Integrative taxonomy of Acrapex stem borers (Lepidoptera : Noctuidae : Apameini): combining morphology and Poisson Tree Process analyses
Bruno P. Le Ru A B N , Claire Capdevielle-Dulac B , Emmanuel F. A. Toussaint C , Desmond Conlong D E , Johnnie Van den Berg F , Beatrice Pallangyo G , George Ong’amo H , Gilson Chipabika I , Richard Molo J , William A. Overholt K , James P. Cuda L and Gael J. Kergoat MA Unité de Recherche IRD 072, icipe-African Insect Science for Food and Health, PO Box 30772, Nairobi, Kenya.
B Unité de Recherche IRD 072, Laboratoire Evolution, Génomes et Spéciation, UPR 9034, 22 CNRS, 91198 – Gif/Yvette, France and Université Paris-Sud 11, 91405 – Orsay, France.
C Zoological State Collection, Münchhausenstraße 21, 81247 – Munich, Germany.
D South African Sugarcane Research Institute, Private Bag X02, Mount Edgecombe, 4300, South Africa.
E School of Biological and Conservation Sciences, University of Kwazulu–Natal, Private Bag X01, Scottsville, Pietermaritzburg, South Africa.
F School of Environmental Sciences and Development, North West University (Potchefstroom Campus), Private Bag X6001, Potchefstroom, 2520, South Africa.
G Biocontrol Programme, PO Box 30031, Kibaha, Tanzania.
H School of Biological Science, College of Physical and Biological Sciences (Chiromo Campus), University of Nairobi, P. O. Box 30197, Nairobi, Kenya.
I Zambia Agriculture Research Institute, Mount Maluku Central Research Station, PO Box 8, Chilanga, Zambia.
J Namulonge Agricultural and Animal Production Research Institute (NAARI), PO Box 7084, Kampala, Uganda.
K Indian River Research and Education Center, University of Florida, Fort Pierce, FL 34945, USA.
L Department of Entomology and Nematology, University of Florida, Gainesville, FL 32611, USA.
M INRA, UMR 1062 CBGP (INRA, IRD, CIRAD, Montpellier SupAgro, Campus de Baillarguet, 34988 Montferrier/Lez, France.
N Corresponding author. Email: bleru@icipe.org
Invertebrate Systematics 28(5) 451-475 https://doi.org/10.1071/IS13062
Submitted: 17 December 2013 Accepted: 16 May 2014 Published: 13 November 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Ten morphologically similar species of Acrapex from eastern and south-eastern Africa belonging to the A. stygiata and A. albivena groups are reviewed. Six species are described as new: A. brunneella, A. mitiwa, A. mpika, A. salmona, A. sporobola and A. yakoba. The Poaceae host plants of eight species are recorded; four species, A. mitiwa. A. subalbissima, A. syscia and A. yakoba, were found developing exclusively on Imperata cylindrica (L.) Beauv., (Andropogoneae); two species, A. sporobola and A. salmona, on I. cylindrica and Sporobolus macranthelus Chiov. (Zoysieae); and A. albivena on I. cylindrica, Miscanthus capensis (Nees) Andersson (Andropogoneae) and Cymbopogon sp. (Andropogoneae). Acrapex stygiata larvae developed on M. capensis and Cymbopogon sp. The host plants of A. brunneella and A. mpika remain unknown. We also conducted molecular phylogenetics and molecular species delimitation analyses on a comprehensive sample of 49 specimens belonging to nine of the studied species. Molecular phylogenetics and molecular species delimitation analyses provided additional evidence of the validity of the six newly described species but also suggested a level of hidden biodiversity for one of them.
Additional keywords: Acrapex, Imperata cylindrica, molecular phylogenetics, molecular species delimitation, Noctuidae, phylogenetics, Poaceae.
Introduction
Acrapex Hampson, 1894 is a tropical genus of stem borers (Lepidoptera: Noctuidae, Apameini, Sesamiina) that consists of at least 83 species (Poole 1989), of which the great majority (~70 species) is distributed in the Afrotropical region (Moyal 2006). However, five Acrapex species described by Laporte (1975, 1984) were recently displaced to Sciomesa and Feraxinia genera (Moyal et al. 2010), thus reducing the total number of Afrotropical Acrapex species to 65. Until 2000, little was known about Acrapex host preferences as specimens had been obtained only from light trap collections. Starting in 2004, extensive surveys were conducted in several sub-Saharan countries, targeting wild habitats rich in Poaceae including open grasslands, forests, banks of streams or rivers and swamps. Poaceae were examined for stem borer infestation and recovered larvae were reared until adult emergence. In addition, light traps were operated in some localities whenever possible. Combining the two collection techniques, we obtained thousands of noctuid stem borer adults and among them several hundred Acrapex specimens. Because the genus Acrapex is speciose, it is beyond the scope of this paper to review the entire genus. Instead, we focus on a small group of morphologically related species, some of which (A. albivena, A. subalbissima, A. syscia, A. mitawa, sp. nov., A. salmona, sp. nov., A. sporobola, sp. nov., A. yakoba, sp. nov.) feed on cogongrass, Imperata cylindrica (L.) Beauv., a rhizomatous perennial grass (Jose et al. 2002; Holzmueller and Jose 2011), which is widely distributed in tropical and subtropical areas of the world and considered to be one of the world’s worst weeds (Holm et al. 1977). The specimens we study here belong to a small subset of two (groups B and C) of the four morphological groups that have been defined by Berio (1973) based on male genitalia. Group B (also referred to at the A. stygiata group) includes A. stygiata, A. subalbissima, A. bruneella, sp. nov. and A. mpika, sp. nov. (the last two species collected only in light traps) and is characterised by the following combination of characters: (i) valve very broad at basal half, rounded along ventral margin, suddenly constricted at middle, terminal half becoming spatulate; (ii) base of costal area heavily sclerotised and produced into a narrow long lobe; and (iii) aedeagus straight, of almost even width, terminating in a tubular vesica with a short, stout cornutus. Group C (also referred to at the A. albivena group) includes A. albivena, A. syscia, A. mitawa, sp. nov., A. salmona, sp. nov., A. sporobola, sp. nov., and A. yakoba, sp. nov. and is characterised by: a flat valve, laminar throughout the extension; valve rather weakly sclerotised, except costal area at basal third, broadly rounded along ventral margin at base, roundly constricted at middle, broadly rounded at apex; and aedeagus simple and crown.
We include, together with the description of the six new species (which have been cross-checked against all Acrapex types preserved in the museum to avoid coinage of synonymies), a supplemental description of the four previously described species with female genitalia presented for the first time.
In a complementary way, we also rely on a molecular dataset to explore the species boundaries of the specimens of interest. Such use of multiple sources of information is now commonly referred to as integrative taxonomy (for more information, see Dayrat 2005; Will et al. 2005; Padial et al. 2010; Schlick-Steiner et al. 2010). To do so, we provide a phylogenetic framework for nine of the studied species and four other species representing four other genera (Busseola Thurau, 1904, Pirateolea Moyal, 2010, Sciomesa Tams & Bowden, 1954 and Sesamia Guenée, 1852) of the subtribe Sesamiina, through the molecular phylogenetic analysis of a six gene molecular dataset. This allows us to implement a recently developed method of molecular species delimitation which is used to assess the congruence of molecular species clusters with the newly described species.
Materials and methods
Insect samples
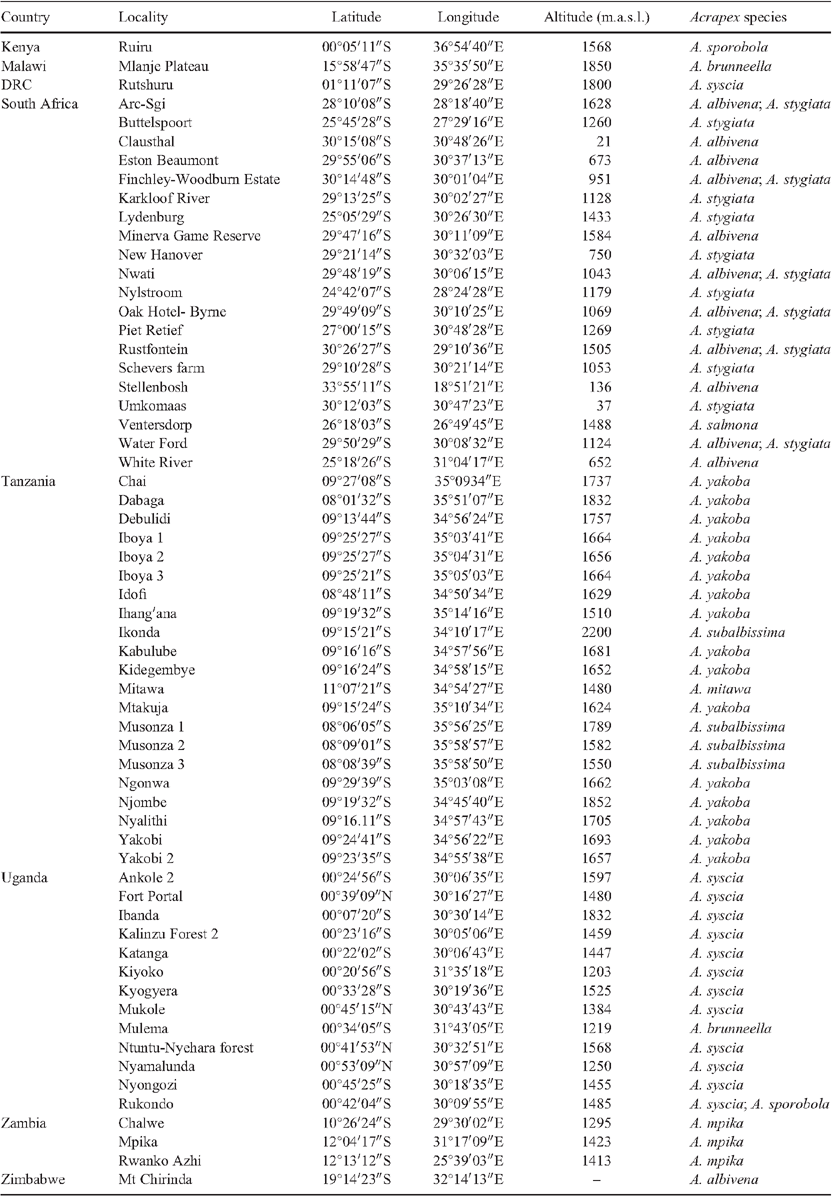
Sampling of visually damaged grasses (Poaceae) in eastern and south-eastern Africa was conducted over eight years (2006–2013) to collect the larval stages of noctuid stem borers within their wild host plants (Le Ru et al. 2006a, 2006b). Larvae were reared on artificial diet (Onyango and Ochieng’Odero 1994) until pupation and emergence of adults (Le Ru et al. 2006a, 2006b). A few adult specimens were also collected in light traps. See Table 1 for collection locations.
DNA extraction and sequencing
For this study, a total of 49 specimens belonging to the group of interest was selected for the molecular analyses. In addition, we included representatives of four other genera in the subtribe Sesamiina as outgroups based on the results of a recent molecular study (Toussaint et al. 2012). DNA was extracted from hind legs using Qiagen DNAeasy tissue kits (Qiagen, Hilden, Germany). PCR amplifications were conducted for four mitochondrial gene fragments, a 658 bp region of the cytochrome oxidase I (COI), 977 bp of the cytochrome b (Cytb), 352 bp of the ribosomal 12S RNA (12S), and 421 bp of the ribosomal 16S RNA (16S). Two nuclear gene regions were also sequenced, 835 bp of the 28S rDNA (28S), and 1240 bp of the elongation factor-1a (EF1a). For both genes, we used the primers and settings detailed in Kergoat et al. (2012). Resulting PCR products were processed by the French sequencing centre Genoscope using a BigDye v3.1 sequencing kit and Applied 3730xl sequencers. Both strands were sequenced for all specimens to minimise PCR artefacts and ambiguities. Sequences of complementary strands were edited and reconciled using Geneious v5.1 software (available at: www.geneious.com/). All the sequences generated in this study were deposited in GenBank (see Table S1, available as Supplementary material, for the accession numbers). Unlike the sequences of coding genes (COI, Cytb, and EF1a), the sequences of ribosomal genes (12S, 16S and 28S) were variable in length. Their alignment was accomplished using MUSCLE (Edgar 2004) with default option settings. For all protein-coding genes, we used Mesquite 2.75 (available at: www.mesquiteproject.org) to check the coding frame for possible errors or stop codons. The combination of the six gene fragments resulted in a combined matrix of 53 specimens and 4683 aligned characters.
Phylogenetic analyses
Phylogenetic analyses were conducted using maximum likelihood (ML) and Bayesian inference (BI). For ML and BI, we carried out partitioned analyses (Nylander et al. 2004) using one partition per gene fragment. For each partition, the best-fit substitution model was selected with jModelTest (Posada 2008) using the corrected Akaike information criterion (AICc; Posada and Buckley 2004).
