12, July 2012. Available at http://www.mobot.org/MOBOT/research/APweb/ [Verified 23 March 2015]
Symonds CL, Cassis G (2013) New species of the lace bug genus Lasiacantha Stål (Insecta: Hemiptera: Heteroptera: Tingidae) from Western Australia. Australian Journal of Entomology 52(1), 53-66.
| Crossref | Google Scholar |
Symonds CL, Cassis G (2018) Systematics and analysis of the radiation of Orthotylini plant bugs associated with callitroid conifers in Australia: description of five new genera and 32 new species (Heteroptera: Miridae: Orthotylinae). Bulletin of the American Museum of Natural History 2018(422), 1-226.
| Crossref | Google Scholar |
Tatarnic N, Cassis G (2012) The Halticini of the world (Insecta: Heteroptera: Miridae: Orthotylinae): generic reclassification, phylogeny, and host plant associations. Zoological Journal of the Linnean Society 164, 558-658.
| Crossref | Google Scholar |
Weirauch C, Schuh RT (2011) Southern hemisphere distributional patterns in plant bugs (Hemiptera: Miridae: Phylinae): Xiphoidellus gen. nov. from Australia and Ampimpacoris gen. nov. from Argentina, show transantarctic relationships. Invertebrate Systematics 24(5), 473-508.
| Crossref | Google Scholar |
Wyniger D (2010) Resurrection of the Pronotocrepini, with revisions of the Nearctic genera Orectoderus Uhler, Pronotocrepis Knight, and Teleorhinus Uhler, and comments on the Palearctic Ethelastia Reuter (Heteroptera: Miridae: Phylinae). American Museum of Natural History 3703, 1-67.
| Crossref | Google Scholar |
Wyniger D, Schuh RT, Henry TJ (2023) Revision of the North American Hallodapini (Insecta: Hemiptera: Heteroptera: Miridae: Phylinae). American Museum Novitates 2023(3994), 1-48.
| Crossref | Google Scholar |