Maximum likelihood analyses were performed with RAxML ver. 7.0.8 (Stamatakis 2006). The best tree was obtained using a heuristic search implementing 100 random-addition replicates. Clade support was then assessed using non-parametric bootstrap values (BV) (1000 replicates were used). Nodes supported by BV ≥ 70% were considered as strongly supported following Hillis and Bull (1993). Bayesian inference analyses were carried out using BEAST ver. 1.7.5 (Drummond et al. 2012). Two distinct runs were carried out with 50 million generations and trees sampled every 5000 generations. In a conservative way, we used a burn-in-period of 12.5 million generations per run. Convergence of runs was assessed by examining the effective sample size (ESS) of parameters with Tracer ver. 1.5 (available from http://beast.bio.ed.ac.uk/Tracer). Clade support was directly provided by the posterior probabilities (PP) estimates, with nodes supported by PP ≥ 0.95 considered as strongly supported (Erixon et al. 2003).
To determine putative molecular species clusters in our dataset, we used the recently developed Poisson tree processes (PTP) method (Zhang et al. 2013). This method does not require an ultrametric tree as input; instead the PTP model uses branch lengths to estimate the mean expected number of substitutions per site between two branching events. The model assumes that each substitution has a small probability of generating a speciation event; hence the number of substitutions between species is expected to be significantly higher than those within species (Zhang et al. 2013). The model then implements two independent classes of Poisson processes (one describing speciation and the other describing within species branching events) and searches for transition points between inter- and the intra-species branching patterns. The latter allows determination of molecular species clusters, which may be used as a potential line of evidence in an integrative taxonomy framework (Dayrat 2005; Padial et al. 2010; Schlick-Steiner et al. 2010; Riedel et al. 2013). That said, it is important to point out that these species clusters are putative; furthermore, similarly to that of other molecular species delimitation methods, the PTP procedure is not error-free and is to be used with caution with unbalanced or single-gene datasets (Zhang et al. 2013). The corresponding analysis was conducted on the web server for PTP (available at http://species.h-its.org/ptp/) using the best ML tree resulting from the RAxML analysis (Zhang et al. 2013).
Morphological study
Genitalia were dissected after immersion of the end of the abdomen in a boiling 10% potash bath for a few minutes, then cleaned, immersed in absolute alcohol for a few minutes and mounted on slides in Euparal (after separating the aedeagus from the rest of the genitalia in the male). Specimens were identified by comparison with types housed in several museums, the Natural History Museum, London (BMNH), the Ditsong National Museum of Natural History (DNMH), South Africa and the Museo Civico di Storia Naturale (MCSN), Milan. Types of new species were deposited in the Museum National d’Histoire Naturelle (MNHN) in Paris, France, except Acrapex brunneella, sp. nov., which was already deposited in the Natural History Museum (BMNH) in London. When possible, paratypes were deposited in the Ditsong National Museum of Natural History (DNMH), South Africa, and in the National Museum of Kenya (NMK) in Nairobi, Kenya.
The species we studied belonged to two very different morphological groups based on male genitalia as defined by Berio (1973). We decided to treat the two morphological groups separately for morphological comparison purposes; the first one named group stygiata considering A. stygiata was the first species of the group described by Hampson in 1910 and the second one named group albivena considering A. albivena was the first species of the group described by Hampson in 1910.
Results
Phylogenetic and molecular species delimitation analyses
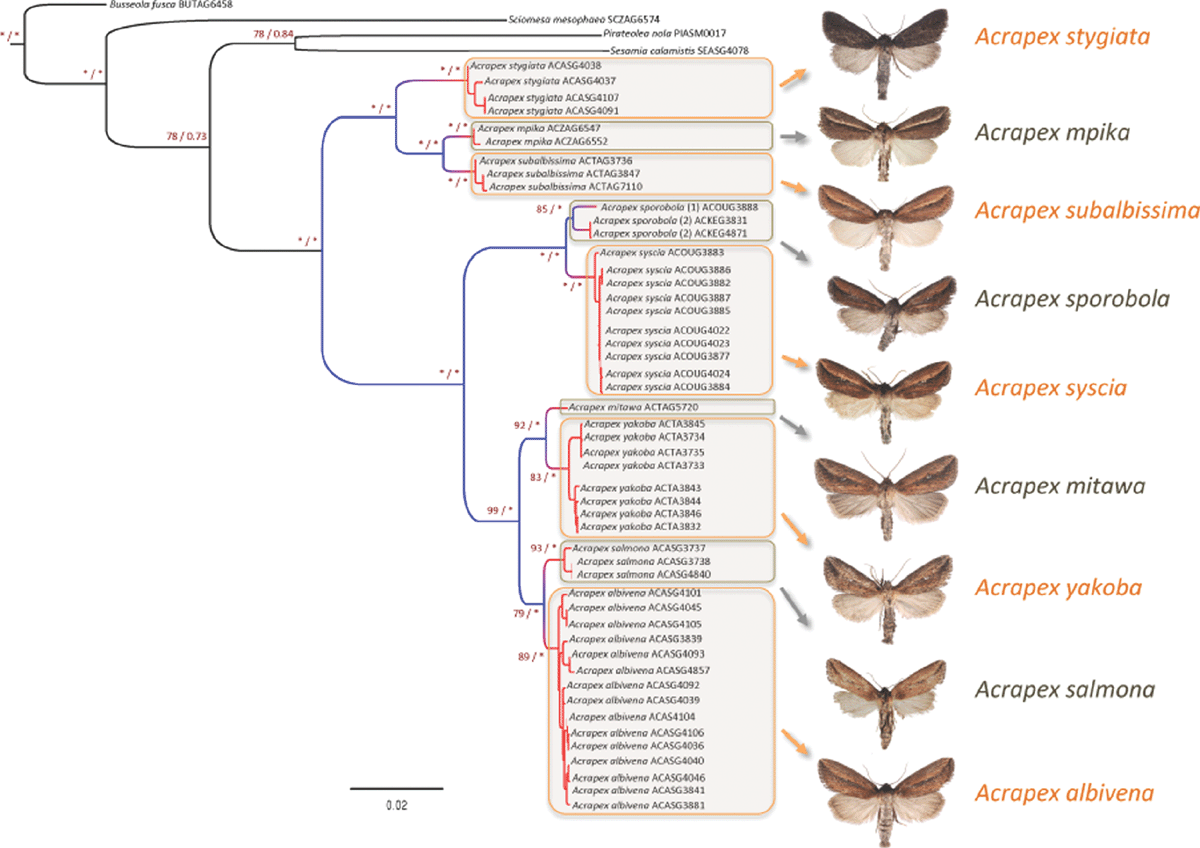
Substitution models belonging to the general time reversible family (assuming gamma rate heterogeneity) were selected for each gene by the AICc. Both ML and BI partitioned analyses generated a similar topology (see Fig. 1). Overall, the corresponding tree is well supported as most interspecific nodes are supported by BV ≥ 70% and PP ≥ 0.95. All the species for which more than one specimen was sampled are recovered monophyletic with a high support. Two major species groups can be distinguished. The first one includes A. stygiata, A. subalbissima and individuals of the newly described A. mpika. The second group includes a clade of two species (A. syscia and the newly described A. sporobola), which clusters together with a larger clade that encompasses A. albivena and the specimens from three newly described Acrapex species (A. mitawa, A. salmona and A. yakoba). Regarding molecular species delimitation, the PTP analysis revealed ten putative species clusters (see Fig. 1). For eight species (A. albivena, A. mitawa, A. mpika, A. salmona, A. stygiata, A. subalbissima, A. syscia and A. yakoba), the molecular species delimitation approach was congruent with the morphological assignation with the exception of A. sporobola, for which the PTP analysis indicated two putative species clusters for the three sampled individuals.
Discussion
Systematics
Of the 10 species treated here, it is clear from both morphological and phylogenetic results that four species (A. brunneella, A. mpika, A. stygiata and A. subalbissima) belong to the Acrapex stygiata group (i.e. group B, Berio 1976) and six species (A. albivena, A. syscia, A. salmona, A. mitawa, A. yakoba and A. sporobola) belong to the Acrapex albivena group (i.e. group C, Berio 1976). Six new species were recognised, two belonging to the A. stygiata group (A. brunneella and A. mpika) and four belonging to the A. albivena group (A. mitawa, A. salmona, A. sporobola and A. yakoba). Our phylogenetic analysis also confirmed the relationships advanced by Berio (1976) based on the morphology of male genitalia. The use of a combined approach based on morphology and molecular species delimitation analyses allowed us to assess with confidence the boundaries of new species in the genus Acrapex. In addition, the molecular species delimitation analyses suggest some level of cryptic genetic diversity in the newly described A. sporobola, which could potentially represent two distinct species. That said, it is worth highlighting that we only managed to sequence three specimens for this species. Therefore we cannot exclude the hypothesis that the additional species cluster of A. sporobola results from an undersampling artefact. Because undersampling can potentially biases the results of species delimitation analyses (Papadopoulou et al. 2008; Lohse 2009; Lim et al. 2012), we think that the decision to describe only one species (i.e. A. sporobola) is a conservative choice.
Among the A. stygiata group, we found two male forewing colours, the dark forewing colour (A. mpika and A. stygiata) and the buff forewing colour (A. brunneella and A. subalbissima); however, species identification is much easier based on male genitalia, in particular the basal half of the valve (which is more-or-less elongated or rounded depending on the species), and the juxta (for which morphology is very characteristic for each of the four species treated in this paper). In spite of its taxonomic value, juxta morphology was not considered by Berio (1976).
Among the A. albivena group, two sub-groups can be distinguished: the sub-group albivena with the veins of the costal area of the forewings adorned with white scales (albivena, salmona, mitawa and yakoba); and the sub-group syscia with the veins without any irroration (syscia and sporobola). Species identification is straightforward within these groups by comparing the shape of the ventral margin at the base of the valves, the ratio between length and width of the valves, and the penis and juxta morphology.
Until now, females were described only for A. subalbissima (Berio 1976) without reference to the genitalia. However, as realised by Pierce (1942), the female genitalia provide good characters for species identification even if sclerotised structures are less developed than in males. Of the10 species treated here, females of eight species are described, including their genitalia. For all species treated here, females are very similar in appearance to that of males and are generally the same size as the males, but with forewings paler and more elongated at the apex. Within the present species, female genitalia allow a clear separation of the A. stygiata group and the A. albivena group: the former is characterised by a globular corpus bursae (subalbissima and mpika) adorned with two elongated signa and by an antrum strongly sclerotised and divided into two bean-shaped lateral plates; the latter by a corpus bursae without signa and by a narrow, band-like post-vaginal plate.
The eight Acrapex species collected in the field as larvae from host-plants were morphologically very similar; ground colour pinkish buff without any markings, with head red-brown, thoracic shield more or less concolorous with head, caudal plate brown and pinacula paler than caudal plate. The A. examinis larva described by Swezey (1927) is slightly different from the larvae described here, but clearly all recorded Acrapex larvae belong to the Sesamia-like species as defined by Le Ru et al. (2006b).
The results presented here highlight the complexity of Acrapex systematics, notably the limitations of morphological taxonomy based upon only one life stage (such as adult moths), in particular when only one sex is available. From the time of Tams and Bowden (1953), male genitalia have been considered as the main criterion for grouping species in Sesamiina genera.
The male genitalia of the ten Acrapex species treated here clearly segregate between two distinct groups as pointed out by Berio (1973). This is further illustrated in the case of A. brunneella, which was first described by Strand (1916) as an aberration of A. brunnea based on wing pattern, then as a paler form of A. stygiata based on both wing pattern and male genitalia (Fletcher 1961). The current study demonstrates the importance of the integration of morphological studies, ecological information and molecular data. Furthermore, it also shows that female genitalia must be taken into account for a complete resolution of the taxonomy of Acrapex and more generally of the subtribe Sesamiina. We recently showed that among Busseola species, female genitalia are more informative than male genitalia for the separation of B. nairobica, B. phaia and B. segeta (Felix et al. 2013).
Host-plant associations
Prior to this study, very little was known about the host-plant associations of Acrapex spp., as there were only two records in the literature: Acrapex examinis (Meyrick) from Panicum torridum Gaudich. (Paniceae) in Hawaii (Swezey 1927), and a misidentified A. syscia from S. macranthelus (Zoysieae) in Kenya (Le Ru et al. 2006b). In addition, Acrapex azumai Sugi was very recently recorded from cogongrass (Andropogoneae) in Japan (Takasu et al. 2014). Our results are consistent with previous host-plant records of Acrapex, which are all from Poaceae. Moreover, our results suggest a high diversity of Acrapex associated with grasses in the tribe Andropogoneae, as the eight species in our study with host records were all found in grasses in this tribe (Imperata cylindrica, Cymbopogon sp. and Miscanthus capensis), although two of the eight species were also found in a grass in the tribe Zoysieae (Sporobolus macranthelus). The feeding habit of Acrapex larvae has been recorded for A. examinis (Swezey 1927) and more recently for A. azumai (Takasu et al. 2014). The feeding habits of the eight Acrapex species we collected as larvae are very similar to that of the ones reported for A. examinis and A azumai, with the typical symptom of plant attack as death of the central tiller, often referred to as ‘dead heart’. In addition, we speculate that Acrapex larvae typically fed on more than one stem before completing their development, as previously suggested by Swezey (1927: p. 180): ‘larvae apparently migrate from one stem to another, for in many bored stems with ‘dead hearts’ not enough eating had been done to suffice for the growth of the larva’. Finally, all the taxa we cover, with the exception of A. brunneella for which there is no information on habitat associations, are markedly hygrophilous species found along banks of streams, rivers and marshes. Until recently, nearly all Acrapex species recorded in literature were collected from light traps and were known from very few localities. Most of species was reported from only one sub-region in Africa (East, Austral, Central or West), suggesting a high rate of endemic species in each sub-region. The restricted distributions and host-plant associations of the ten Acrapex species considered in our study confirm the high rate of endemism of Acrapex in Africa. During the last ten years, we have collected almost 60 000 stem borer larvae from nearly 180 different host plants in 1200 localities belonging to 16 sub-Saharan countries, and therefore the limited geographic ranges and host plant affinities reported in our study cannot be attributed to sampling bias or insufficient sampling. Moreover, our results suggest that most Acrapex species are probably specialist feeders on one or only a few grasses. In general, noctuid stem borers with the most extensive geographic distributions are the most polyphagous (Le Ru et al. 2006b; Moyal et al. 2010), and none of these widely distributed, polyphagous species belongs to the genus Acrapex.
Taxonomy
Group stygiata: A. stygiata
Acrapex brunneella Le Ru, sp. nov.
(Figs 2A, C, 7A, E, 11)
Material examined
Holotype. Male, Uganda: Mulema, 5000 Ft, May 1903, Noctuidae genitalia slide N° 2459, Doggett, W.L. coll., 1904–23, BMNH, London.
Paratype. MALAWI (Nyasaland): Mlanje Plateau, 6500 Ft, Dec 1913, Neave S.A. coll., 1M, Noctuidae genitalia slide N° 2474.
Description
Male (Fig. 2A, B): antennae ochraceous–buff, filiform and slightly ciliate, flagellum adorned dorsally with fuscous scales, palpus light ochreous–buff; colour of head, thorax and forewing light ochreous–buff; head and prothorax strongly tinged with fuscous; eyes fuscous. Legs light ochreous–buff, buff in inner surface. Forewing: costal area broadly irrorated with fuscous scales, diffusely edged on the lower side and extended beyond the upper median with all the veins of the ground colour; irrorated median area extended on distal side to termen, ending obliquely well before termen; no transverse lines, a narrow fuscous longitudinal area from the base to the termen with veins in that area and below irrorated with fuscous. Fringe ochraceous–buff adorned with a narrow buff line and a narrow fuscous line. Hindwing white, veins irrorated with ochreous–buff scales, costa and apex slightly suffused with fuscous scales; fringe white adorned with a thick white line and a narrow fuscous line. Underside of forewing with white ground-colour suffused with fuscous scales, more densely suffused on the costal area, apex and termen; Underside of hindwing white with costa and apex suffused with fuscous scales and veins irrorated with ochreous–buff scales.
Wingspan: 23–25 mm (2 males)
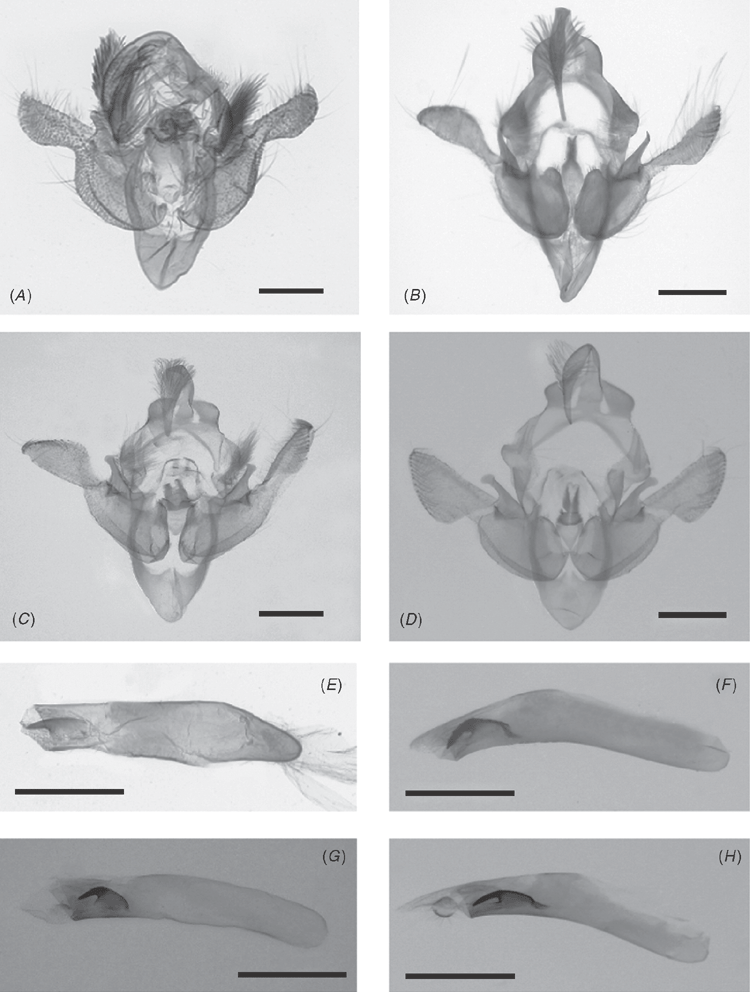
Male genitalia (Fig. 7A, E): uncus long and narrow, tapering to a fine point and tufted with long hair on the upperside; valves very broad at basal half; terminal half spatulate. Terminal half weakly sclerotised and basal half more sclerotised with a broad sacculus; costal area at base heavily clerotized and produced into a narrow long lobe, roundly pointed and curved inwardly; the juxta large, oblong elongated pear-shaped without sclerotisation at the base with a long and wide sclerotised neck very shortly bifid. Aedeagus straight, terminating in a tubular vesica with a stout, broadly based, pointed cornutus.
Bionomics
Biology unknown.
Distribution
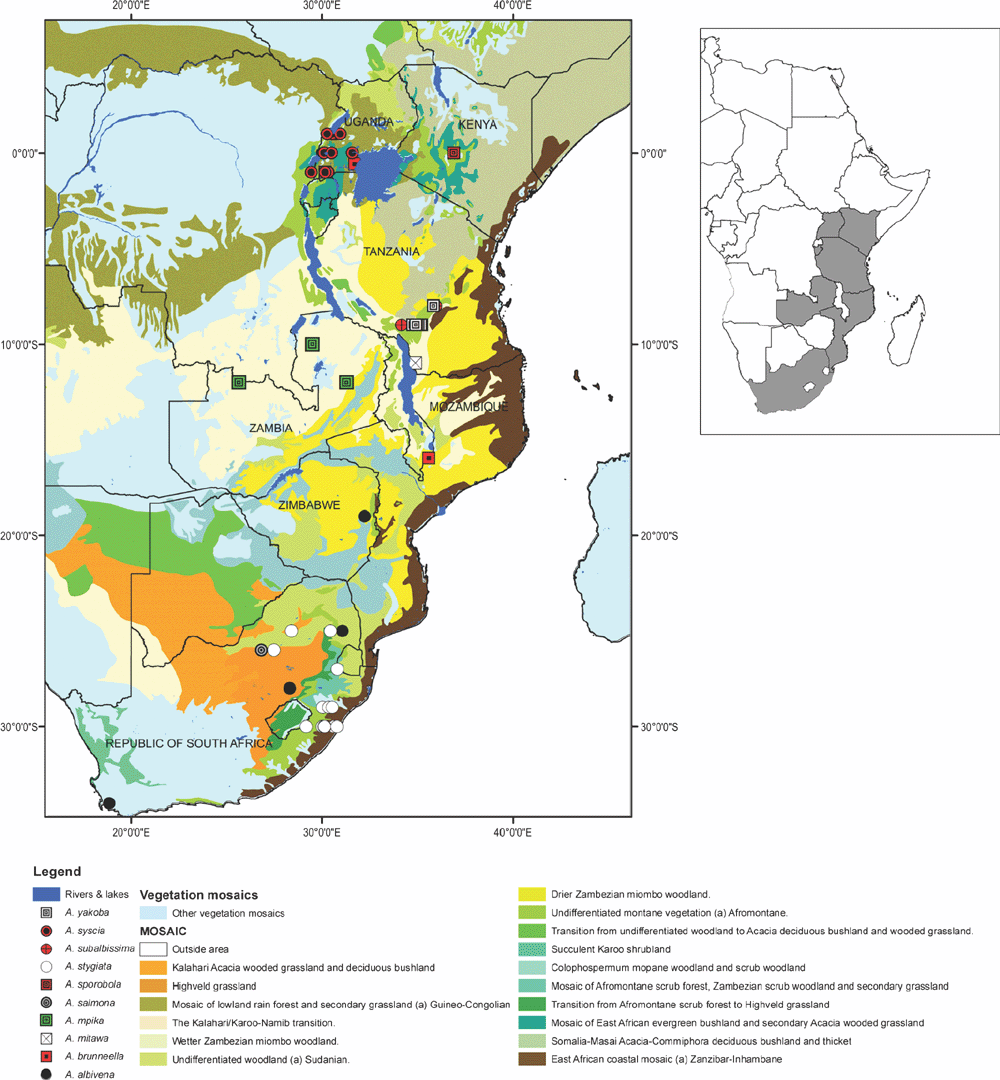
Uganda and Malawi. The two records are from Afromontane (Mosaic no 19) vegetation mosaic (White 1983) (Fig. 11).
Remarks
Easily separated from A. stygiata and A. mpika by the forewing which is ochreous–buff in stygiata and fuscous in mpika; separated from A. subalbissima by having a juxta much larger and wider.
Etymology
Named after the ab. brunnella name given by Strand (1916).
Acrapex mpika Le Ru, sp. nov.
Material examined
Holotype. Male, Zambia: Northern: Mpika, 12°04.288ʹS, 31°17.155ʹE, 1423 m, 15 March 2012, B. Le Ru leg., 1M gen. prep. LERU Bruno/G579, MNHN, Paris.
Paratypes. ZAMBIA: North-Western: Rwanko Azi, 12°13.212ʹS, 25°39.064ʹE, 1413 m, 20 March 2012, ex light trap, B. Le Ru leg., 1F gen. prep. LERU Bruno/G116), 1M gen. prep. LERU Bruno/G124, 4M, MNHN, Paris; Northern: Chalwe, 10°26.395ʹS, 29°30.039ʹE, 1295 m, 23 March 2012, B. Le Ru leg., 1M, MNHN, Paris.
Description (Fig. 2D–F)
Both male and female are similar, the female paler than the male; antennae ochreous–buff, filiform in both sexes and slightly ciliate in males, flagellum adorned dorsally with fuscous scales, palpus fuscous; head and base of thorax fuscous, thorax becoming gradually buff; eyes fuscous. Legs fuscous ringed with white, black fuscous in inner surface; abdomen grey irrorated with fuscous scales. Forewing; ground-colour bright fuscous with costal area irrorated with brown fuscous and white scales, more diffuse on the lower side; irrorated buff median area extended on distal side to termen; no transverse lines. The cell is externally adorned with some black markings, variable in extent and intensity; a longitudinal fuscous fascia from base along lower margin of cell ending to an oblique line of four black triangular markings variable in extent. A curved postmedial row of spots, each spot located on the veins; fringe fuscous adorned with a narrow white line. Hindwing: ground colour white, veins slightly irrorated with fuscous scales, costa and apex slightly suffused with fuscous scales; fringe white adorned with a narrow light fuscous line. Underside of the forewing with ground-colour grey, uniformly suffused with fuscous scales and some white scales on the costal area. Underside of hindwing white suffused with fuscous scales on costa, apex and termen; veins slightly irrorated with fuscous scales.
Wingspan: 23–25 mm (males) (n = 7); 24 mm (females) (n = 1).
Male genitalia (Fig. 7B, F): uncus narrow and long tapering in a very fine and long point, tufted with long hair on upperside; valves with terminal half weakly sclerotised and basal half better sclerotised with a broad sacculus; base of the costal area with a pronounced protuberance and a narrow long duck-billed lobe, slightly curved inwardly; juxta oblong elongated pear-shaped without sclerotisation at the base with a long and very narrow sclerotised neck slightly bifid at the apex. Aedeagus long, strongly curved in the middle, terminating in a tubular vesica with a stout, broadly based, strongly curved and pointed cornutus.
Female genitalia (Fig. 9A): corpus bursae short and globular with two small elongated signa; ductus bursae about the same length as corpus bursae, without sclerotisation near the bursa, globular near the ostium with a strongly sclerotised connection. Antrum sclerotised, with two bean-shaped lateral plates separated in their middle with a deep depression; the posterior lip v-shaped and the anterior u-shaped; anterior apophyses as long as posterior ones; ovipositor lobes relatively short and wide (2.1 times longer than wide) with dorsal surface bearing numerous short and stout setae, the ventral side of each lobe curved and tooth-shaped.
Bionomics
Biology unknown. The moths were caught in a light trap in grasslands near marshes.
Distribution
Zambia, Northern and North-west Province. Moths were found in wet Zambezian miombo woodland (dominated by Brachystegia, Julbernardia and Isoberlinia) (Mosaic no 25) vegetation mosaic (White 1983) (Fig. 11).
Remarks
Forewing fuscous like A. stygiata but the species may easily be separated after the male genitalia; the upper part of the juxta is sclerotised and bifid in A. stygiata whereas there is no sclerotised area in mpika; also basal half of valves much more globular in mpika than in stygiata; corpus bursae with two small elongated signa in A. mpika whereas there are no signa in A. stygiata.
Etymology
Named after the town of Mpika in Zambia.
Acrapex stygiata (Hampson)
(Figs 3A–D, 7C, G, 9B, 10A, 11)
Material examined
Holotype. Male, South Africa: Transvaal: Piet Retief, 15 Sept 1903, R. Crawshay leg. (1903–314), gen. prep. 2275, BMNH, London.
Other material examined. SOUTH AFRICA: Kwazulu–Natal: Karkloof River, 29°13.416ʹS, 30°02.456ʹE, 1128 m asl, 24 Nov 2009, ex larva (in stem of Miscanthus capensis (Nees) Andersson), B. Le Ru leg., 1F gen. prep. LERU Bruno/G344, MNHN, Paris; Eston Beaumont, 29°55.102ʹS, 30°37.222ʹE, 673 m, 25 Nov 2009, ex larva (in stem of Cymbopogon sp), B. Le Ru leg., MNHN, Paris ; 1M, 1M gen. Prep. LERU Bruno/G342; Waterford, 29°50.471ʹS, 39°08.523ʹE, 1124 m, 27 Nov 2009, B. Le Ru leg., 1F, MNHN, Paris; Schevers Farm, 29°10.448ʹS, 30°21.243ʹE, 1053 m, 27 Nov 2009, ex larva (in stem of Miscanthus capensis (Nees) Andersson), B. Le Ru leg., 1M gen. prep. LERU Bruno/G343, MNHN, Paris.
Supplementary description
The male was described in sufficient detail by Hampson (1910) and Janse (1939). The female is described here for the first time; it looks very similar to that of the male, however the general shape of the female’s forewing is more elongated at the apex that that of the male. Additions to two previous descriptions (Fig. 3A–D): antennae fuscous, filiform in both sexes and slightly ciliate in males, flagellum adorned dorsally with fuscous scales, palpus fuscous. Forewing: in some specimens a longitudinal ochreous-brown fascia from apex to before end of cell, sometimes reaching the base, getting narrower to the base; in some specimens the fuscous ground colour is suffused with ochreous-brown scales. Fringe fuscous adorned successively with a narrow white line, a thick fuscous line, another narrow white line and 7 black elongated markings at the base. Hindwing; white to buff, with veins, costa and apex strongly suffused with fuscous scales; fringe white to buff with a narrow basal white or buff line highlighted at the base with a narrow black line. Underside of the forewing uniformly grey; in some specimens suffused with brown scales. Underside of hindwing white uniformly suffused with fuscous scales but more densely on costa and apex.
Wingspan: 26–27 mm (males) (n = 3); 27–29 mm (females) (n = 2).
Male genitalia (Fig. 7C, D): the male genitalia was described by Janse (1939) with sufficient detail; however in addition to the information provided by Janse, the juxta is oblong, elongated, pear-shaped without sclerotisation at the base with a short wide sclerotised neck shortly bifid.
Female genitalia (Fig. 9B): corpus bursae short and globular without signa; ductus bursae without sclerotisation, about the same length as corpus bursae, narrow near the bursa and wide and globular near the ostium, the junction with the ostium bulb-shaped and slightly sclerotised. Antrum strongly sclerotised, the posterior lip straight and slightly leaning on the back, the anterior lip w-shaped; anterior apophyses as long as posterior ones; ovipositor lobes relatively short and wide with dorsal surface bearing numerous short and stout setae, the ventral side of each lobe curved and tooth-shaped.
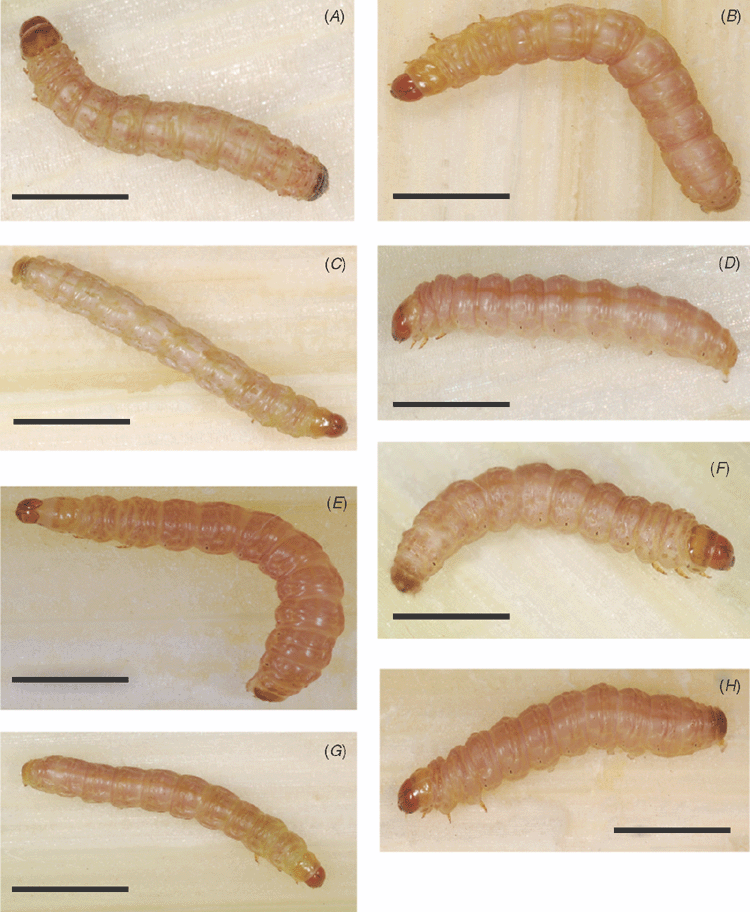
Larvae L5 instar (Fig. 10A): length, 25–30 mm, width, 3.0 mm; head smooth, brown, prothoracic shield pale yellow brown; body with ground colour buff, dorsally suffused with pink, pinacula brown and caudal plate black. Young larvae are very similar in appearance to that of mature ones.
Bionomics
Acrapex stygiata is a markedly hygrophilous species inhabiting grasses along banks of streams, rivers and marshes. Larvae were collected at the bottom of young stems of Miscanthus capensis (Nees) Andersson and Cymbopogon sp. stems, always solitary. Typically, plants exhibiting signs of infestation by A. stygiata larvae have a curled, brown, central leaf. Damaged stems had a small hole (ca. 2 mm diameter) located ~10 cm from ground level. Stems found with damage but no stemborer also had a larger hole near the bottom of the stem just above the junction of the stem and the rhizome. We suspect that the small holes at the top of the stem were borer entry holes while the larger holes at bottom of the stem were exit holes. No pupae were found in stems, and therefore borers probably pupate in the soil near exit holes. Based on the small amount of nutrients available in most stems, we suspect that one borer may feed on multiple stems before pupating.
Distribution
South Africa in Kwazulu–Natal, Mpumalanga, Limpopo and North West regions (Fig. 11). It has been found in Afromontane (Mosaic no 19), transition from Afromontane scrub forest to Highveld grassland (Mosaic no 20), Highveld grassland (Mosaic no 58) and wetter Zambezian miombo woodland (dominated by Brachystegia, Julbernardia and Isoberlinia) (Mosaic no 25) vegetation mosaics (White 1983).
Remarks
Fletcher (1961) recorded A. stygiata from Uganda and Nyasaland and listed Acrapex brunnea Hampson, 1910 (tom. cit. p 318, ab.2.) and Acrapex brunnea ab. brunneella Strand 1916 (Arch. Naturgesh., 82 A2:87) as synonyms. We did not find the male specimen collected by Fletcher in Uganda, Ibanda, 4700 Ft, however careful cross checking of both adults and genitalia slides kept in the BMNH, clearly indicate these two taxa are not synonyms of A. stygiata. The first taxon belongs to A. brunnea as described first by Hampson (1910) and later with description of genitalia by Janse (1939). The second taxon is not related to Acrapex brunnea but belongs to a new species related to A. stygiata but with clear morphological differences in both wing pattern and genitalia.
Acrapex subalbissima Berio
(Figs 3E–H, 7D, H, 9C, 10B, 11)
Material examined
Holotype. Male, Tanzania: Iringa region: Kipengere Mountain, Ikonda, 07 Apr 1971, 1M, gen. prep. Berio N° 5080, MCSN, Milan. Allotype: TANZANIA, Ikonda, 01 Apr 1971, 1F, MCSN, Milan.
Paratypes. TANZANIA: Ikonda: 10 Mar – 04 Apr 1971, 3M, 9F, MCSN, Milan.
Other material examined. TANZANIA: Iringa region, Uzungwa Mountain, Musonza, 08°06.090’S, 35°56.421’E, 1789 m, 24 Mar 2009, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., 1M, 4F, 2F gen. prep. LERU Bruno/G345-G346, 1M gen. prep. LERU Bruno/G347, MNHN, Paris.
Redescription (Fig. 3e–h)
Both male and female are very similar; antennae ochreous–buff, filiform in both sexes and slightly ciliate in males, flagellum adorned dorsally with fuscous scales, palpus fuscous; head and base of thorax fuscous brown heavily suffused with white scales, thorax becoming gradually ochreous–buff; eyes fuscous. Legs fuscous brown ringed with white and suffused with white scales, black fuscous in inner surface; abdomen grey brow. Forewing; ground-colour ochreous–buff with costal area irrorated with fuscous scales, diffusely edged on the lower side and extended beyond the upper median with all the veins of the ground colour; irrorated buff median area extended on distal side to termen; no transverse lines. The cell is externally adorned with some black markings, variable in extent and intensity; a longitudinal fuscous fascia from base along lower margin of cell ending to an oblique line of four black triangular markings variable in extent. The inner margin irrorated with fuscous scales. A curved postmedial row of spots, each spot located on the veins; fringe fuscous adorned with a narrow white line. Hindwing: ground colour white, veins slightly irrorated with ochreous–buff scales, fringe white adorned with a narrow light buff line. Underside of forewing with white ground-colour suffused with fuscous scales, more densely suffused on the costal area, apex and termen; Underside of hindwing white with costa and apex suffused with fuscous scales and veins irrorated with fuscous scales.
Wingspan: 23–25 mm (males) (n = 4); 23–26 mm (females) (n = 7).
Male genitalia (Fig. 7D, H): uncus very similar to that of A. stygiata; valves very broad at basal half but more thickset, almost globular, than in stygiata; terminal half spatulate like in A. stygiata, terminal half weakly sclerotised and basal half more sclerotised with a broad sacculus; costal area at base heavily sclerotised and produced into a narrow long lobe, roundly pointed and curved inwardly very similar to that of A. stygiata; the juxta is similar with that of A. stygiata; oblong, elongated pear-shaped without sclerotisation at the base but shorter than in A. stygiata with a shorter and narrower sclerotised neck than in stygiata and longly bifid. Aedeagus long, curved in the middle, terminating in a tubular vesica with a stout, broadly based, curved and pointed cornutus.
Female genitalia (Fig. 9C): corpus bursae short and globular with two small, elongated signa; ductus bursae about the same length as corpus bursae, without sclerotisation near the bursa and slightly sclerotised near the ostium. Antrum sclerotised, with two bean-shaped lateral plates separated in the middle by an incomplete fissure; the posterior lip y-shaped and the anterior u-shaped; anterior apophyses as long as posterior ones; ovipositor lobes relatively short and wide (2.2 times longer than wide) with dorsal surface bearing numerous short and stout setae, the ventral side of each lobe curved and tooth-shaped.
Larvae L5 instar (Fig. 10B): length, 20–25 mm, width, 2.5 mm; head smooth, orange brown, prothoracic shield yellow buff; body with ground colour yellow buff, dorsally suffused with pink, pinacula and caudal plate yellow buff. Young larvae are very similar to that of mature ones.
Bionomics
Like A. stygiata, A. subalbissima is a markedly hygrophilous species found on banks of streams and rivers. Larvae were collected at the bottom of stems of cogongrass, always solitary like A. stygiata; for the biology refer to A. stygiata.
Distribution
Tanzania in Kipengere and Uzungwa mountains (Fig. 11). Records are from Afromontane (Mosaic no 19) vegetation mosaic (White 1983).
Remarks
Forewing ochreous–buff like A. brunneella but the species may easily be separated after the male genitalia; the juxta is much larger and wider in A. brunneella than in A. subalbissima; also basal half of valves much more globular in A. subalbissima than in A. brunneella.
Group albivena: A. albivena
Acrapex albivena Hampson
(Figs 4A–D, 8A, F, 9D, 10C, 11)
Material examined
Holotype. Male, South Africa: Transkei: 99.26, Miss F. Barrett, 1M, Noctuidae genitalia slide N° 2462, BMNH, London.
Other material examined. SOUTH AFRICA: Kwazulu–Natal: Durban, Bowker coll., 1M, Noctuidae genitalia slide N° 2473, BMNH, London; Eston Beaumont, 29°55.102’S, 30°37.222’E, 673 m, 03 Feb 2009, ex larva (in stem of Cymbopogon sp.), B. Le Ru leg., 2M, 5F, 3M gen. prep. LERU Bruno/G332-G337-G467, 1F gen. prep. LERU Bruno/G333; Eston Beaumont, 29°55.102’S, 30°37.222’E, 673 m, 03 Feb 2009, ex larva (in stem of Miscanthus capensis (Nees) Andersson), B. Le Ru leg., 1M, 1F, 1F gen. prep. LERU Bruno/G334; Mwati, 29°48.324ʹS, 30°06.244ʹE, 1043 m, 27 Nov 2009, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., 1M,1M gen. prep. LERU Bruno/G339, 1F gen. prep. LERU Bruno/G340; Waterford, 29°50.471’S, 30°08.523’E, 1124 m, 27 Nov 2009, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., 2M, 1M gen. prep. LERU Bruno/G341.
Supplementary description
The female is described here for the first time. It is similar in appearance to that of the male, however the general shape of the female forewing is more elongated at the apex than in the male. Additions to the two previous descriptions (Hampson 1910; Janse 1939) (Fig. 4A–D): antennae ochreous, filiform in both sexes and slightly ciliate in males, flagellum adorned dorsally with black scales, palpus fuscous, eyes fuscous. Legs fuscous ringed with white, black fuscous on inner surface; abdomen grey irrorated with fuscous scales. Forewing: ground-colour ochreous suffused with fuscous scales in the costal area, white scales in other areas and with the veins adorned with white scales highlighted with fuscous scales; reniform indicated by few white scales, preceded by some black scales, sometimes absent; a longitudinal black-fuscous fascia along lower external margin of the cell, then ending obliquely to apex; in line with the oblique part of the black fascia, 3 black marks on each of the 3 veins CuA1, CuA2 and 1A+2A; a postmedial row of black elongated spots between the veins; fringe fuscous brown adorned with a narrow ochreous line. Hindwing: ground colour white, veins slightly irrorated with fuscous scales, terminal area more heavily suffused with fuscous scales; male hindwing more suffused with fuscous scales than female hindwing; fringe white adorned with a narrow fuscous line. Underside of the forewing with ground-colour ochreous brown, uniformly suffused with fuscous scales and some white scales in the costal area. Underside of hindwing white suffused with fuscous scales on costa, apex and termen; veins slightly irrorated with fuscous scales.
Wingspan: 23–26 mm (males) (n = 13); 24–26 mm (females) (n = 9).
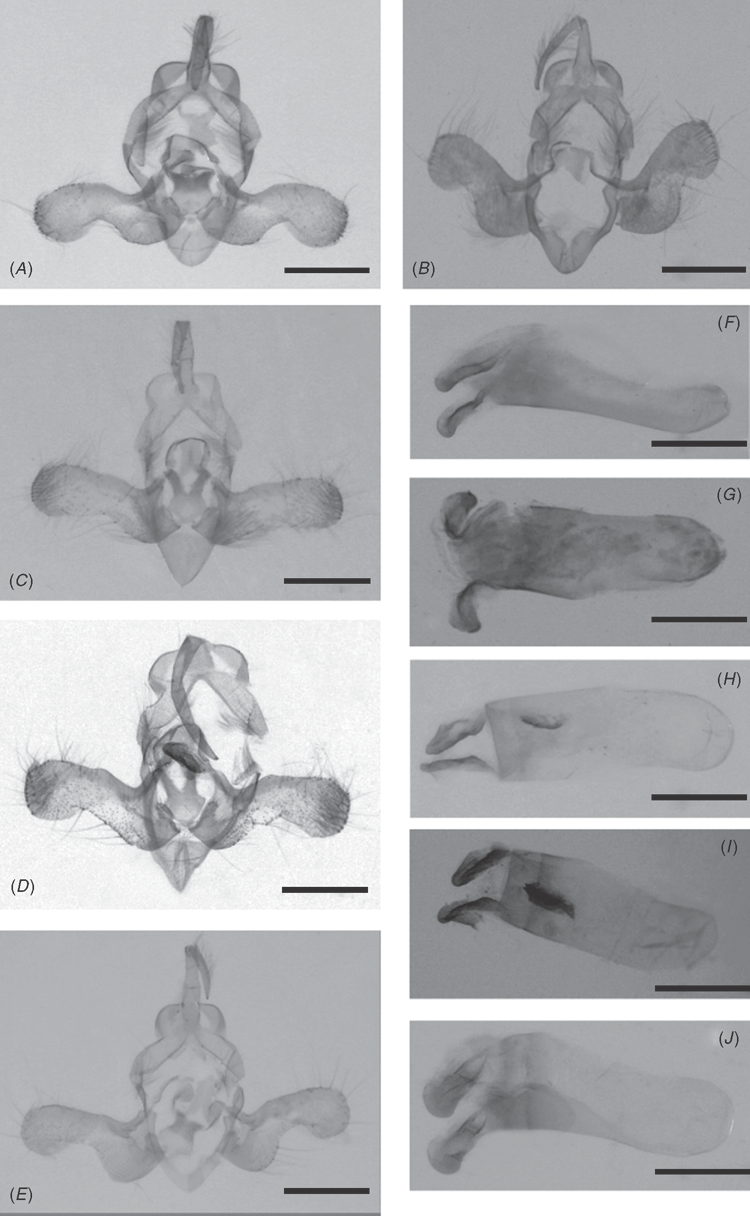
Male genitalia (Fig. 8A, F): the description of male genitalia was made by Janse (1939); additional description: uncus long and narrow, tapering in a blunt point, tufted with hairs on the upper half; the juxta rounded without sclerotisation at the base with a short wide sclerotised neck very shortly bifid; aedeagus slightly curved, short and stout, manica with a two-lobed sclerotisation, almost one-third length of the aedeagus, vesica without cornuti.
Female genitalia (Fig. 9D): corpus bursae short and globular without signa; ductus bursae without sclerotisation, ~1.5 the length of corpus bursae, widening on the ostium side, ending in a narrow sclerotised ring. Antrum narrow band-like slightly leaning on the back; anterior apophyses as long as posterior ones; ovipositor lobes relatively short and wide (2 times longer than wide) with dorsal surface bearing numerous short and stout setae, the ventral side of each lobe curved and tooth-shaped.
Larvae L5 instar (Fig. 10C): length, 20–25 mm, width, 2.5 mm; head smooth, orange brown, prothoracic shield yellow buff; body with ground colour yellow buff, dorsally suffused with pink, pinacula and caudal plate yellow buff. Young larvae are very similar to that of mature ones.
Bionomics
Acrapex albivena is a markedly hygrophilous species found of banks of streams, rivers and marshes. We found A. albivena larvae in the same wetlands habitats as A. stygiata, but A. albivena was more common; they were collected from Miscanthus capensis (Nees) Andersson, Cymbopogon sp. and cogongrass stems. For the biology refer to A. stygiata.
Distribution
South Africa in Kwazulu–Natal, Mpumalanga, Free State, Eastern and Western Cape regions and Zimbabwe in Manicaland (Fig. 11). The species was found in Afromontane (Mosaic no 19), transition from Afromontane scrub forest to Highveld grassland (Mosaic no 20), Highveld grassland (Mosaic no 58), wetter Zambezian miombo woodland (dominated by Brachystegia, Julbernardia and Isoberlinia) (Mosaic no 25) and Cape shrubland (Fynbos) (Mosaic no 50) vegetation mosaics (White 1983).
Remarks
Janse (1939) indicated that A. albivena is very similar to, if not the same species as, to that of A. hemiphlebia Hampson 1914, however reporting: ‘I regret to say that I have so far been unable to secure the reference to the original description of this species’. Having seen the original description of A. hemiphlebia by Hampson and the type specimen (both adult and genitalia) preserved in BMNH, we can definitely conclude that A. albivena is not the same species as A. hemiphlebia, which belongs to the Acrapex unicolora species group. Specimens of Acrapex hemiphlebia described by Janse (1939) either belong to a new species to be named or correspond to a particular form of A. albivena; its taxonomic status will be investigated in a future study. Janse’s misidentification was noted by Fletcher (1961).
Acrapex mitawa Le Ru, sp. nov.
(Figs 4E, F, 8B, G, 10D, 11)
Material examined
Holotype. Male, Tanzania: Ruvuma region: Mitawa, 11°07.356ʹS, 34°54.463ʹE, 1480 m, 03 Jun 2010, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., gen. prep. LERU Bruno/G331, MNHN, Paris.
Description (Fig. 4E, F)
Male: antennae bright fuscous dorsally and buff ventrally, filiform and slightly ciliate, flagellum adorned dorsally with fuscous scales, palpus fuscous, eyes fuscous. Head and base of thorax fuscous tinged with buff, thorax becoming gradually buff. Legs fuscous, ringed with buff, black fuscous in inner surface; abdomen fuscous irrorated with buff scales. Forewing: ground-colour ochreous, suffused with buff and fuscous scales, more heavily in the costal area; reniform indicated by few white scales, surrounded with black scales; a longitudinal black fuscous median fascia along lower external margin of the cell, ending obliquely at the apex; the veins to the apex suffused with buff scales, the veins below the cell adorned with fuscous, white and black scales; a postmedial row of black elongated spots between the veins; fringe fuscous externally, buff suffused with black internally. Hindwing: ground colour white suffused with fuscous scales, veins suffused with fuscous scales, costa and apex more heavily suffused with fuscous scales; fringe white suffused with fuscous and adorned with a narrow fuscous line. Underside of the forewing with ground-colour fuscous, suffused with buff scales on the costal area. Underside of hindwing white uniformly suffused with fuscous scales but more heavily on costa and apex; veins irrorated with fuscous scales.
Wingspan: 23 mm (male) (n = 1).
Male genitalia (Fig. 8B, G): uncus long and narrow, tapering in truncate apex, tufted with hairs on the upper half; valves resembling those of albivena, with a length/width ratio of 2.1, rounded along ventral margin but with a more open-angle at base, less constricted at middle, broadly rounded at apex with a corona, ventral surface covered with papillated bristly short hairs; aedeagus short and stout, manica with a two-lobed sclerotisation strongly rounded at tip, almost one quarter length of the aedeagus, vesica without cornuti.
Larvae L5 instar (Fig. 10D): length, 20–25mm, width, 2.5 mm; head smooth, orange brown, prothoracic shield very pale buff; body with ground colour pink suffused with buff, pinacula and caudal plate pale buff. Young larvae are very similar to that of mature ones.
Bionomics
It is a hygrophilous species of banks of streams, rivers and marshes. Larvae were collected in cogongrass stems. For the biology of larvae refer to A. stygiata.
Distribution
Tanzanian, Ruvuma region, south of Songea area (Fig. 11). The species was found in wet Zambezian miombo woodland (dominated by Brachystegia, Julbernardia and Isoberlinia) (Mosaic no 25) vegetation mosaic (White, 1983).
Remarks
Acrapex mitawa is externally very similar to that of albivena; separation of the species is only possible with the genitalia, much more compact in mitawa with a length/width ratio of 2.1 compared to 2.5 for A. albivena; also the two-lobed sclerotisation of the manica much more rounded at tip in mitiwa compared to A. albivena.
Etymology
Named after the village of Mitawa in Tanzania.
Acrapex salmona Le Ru, sp. nov.
(Figs 5A, B, 9E, 10E, 11)
Material examined
Holotype. Female, South Africa: North West region: Ventersdorp, 26°18.078ʹS, 26°49.746ʹE, 1488 m, 02 Feb 2007, ex larva (in stem of Sporobolus macranthelus Chiov.), B. Le Ru leg., gen. prep. LERU Bruno/G508, MNHN, Paris.
Description (Fig. 5A, B)
Female: antennae buff, filiform, flagellum adorned dorsally with fuscous scales, palpus fuscous, eyes fuscous; head and base of thorax brown-black, thorax becoming gradually salmon. Legs salmon ringed with fuscous, bright fuscous on inner surface; ground-colour of abdomen salmon with fuscous areas. Forewing: ground-colour salmon slightly suffused with ochreous, fuscous and black scales, more heavily on the costal area; reniform indicated by few white scales, preceded by some black scales; a longitudinal and narrow white-fuscous median fascia along lower external margin of the cell, ending obliquely black to apex; the veins below the cell adorned with white, fuscous and black scales; a postmedial row of black elongated spots between the veins; fringe salmon-fuscous. Hindwing: ground colour white, veins slightly suffused with fuscous scales, costal, apex and termen more heavily suffused with fuscous scales; fringe white adorned with a narrow salmon line. Underside of the forewing with ground-colour bright ochreous suffused with brown-fuscous scales on the costal and apex margin areas. Underside of hindwing white suffused with fuscous scales but more heavily on costa, apex and termen; veins slightly irrorated with fuscous scales.
Wingspan: 24 mm (female) (n = 1).
Female genitalia (Fig. 9E): corpus bursae elongated ovoid without signa; ductus bursae short about one third the length of corpus bursae without sclerotisation at base, widening on the ostium side, ending in a narrow sclerotised ring similar to that of albivena. Antrum narrow band-like without sclerotisation, slightly leaning on the back; anterior apophyses as long as posterior ones; ovipositor lobes 2.4 times longer than wide, with dorsal surface bearing numerous short ands stout setae, the ventral side of each lobe curved and tooth-shaped.
Larvae L5 instar (Fig. 10E): length, 20–25 mm, width, 2.5 mm; head smooth, orange brown, prothoracic shield very pale; body with ground colour yellow buff, heavily suffused with pink, pinacula and caudal plate bright brown. Young larvae are very similar to that of mature ones.
Bionomics
Similar to that of previous species, A. salmona is a hygrophilous species found on banks of marshes. Larvae were collected in Sporobolus macranthelus Chiov. and cogongrass stems. For the biology of larvae collected on cogongrass, refer to A. stygiata. The biology of the larvae collected from S. macranthelus was different; larvae were found at the top of young flowering stems, usually at the bottom of the inflorescence, always solitary. Typically, stems exhibiting signs of infestation by A. salmona larvae presented dead heart inflorescences. Damaged stems had a small hole (ca. 2 mm diameter) located ~10–20 cm below the top of the inflorescence; we did not observed any additional holes as seen in infested cogongrass. No pupae were found in stems, and therefore borers probably pupate in the soil. Like in cogongrass, we suspect that one larva may feed on multiple stems before pupating.
Distribution
South Africa in North-west region (Fig. 11). The species was found in wetter Zambezian miombo woodland (dominated by Brachystegia, Julbernardia and Isoberlinia) (Mosaic no 25) vegetation mosaic (White 1983).
Remarks
Acrapex salmona is very similar to that of A. albivena. Forewings of A. salmona females are paler than A. albivena and the veins of the later in the costal and apex areas are adorned with white scales. Post-vaginal plate without sclerotisation in A. salmona and wider than in A. albivena; ovipositor lobes longer in A. salmona than in A. albivena.
Etymology
Named after the ground colour of the upperside of the forewing.
Acrapex sporobola Le Ru, sp. nov.
(Figs 5C–F, 8C, H, 9F, 10F, 11)
Material examined
Holotype. Male, Kenya: Central: Ruiru, 00°05.459ʹS, 36°54.671ʹE, 1568 m, May 2005, ex larva (in stem of Sporobolus macranthelus Chiov.), B. Le Ru leg., gen. prep. LERU Bruno/G50, MNHN, Paris.
Paratypes. Same locality as holotype, 2M May 2005, 3F May 2005, 1F May 2005, gen. prep. LERU Bruno/G51, 1F Dec 2010, gen. prep. LERU Bruno/G357, 1M Dec 2010, gen. prep. LERU Bruno/G359, MNHN, Paris; same data as holotype, 1M May 2005, 1F Dec 2010, NMK, Kenya.
Description (Fig. 5C–F)
Both sexes look similar, however the general shape of the female forewing is more elongated at the apex than in the male and is paler; antennae bright fuscous dorsally and buff ventrally, filiform and slightly ciliate in the male; flagellum adorned dorsally with black scales, palpus fuscous black, eyes fuscous. Head and base of thorax black, thorax becoming gradually fuscous in male; head and base of thorax fuscous tinged with buff, thorax becoming gradually buff in female. Legs fuscous ringed with buff, black fuscous on inner surface; abdomen fuscous irrorated with buff scales. Forewing: ground-colour ochreous brown in male, buff in female, suffused with fuscous scales in the costal area in male, with ochreous and fuscous scales in the female; reniform indicated by few white scales, surrounded by some black scales, sometimes absent; a longitudinal black-brown median fascia along lower external margin of the cell, variable in area, ending obliquely at apex; the veins below the cell adorned with white, fuscous and black scales; a postmedial row of black elongated spots between the veins; fringe fuscous externally, black fuscous internally in male, fuscous and buff in female. Hindwing: ground colour white suffused with fuscous scales, veins irrorated with fuscous scales, apex more heavily suffused with fuscous scales; fringe white with some fuscous scales, adorned with a narrow fuscous line. Underside of the forewing with ground-colour fuscous-black suffused with fuscous scales in male, bright fuscous suffused with buff scales in female. Underside of hindwing white uniformly suffused with fuscous scales but more heavily on costa, apex and termen; veins irrorated with fuscous scales.
Wingspan: 23–24 mm (males) (n = 5); 23–25 mm (females) (n = 6).
Male genitalia (Fig. 8C, H): uncus long and narrow, tapering in truncate apex, tufted with hairs on the upper half; valves similar to that of A. albivena but less rounded along ventral margin at base, slightly constricted at middle but not rounded, broadly rounded at apex with a corona, ventral surface covered with papillated bristly short hairs; the juxta slightly pyriform without sclerotisation at the base, with a short wide sclerotised neck longly bilobate, each lobe curved to the exterior, the same width along the full length, with the apex truncate; aedeagus short and massive, three times as long as its shortest width, manica with a two-lobed sclerotisation, less than one third length of the aedeagus, vesica with an elongated cornutus shorter than the manica lobe, plate-like without acuminated edge.
Female genitalia (Fig. 9F): corpus bursae elongated ovoid without signa; ductus bursae about one third length of corpus bursae, not sclerotised on bursa side, widening and sclerotised on the ostium side. Antrum narrow band-like, slightly sclerotised and leaning on the back; anterior apophyses as long as posterior ones; ovipositor lobes relatively short and wide (less than 2 times longer than wide) with dorsal surface bearing numerous short ands stout setae, the ventral side of each lobe curved and tooth-shaped.
Larvae L5 instar (Fig. 10F): length, 20–25 mm, width, 2.5 mm; head smooth, orange brown, prothoracic shield very buff-brown; body with ground colour buff, suffused with pink, pinacula pale buff and caudal plate brown. Young larvae are very similar to that of mature ones.
Bionomics
Acrapex sporobola is a markedly hygrophilous species of banks of streams, rivers and marshes. Acrapex sporobola larvae were found in one locality in Western Uganda in cogongrass stems and in one locality in Central Kenya in Sporobolus macranthelus stems. For the biology refer to A. stygiata for larvae collected on cogongrass and to A. salmona for those ones collected in Sporobolus.
Distribution
Central Kenya Central and western Uganda (Fig. 11). The species was found in Afromontane (Mosaic no 19) and transitional East African evergreen bushland and secondary Acacia wooded grassland (Mosaic no 45) vegetation mosaics (White 1983).
Remarks
Acrapex sporobola is very similar to that of A. syscia. The females of both species cannot be separated morphologically. Only males can be differentiated at genitalia level; the external margin of the constricted middle of the valves forming an elbow bend in A. syscia, absent or attenuated in sporobola; the two lobes of the juxta narrowing to the apex in A. syscia, with the same width along the all length in sporobola; the cornutus on the vesica is much smaller in A. sporobola than in A. syscia. The close relationships of the two species is also supported by the results of the molecular analyses, which groups the two species with a very high level of support (BV of 100% and CPP of 1.0).
Etymology
Named after the host-plant Sporobolus macranthelus in Kenya.
Acrapex syscia Fletcher
(Figs 6A–D, 8D, I, 9G, 10G, 11)
|
|

|
|
Material examined
Holotype. Male, Uganda: Ruwenzori Range: Ibanda, 4700 ft, 04–12 Sep 1952, Fletcher coll., Ruwenzori Exped. B. M. 1952–566, Noctuidae genitalia slide N° 2464, BMNH, London.
Paratypes. Same data as holotype, 2M, Noctuidae genitalia slide N° 2432–2465, BMNH, London.
Other material examined. UGANDA: Fort Portal: 5000 ft, Dec 1934–Jan1935, Edwards coll., 1M, BMNH, Londres. RDC: Rutshuru, Kilinga, Jun 1936, 1M, gen. prep. Berio N° 5100, MCSN, Milan. UGANDA: Kyogyera, 00°33.453’S, 30°19.593’, 1525 m, 14 May 2009, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., 1F gen. prep. LERU Bruno/G351, 1F, MNHN, Paris; Ntuntu, 00°41.870ʹS, 30°32.858ʹE, 1568 m, 17 May 2009, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., 1F gen. prep. LERU Bruno/G353, 1M gen. prep. LERU Bruno/G354, 1M, 1F, MNHN, Paris; Nyongozi, 00°45.437’S, 30°18.580’E, 1455 m, 12 May 2009, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., 1M gen. prep. LERU Bruno/G468, 2F, MNHN, Paris.
Supplementary description
The female is described here for the first time, it looks similar to that of the male, however the general shape of the female forewing is more elongated at the apex than in the male and is paler. Additions to previous description (Fig. 6a–d): antennae ochreous, filiform in both sexes and almost serrate in males, flagellum adorned dorsally with black scales, palpus fuscous, eyes fuscous. Legs fuscous brown ringed with white, black fuscous on inner surface; abdomen fuscous irrorated with ochreous scales. Forewing: ground-colour ochreous in male, ochreous–buff in female, suffused with fuscous and white scales in the costal area and with areas between veins suffused with brown scales; reniform indicated by few white scales, preceded by some black scales, sometimes absent; a longitudinal black-brown median fascia along lower external margin of the cell, variable in area, widening to the termen and ending obliquely at apex; the veins below the cell adorned with white, fuscous and black scales; a postmedial row of black elongated spots between the veins; fringe black fuscous. Hindwing: ground colour white to white, veins slightly irrorated with fuscous scales, termen more heavily suffused with fuscous scales; male hindwing more suffused with fuscous scales than female hindwing; fringe bright fuscous in male, white in female, adorned with a narrow fuscous line. Underside of the forewing with ground-colour fuscous- brown, suffused with fuscous and white scales on the costal and apex areas. Underside of hindwing white uniformly suffused with fuscous scales but more heavily on costa, apex and termen; veins slightly irrorated with fuscous scales.
Wingspan: 23–25 mm (males) (n = 13); 23–25 mm (females) (n = 9).
Male genitalia (Fig. 8D, I): the description of male genitalia was made by Fletcher (1961); additional description: uncus long and narrow, tapering in truncate apex, tufted or not with hairs on the upper half; valves similar to that of albivena but less rounded along ventral margin at base, not roundly constricted at middle, broadly rounded at apex, the external margin of the constricted middle forming an elbow bend; the juxta slightly pyriform without sclerotisation at the base, with a short, wide, sclerotised neck longly bilobate, each lobe slightly curved to the exterior and narrowing to the apex almost truncate; aedeagus short and massive, three times as long as its shortest width, manica with a two-lobed sclerotisation, less than one third length of the aedeagus, vesica with an elongated cornutus same length as the manica lobe, ending with two small teeth.
Female genitalia (Fig. 9G): corpus bursae elongated ovoid without signa; ductus bursae about one-third length of corpus bursae, non sclerotised on bursa side, widening and sclerotised on the ostium side. Antrum narrow band-like, slightly sclerotised and leaning on the back; anterior apophyses as long as posterior ones; ovipositor lobes relatively short and wide (less than 2 times longer than wide) with dorsal surface bearing numerous short and stout setae, the ventral side of each lobe curved and tooth-shaped.
Larvae L5 instar (Fig. 10G): length, 20–25 mm, width, 2.5 mm; head smooth, orange brown, prothoracic shield very pale buff; body with ground colour white, slightly suffused with pink, pinacula pale buff and caudal plate brown. Young larvae are very similar to that of mature ones.
Bionomics
Similar to that of previous species, A. syscia is a markedly hygrophilous species of banks of streams, rivers and marshes. A. syscia larvae were common in Western Uganda in cogongrass stems. For the biology refer to A. stygiata.
Distribution
Western Uganda and eastern Democratic Republic of Congo (Fig. 11). The species was found in Afromontane (Mosaic no 19), transitional East African evergreen bushland and secondary Acacia wooded grassland (Mosaic no 45) and lowland rain forest and secondary grassland (Mosaic no 11) vegetation mosaics (White 1983).
Remarks
In contrast to A. albivena, the veins of the coastal area of the forewings of A. syscia are not adorned with white scales. The sclerotised neck of the juxta is longly bilobate in A. syscia whereas it is shortly bilobate in A. albivena; post-vaginal plate much more developed and wide in A. albivena than in A. syscia.
Acrapex yakoba Le Ru, sp. nov.
(Figs 6E–H, 8E, J, 9H, 10H, 11)
Material examined
Holotype. Male, Tanzania: Iringa region: Yakobi, 09°24.688ʹS, 34°56.374ʹE, 1693 m, 11 March 2009, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., gen. prep. LERU Bruno/G327, MNHN, Paris.
Paratypes. Same data as holotype, 2M, 3F, 1M gen. prep. LERU Bruno/G466, 2F gen. prep. LERU Bruno/G328–564, MNHN, Paris; same data as holotype, 2M, 2F, NMK, Kenya; Idofi, 08°48.185ʹS, 34°50.570ʹE, 1629 m, 14. Mar 2009, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., 2M, 3F, 1M gen. prep. LERU Bruno/G329, MNHN, Paris; Njombe, 09°19.548ʹS, 34°45.698ʹE, 1852 m, 1 Mar 2008, ex larva (in stem of Imperata cylindrica (L.) P. Beauv.), B. Le Ru leg., 1M gen. prep. LERU Bruno/G326, MNHN, Paris.
Description (Fig. 6e–h)
Males and females look similar, however the general shape of the female forewing is more elongated at the apex than in the male and is paler; antennae bright fuscous dorsally and buff ventrally, filiform in both sexes and slightly ciliate in males, flagellum adorned dorsally with fuscous scales, palpus fuscous, eyes fuscous. Head and base of thorax brown-black tinged with buff, thorax becoming gradually buff. Legs fuscous, ringed with buff, buff on the inner surface; abdomen fuscous irrorated with ochreous scales. Forewing: ground-colour buff in both sexes, suffused with ochreous and fuscous scales, more heavily in the costal area; reniform indicated by few white scales, preceded by some black scales, sometimes absent; a longitudinal black median fascia along lower external margin of the cell, variable in area, ending obliquely at the apex; the veins below the cell adorned with white, fuscous and black scales; a postmedial row of black elongated spots between the veins; fringe fuscous externally, buff suffused with black internally. Hindwing: ground colour white to white, veins slightly irrorated with fuscous scales, costa and apex more heavily suffused with fuscous scales; male hindwing much more suffused with fuscous scales than female; fringe white suffused with fuscous and adorned with a narrow fuscous line. Underside of the forewing with ground-colour fuscous, suffused with white scales in the costal area. Underside of hindwing white uniformly suffused with fuscous scales but more heavily on costa and apex; veins slightly irrorated with fuscous scales.
Wingspan: 24–26 mm (males) (n = 10); 25–28 mm (females) (n = 8).
Male genitalia (Fig. 8E, J): uncus long and narrow, tapering to a fine point, tufted with hairs on the upper half; valves similar to that of albivena, rounded along ventral margin but with a tight angle at base, roundly constricted at middle, the external margin of the constricted middle forming an elbow bend like in A. syscia, broadly rounded at apex with a corona, ventral surface covered with papillated bristly short hairs; the juxta rounded without sclerotisation at the base, with a short sclerotised neck, very shortly bilobate, each lobe slightly curved to the exterior; aedeagus slightly curved, short and stout, manica with a two-lobed sclaerotization strongly rounded at tip, almost one third length of the aedeagus, vesica without cornuti.
Female genitalia (Fig. 9H). Corpus bursae long, globular at the tip, ovoid at the base, without signa; ductus bursae about one third length of corpus bursae, non sclerotised on bursa side, widening and ending in a narrow sclerotised ring on the ostium side. Antrum narrow band-like slightly sclerotised, leaning on the back and adorned with a very narrow and strongly sclerotised plate in the middle; anterior apophyses as long as posterior ones; ovipositor lobes 2.3 times longer than wide, with dorsal surface bearing numerous short and stout setae, the ventral side of each lobe curved and tooth-shaped.
Larvae L5 instar (Fig. 10H): length, 20–25 mm, width, 2.5 mm.; head smooth, orange brown, prothoracic shield very pale buff; body with ground colour buff, heavily suffused with pink, pinacula and caudal plate brown. Young larvae are very similar to that of mature ones.
Bionomics
It is a hygrophilous species of banks of streams, rivers and marshes Larvae were collected in cogongrass stems; for the biology of larvae refer to A. stygiata.
Distribution
Tanzania, Iringa region in Njombe area (Fig. 11). The species was found in wetter Zambezian miombo woodland (dominated by Brachystegia, Julbernardia and Isoberlinia) (Mosaic no 25) vegetation mosaic (White 1983).
Remarks
Acrapex yakoba is externally similar to that of A. albivena, however ground colour of forewings is ochreous in A. albivena and buff in A. yakoba; the neck of the juxta is wider in A. albivena than in A. yakoba; the two-lobed sclerotisation of the manica are much more strongly rounded in A. yakoba than in A. albivena. Post-vaginal plate wider and less sclerotised in A. yakoba; ovipositor lobes shorter and wider in A. albivena.
Etymology
Named after the village of Yakobi in Tanzania.
Final considerations
This study documented six new species of Acrapex in two species groups. Considering that Acrapex is by far the most diverse genus of the Sesamiina with at least 83 species (71 known species in Africa), it seems very likely the Acrapex species diversity in Sub-Saharan Africa is greatly underestimated. Among the many Acrapex specimens collected by our team during the last ten years from both host-plants and in light traps, many additional new species have been collected and will be described in future papers. The new information presented here on the ecology and taxonomy of noctuid stem borers not only increases our knowledge of insect biodiversity in Africa, but may also have application for biological control of cogongrass in the south-eastern USA, where it is highly invasive. Historically, gramineous weeds have not been considered good targets for biological control because of a perceived lack of specialised insect herbivores (Bernays 1985; Tscharntke and Greiler, 1995; Massey et al. 2006) and the high economic value of crop grasses (Wapshere 1990; Pemberton 2002). However, investigations on several weedy grasses, including Phragmites australis (Cav.) Trin ex Steudal (Tewksbury et al. 2002), Spartina alterniflora Loisel (Grevstad et al. 2003), Arundo donax L. (Goolsby and Moran 2009; Goolsby et al. 2009), and Hymenachne amplexicaulis (Rudge) Nees (Diaz et al. 2009, 2010) have demonstrated the presence of specialised insect herbivores. Moreover, an investigation in Java identified a specialist stem-galling cecidoymid on cogongrass (Mangoendihardjo 1980). In the present study, seven of the Acrapex species examined fed on cogongrass, and four of them (A. mitawa, A. subalbissima, A. syscia, A. yakoba) were found exclusively on cogongrass, despite sampling of several other grasses concurrently at the same locations. Similarly, in Japan A. azumai is thought to feed exclusively on cogongrass (Takasu et al. 2014). Thus, one or more Acrapex spp. may have value as biological control agents. As with all introduced weed biological control agents in the USA, extensive host range testing in a quarantine laboratory, would be required before approval for field release.
|
Acknowledgements
We would like to thank the associate editor, Pr. Rudolf Meier and two anonymous reviewers for their useful and constructive comments. We are grateful to the Natural History Museum of London (M. Honey), to the Museo Civico di Storia Naturale (Dr Fabrizio Rigato) and to the Ditsong National Museum of Natural History (Dr M. Krüger) for the permission to study and photograph the types. Financial support was provided by the Institut de Recherche pour le Développement, by icipe, African Insect Science for Food and Health (Kenya), by the Florida Fish and Wildlife Conservation Commission through a grant to the University of Florida (USA) and laboratory facilities by icipe, African Insect Science for Food and Health (Kenya) and the Laboratory Evolution Génomes Spéciation of the Centre National de la Recherche Scientifique in Gif-Sur-Yvette (France). Part of the sequencing was also supported by the program ‘Bibliothèque du Vivant’ (Project Noctuid Stem Borer Biodiversity; NSBB) supported by a joint CNRS, INRA and MNHN consortium. All specimens were collected under appropriate collection permits from the different countries surveyed and no conflicts of interest were discovered.
References
Berio, E. (1973). Nuove species e generi di noctuidae africane e asiatiche e note sinonimiche. Parte II. Annali del museo civico di storia naturale, Giacomo doria 79, 126–171.Berio, E. (1976). Nuovi generi e specie di noctuidae dell‘Africa equatoriale. Annali del Museo civico di storia naturale, Giacomo doria 81, 96–123.
Bernays, E. A. (1985). Arthropods for weed control in IPM systems. In ‘Biological Control in Agricultural Integrated Pest Management Systems’ . (Eds M. A. Hoy, D. C. Herzog.) pp. 373–391. (Academic Press: Burlington, MA.)
Dayrat, B. (2005). Toward integrative taxonomy. Biological Journal of the Linnean Society. Linnean Society of London 85, 407–415.
| Toward integrative taxonomy.Crossref | GoogleScholarGoogle Scholar |
Diaz, R., Overholt, W., Cuda, J., Pratt, P., and Fox, A. (2009). Host specificity of Ischnodemus variegatus, an herbivore of West Indian marsh grass (Hymenachne amplexicaulis). BioControl 54, 307–321.
| Host specificity of Ischnodemus variegatus, an herbivore of West Indian marsh grass (Hymenachne amplexicaulis).Crossref | GoogleScholarGoogle Scholar |
Diaz, R., Overholt, W. A., Heard, T. A., Van Klinken, R., and Samayoa, A. (2010). Life history parameters of Ischnodemus variegatus (Signoret) (Hemiptera: Blissidae) reared on two closely related grasses. Biological Control 55, 219–224.
| Life history parameters of Ischnodemus variegatus (Signoret) (Hemiptera: Blissidae) reared on two closely related grasses.Crossref | GoogleScholarGoogle Scholar |
Drummond, A. J., Suchard, M. A., Xie, D., and Rambaut, A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29, 1969–1973.
| Bayesian phylogenetics with BEAUti and the BEAST 1.7.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFagu7fO&md5=beec25ccf0d2a98903aa512fd45babe5CAS | 22367748PubMed |
Edgar, R. C. (2004). MUSCLE : a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 1–19.
Erixon, P., Svennblad, B., Britton, T., and Oxelman, B. (2003). Reliability of Bayesian posterior probabilities and bootstrap frequencies in phylogenetics. Systematic Biology 52, 665–673.
| Reliability of Bayesian posterior probabilities and bootstrap frequencies in phylogenetics.Crossref | GoogleScholarGoogle Scholar | 14530133PubMed |
Felix, A.-E., Calatayud, P.-A., Le Ru, B., Capdevielle-Dulac, C., Ong’amo, G., Silvain, J.-F., and Frerot, B. (2013). To be or not to be a species: use of reproductive isolation experiments and genetic analysis to clarify the taxonomic status of two Busseola (Lepidoptera: Noctuidae) species in Kenya. Annales de la Société Entomologique de France 49, 345–354.
Fletcher, D. S. (1961). ‘Noctuidae. Ruwenzori Expedition 1952.’ Vol. 1, pp. 177–323. British Museum (Natural History), London.
Gaede, M. (1935). Noctuidae. In ‘Die Gross-Schmetterlinge der Erde. Macrolepidoptera of the world. a systematic description of the hitherto known Macrolepidoptera. A systematic description of the hitherto known Macrolepidoptera’. (Ed. A. Seitz.) Die Afrikanischen Spinner und Schwärmer. xv. p. 95, pl. 10.
Goolsby, J. A., and Moran, P. (2009). Host range of Tetramesa romana (Hymenoptera: Eurytomidae), a potential biological control agent of giant reed, Arundo donax L. in North America. Biological Control 49, 160–168.
| Host range of Tetramesa romana (Hymenoptera: Eurytomidae), a potential biological control agent of giant reed, Arundo donax L. in North America.Crossref | GoogleScholarGoogle Scholar |
Goolsby, J. A., Moran, P. J., Adamczyk, J. J., Kirk, A. A., Jones, W. A., Marcos, M. A., and Cortés, E. (2009). Host range of the European, rhizome-stem feeding scale Rhizaspidiotus donacis (Hemiptera: Diaspididae), a candidate biological control agent for giant reed, Arundo donax (Poales: Poaceae) in North America. Biocontrol Science and Technology 19, 899–918.
| Host range of the European, rhizome-stem feeding scale Rhizaspidiotus donacis (Hemiptera: Diaspididae), a candidate biological control agent for giant reed, Arundo donax (Poales: Poaceae) in North America.Crossref | GoogleScholarGoogle Scholar |
Grevstad, F. S., Strong, D. R., Garcia-Rossi, D., Switzer, R. W., and Wecker, M. S. (2003). Biological control of Spartina alterniflora in Willapa Bay, Washington using the planthopper Prokelisia marginata: agent specificity and early results. Biological Control 27, 32–42.
| Biological control of Spartina alterniflora in Willapa Bay, Washington using the planthopper Prokelisia marginata: agent specificity and early results.Crossref | GoogleScholarGoogle Scholar |
Hampson, G. F. (1910). ‘Catalogue of the Noctuidae in the Collection of the British Museum.’ (Taylor and Francis: London.)
Hillis, D. M., and Bull, J. J. (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42, 182–192.
| An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis.Crossref | GoogleScholarGoogle Scholar |
Holm, L. G., Donald, P., Pancho, J. V., and Herberger, J. P. (1977). ‘The World’s Worst Weeds: Distribution and Biology.’ (The University Press of Hawaii: Honolulu, Hawaii.)
Holzmueller, E. G., and Jose, S. (2011). Invasion success of cogongrass, an alien C4 perennial grass, in the Southeastern United States: Exploration of the ecological basis. Biological Invasions 13, 435–442.
| Invasion success of cogongrass, an alien C4 perennial grass, in the Southeastern United States: Exploration of the ecological basis.Crossref | GoogleScholarGoogle Scholar |
Janse, A. J. T. (1939). The Moths of South Africa. Cymatophoridae, Callidulidae and Noctuidae (partim). Vol. 3, pp. 307–435. (E.P. and Commercial Printing Co. Ltd: Durban.)
Jose, S., Cox, J., Miller, D. L., Shilling, D. G., and Merit, S. (2002). Alien plant invasions: the story of cogongrass in Southeastern forests. Journal of Forestry 100, 41–44.
Kergoat, G. J., Prowell, D. P., Le Ru, B. P., Mitchell, A., Dumas, P., Clamens, A.-L., Condamine, F. L., and Silvain, J.-F. (2012). Disentangling dispersal and vicariance patterns in armyworms: evolution and historical biogeography of the pest genus Spodoptera (Lepidoptera: Noctuidae). Molecular Phylogenetics and Evolution 65, 855–870.
| Disentangling dispersal and vicariance patterns in armyworms: evolution and historical biogeography of the pest genus Spodoptera (Lepidoptera: Noctuidae).Crossref | GoogleScholarGoogle Scholar | 22939903PubMed |
Kfir, R., Overholt, W. A., Khan, Z. R., and Polaszek, A. (2002). Biology and management of economically important lepidopteran cereal stem borers in Africa. Annual Review of Entomology 47, 701–731.
| Biology and management of economically important lepidopteran cereal stem borers in Africa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvVKqtQ%3D%3D&md5=c20f0d066a1470f8778bae94e1ea2144CAS | 11729089PubMed |
Laporte, B. (1975). Diagnoses de 17 nouvelles espèces de Noctuidae d’Ethiopie et du Kenya (Lepidoptères). Bulletin de la Société Linnéenne de Lyon 8, 277–287.
Laporte, B. (1984). Noctuidae in Rougeot, P.-C. (Ed.) Missions Entomologiques en Ethiopie 1976–1982. Mémoires du Museum National d’Histoire Naturelle (Nouvelle Série) 128, 12–51.
Le Ru, B. P., Ong’amo, G. O., Moyal, P., Muchungu, E., Ngala, L., Musyoka, L., Abdullah, B., Kauma, Z., Matama, T., Lada, V. Y., Pallangyo, K., Sidumo, A., Omwega, C., Schulthess, F., Calatayud, P.-A., and Silvain, J.-F. (2006a). Major ecological characteristics of East African noctuid stem borers. Annales de la Société Entomologique de France 42, 353–362.
Le Ru, B., Ong’amo, G. O., Moyal, P., Ngala, L., Musyoka, B., Abdullah, Z., Cugala, D., Defabachew, B., Haile, T. A., Kauma Matama, T., Lada, V. Y., Negassi, B., Pallangyo, K., Ravololonandrianina, J., Sidumo, A., Omwega, C., Schulthess, F., Calatayud, P.-A., and Silvain, J.-F. (2006b). Diversity of lepidopteran stem borers on monocotyledonous plants in eastern Africa and the islands of Madagascar and Zanzibar revisited. Bulletin of Entomological Research 96, 555–563.
| Diversity of lepidopteran stem borers on monocotyledonous plants in eastern Africa and the islands of Madagascar and Zanzibar revisited.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s%2Fgs1arsQ%3D%3D&md5=a4a1da8f9e35847ce9349c0cfacf35afCAS | 17201973PubMed |
Lim, G. S., Balke, M., and Meier, R. (2012). Determining species boundaries in a world full of rarity: singletons, species delimitation methods. Systematic Biology 61, 165–169.
| Determining species boundaries in a world full of rarity: singletons, species delimitation methods.Crossref | GoogleScholarGoogle Scholar | 21482553PubMed |
Lohse, K. (2009). Can mtDNA barcodes be used to delimit species? A response to Pons et al. (2006). Systematic Biology 58, 439–442.
| Can mtDNA barcodes be used to delimit species? A response to Pons et al. (2006).Crossref | GoogleScholarGoogle Scholar | 20525596PubMed |
Mangoendihardjo, S. (1980). Some notes on the natural enemies of Alang-alang (Imperata cylindrica (L.) Beauv.) in Java. In ‘Proceedings of BTROP Workshop on Alang-alang, Bogor, Indonesia, 27–29 July 1976’. (Ed. B. Soewardi.) pp. 47–55. (BIOTROP SEAMO Regional Center for Tropical Biology: Bogor, Indonesia.)
Massey, F. P., Ennos, A. R., and Hartley, S. E. (2006). Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and a phloem feeder. Journal of Animal Ecology 75, 595–603.
| Silica in grasses as a defence against insect herbivores: contrasting effects on folivores and a phloem feeder.Crossref | GoogleScholarGoogle Scholar | 16638012PubMed |
Moyal, P. (2006). History of the systematics of the Sesamia sensu lato group of African Noctuid stem borers of monocotyledonous plants (Lepidoptera). Annales de la Société Entomologique de France 42, 285–291.
| History of the systematics of the Sesamia sensu lato group of African Noctuid stem borers of monocotyledonous plants (Lepidoptera).Crossref | GoogleScholarGoogle Scholar |
Moyal, P., Le Ru, B., Conlong, D., Cugala, D., Defabachew, B., Matama-Kauma, T. B., Pallangyo, B., and ‘, J. (2010). Systematics and molecular phylogeny of two African stem borer genera, Sciomesa Tams and Bowden and Carelis Bowden (Lepidoptera: Noctuidae). Bulletin of Entomological Research 100, 641–659.
| Systematics and molecular phylogeny of two African stem borer genera, Sciomesa Tams and Bowden and Carelis Bowden (Lepidoptera: Noctuidae).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cbkslKqtA%3D%3D&md5=cc9f1e8fbb7d6e99b4a44b3583bf9ef1CAS | 20576171PubMed |
Moyal, P., Tokro, P., Bayram, A., Savopoulou-Soultani, M., Conti, E., Eizaguirre, M., Le Ru, B., Avand-Faghih, A., and Andreadis, S. (2011). Origin and taxonomic status of the Palearctic population of the stem borer Sesamia nonagrioides (Lefèbvre) (Lepidoptera: Noctuidae). Biological Journal of the Linnean Society. Linnean Society of London 103, 904–922.
| Origin and taxonomic status of the Palearctic population of the stem borer Sesamia nonagrioides (Lefèbvre) (Lepidoptera: Noctuidae).Crossref | GoogleScholarGoogle Scholar |
Nylander, J. A. A., Ronquist, F., Huelsenbeck, J. P., and Nieves-Aldrey, J. L. (2004). Bayesian phylogenetic analysis of combined data. Systematic Biology 53, 47–67.
| Bayesian phylogenetic analysis of combined data.Crossref | GoogleScholarGoogle Scholar |
Onyango, F. O., and Ochieng’Odero, J. P. R. (1994). Continuous rearing of the maize stem borer Busseola fusca on an artificial diet. Entomologia Experimentalis et Applicata 73, 139–144.
| Continuous rearing of the maize stem borer Busseola fusca on an artificial diet.Crossref | GoogleScholarGoogle Scholar |
Padial, J. M., Miralles, A., De la Riva, I., and Vences, M. (2010). The integrative future of taxonomy. Frontiers in Zoology 7, 16.
| The integrative future of taxonomy.Crossref | GoogleScholarGoogle Scholar | 20500846PubMed |
Papadopoulou, A., Bergsten, J., Fujisawa, T., Monaghan, M. T., Barraclough, T. G., and Vogler, A. P. (2008). Speciation and DNA barcodes: testing the effects of dispersal on the formation of discrete sequence clusters. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363, 2987–2996.
| Speciation and DNA barcodes: testing the effects of dispersal on the formation of discrete sequence clusters.Crossref | GoogleScholarGoogle Scholar | 18522916PubMed |
Pemberton, R. W. (2002). Selection of appropriate future target weeds for biological control. In ‘Biological Control of Invasive Plants in the Eastern United States’. (Eds R. Van Driesche, S. Lyon, B. Blossey, M. Hoddle and R. Reardon.) pp. 375–86. USDA Forest Service Publication FHTET-2002–04.
Pierce, F. N. (1942). The genitalia of the group Noctuidae of the Lepidoptera of the British Islands. (Female). (Classey, Farringdon).
Poole, R. W. (1989). Noctuidae; Lepidopterorum catalogus (Lepidopterorum Catalogus New Series, Fasc. 118), Part 1 and 2).
Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25, 1253–1256.
| jModelTest: phylogenetic model averaging.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotlKgsb4%3D&md5=a227fa0ae4120a3a8c06cab96986f12aCAS | 18397919PubMed |
Posada, D., and Buckley, T. R. (2004). Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53, 793–808.
| Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests.Crossref | GoogleScholarGoogle Scholar | 15545256PubMed |
Riedel, A., Sagata, K., Suhardjono, Y. R., Tänzler, R., and Balke, M. (2013). Integrative taxonomy on the fast track – towards more sustainability in biodiversity research. Frontiers in Zoology 10, 15.
| Integrative taxonomy on the fast track – towards more sustainability in biodiversity research.Crossref | GoogleScholarGoogle Scholar | 23537182PubMed |
Schlick-Steiner, B. C., Steiner, F. M., Seifert, B., Stauffer, C., Christian, E., and Crozier, R. H. (2010). Integrative taxonomy: a multisource approach to exploring Biodiversity. Annual Review of Entomology 55, 421–438.
| Integrative taxonomy: a multisource approach to exploring Biodiversity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptVShtQ%3D%3D&md5=225cd409618e3867a1f7bfe80eed831aCAS | 19737081PubMed |
Stamatakis, A. (2006). RaxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690.
| RaxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFKlsbfI&md5=b0df768ad6679df0c2d42f77d14048ddCAS | 16928733PubMed |
Strand, E. (1916). Neue Nebenformen exotischer Heterocera. Archiv für Naturgeschichte 82, 147–157.
Swezey, O. H. (1927). Insect fauna of Panicum torridum, a native grass in Hawaii. Proceeding of the of the Hawaii Entomological Society 7, 179–182.
Takasu, K., Yoshiyasu, Y., Burrell, A. M., Klein, P. E., Racelis, A., Goosby, J. A., and Overholt, W. A. (2014). Acrapex azumai Sugi (Lepidoptera: Noctuidae) as a possible biological control agent of the invasive weed Imperata cylindrica (L.) Beauv. (Poaceae) in the United States. Lepidoptera Science 65, .
Tams, W. H. T., and Bowden, J. (1953). A revision of the African species of Sesamia Guenée and related genera (Agrotidae-Lepidoptera). Bulletin of Entomological Research 43, 645–679.
Tewksbury, L., Casagrande, R., Blossey, B., Häfliger, P., and Schwarzländer, M. (2002). Potential for biological control of Phragmites australis in North America. Biological Control 23, 191–212.
| Potential for biological control of Phragmites australis in North America.Crossref | GoogleScholarGoogle Scholar |
Toussaint, E. F. A., Condamine, F. L., Kergoat, G. J., Silvain, J.-F., Capdevielle-Dulac, C., Barbut, J., and Le Ru, B. P. (2012). Palaeoenvironmental shifts drove the adaptive radiation of a noctuid stemborer tribe (Lepidoptera, Noctuidae, Apameini) in the Miocene. PLoS ONE 7, e41377.
| Palaeoenvironmental shifts drove the adaptive radiation of a noctuid stemborer tribe (Lepidoptera, Noctuidae, Apameini) in the Miocene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFGgtr7N&md5=ddd26eb09f81a29b30f37e931c467ecbCAS |
Tscharntke, T., and Greiler, H. J. (1995). Insect communities, grasses, and grasslands. Annual Review of Entomology 40, 535–558.
| Insect communities, grasses, and grasslands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXjtVWlt7k%3D&md5=60bf9a11eb71bc1abaf5d5da9b983f23CAS |
Wapshere, A. J. (1990). Biological control of grass weeds in Australia: an appraisal. Plant Protection Quarterly 5, 62–75.
White, F. (1983). The vegetation of Africa, a descriptive memoir to accompany the UNESCO/AETFAT/UNSO vegetation map of Africa. UNESCO. Natural Resources Research 20, 1–356.
| 1:STN:280:DC%2BD283pvVGquw%3D%3D&md5=c721a66331721ee5787e5afd2577d7a2CAS |
Will, W. W., Mishler, B. D., and Wheeler, Q. D. (2005). The perils of DNA Barcoding and the need for integrative taxonomy. Systematic Biology 54, 844–851.
| The perils of DNA Barcoding and the need for integrative taxonomy.Crossref | GoogleScholarGoogle Scholar |
Zhang, J., Kapli, P., Pavlidis, P., and Stamatakis, A. (2013). A general species delimitation method with applications to phylogenetic placements. Bioinformatics , .
| A general species delimitation method with applications to phylogenetic placements.Crossref | GoogleScholarGoogle Scholar | 24344194PubMed |