The effect of cranioplasty on outcomes and complications of unresponsive wakefulness syndrome and minimally responsive state
Elena Aidinoff A B * , Hiela Lehrer A , Ilana Gelernter B , Ilil Dayan A , Adi Kfir A , Lilach Front A , Ana Oksamitny A and Amiram Catz A B
A B * , Hiela Lehrer A , Ilana Gelernter B , Ilil Dayan A , Adi Kfir A , Lilach Front A , Ana Oksamitny A and Amiram Catz A B
A
B
Abstract
Studies that have shown neurological improvement following cranioplasty (CP) after decompressive craniectomy (DC) in patients with unresponsive wakefulness syndrome (UWS) and minimally conscious state (MCS) did not include control groups. The aim of this study was to assess the justification of CP for these patients.
Data were collected from medical records of inpatients with UWS and MCS admitted between 2002 and 2018.
Of the 144 participants (mean age 40 years, 76% males, 75% in UWS), 37% had CP following DC. The Loewenstein Communication Scale (LCS) gain was 12 ± 17 and 16 ± 17 for the control and study patients, respectively. The corresponding consciousness recovery rate (based on Coma Recovery Scale-Revised scores) was 51% and 53%, respectively. One-year survival rates were 0.80 and 0.93, and 5-year survival rates were 0.67 and 0.73, respectively. Mean outcome values were higher for the study group, but the differences between the groups did not reach statistical significance.
The study did not demonstrate that CP increases brain recovery or survival. Nevertheless, it showed that CP did not decrease them either, and it did not increase complications rate. The findings, therefore, support offering CP to patients with UWS and MCS as CP does not increase risks and can achieve additional goals for these patients.
Keywords: cranioplasty, decompressive craniectomy, disorders of consciousness, minimal consciousness state, minimally responsive state, outcomes, unresponsive wakefulness syndrome, vegetative state.
Introduction
Prolonged disorders of consciousness (DOC) caused by brain damage include unresponsive wakefulness syndrome (UWS) and minimally conscious state (MCS). In UWS, patients have sleep–wake cycles but lack noticeable awareness. Patients with UWS may present eye-opening spontaneously or following stimulation. They may also show reflexive and spontaneous behaviour, such as strong noise response, spontaneous swallowing of saliva and munching movements – a pathological chewing pattern. In MCS, patients also have limited and inconsistent but reproducible signs of awareness of themselves or their environment. The presence of behaviours associated with conscious awareness can distinguish MCS from UWS (Giacino et al. 2002; Royal College of Physicians 2013).
UWS and MCS may result from traumatic brain injury (TBI) or non-traumatic brain injury (NTBI) involving conditions such as hypoxia, encephalopathy, stroke, intracranial infection, haemorrhage, or tumour (Pisa et al. 2014). Following TBI or NTBI, there is a risk of increased intracranial pressure (ICP) (Whitfield et al. 2001; Beez et al. 2019). When conservative treatment does not reduce increased ICP, decompressive craniectomy (DC) is often used. DC, in which a portion of the skull is removed to relieve ICP, may be a life-saving procedure (Whitfield et al. 2001; Aarabi et al. 2006; Hutchinson et al. 2016; Zhang et al. 2017; Beez et al. 2019).
Cranioplasty (CP) is often performed following DC to restore cosmesis, improve cerebrospinal fluid dynamics, provide protection to the brain, and prevent sinking skin flap syndrome (Iaccarino et al. 2020). Certain publications have reported that CP may also enhance neurological or functional recovery and improve consciousness (Yamaura and Makino 1977; Agner et al. 2002; Stiver et al. 2008; Di Stefano et al. 2012; Honeybul et al. 2013; Malcolm et al. 2018; Singh et al. 2019). However, CP can also be associated with complications, including seizures, hydrocephalus, infection, epidural collection, intracerebral haemorrhage, unintended intraoperative durotomy, impaired wound healing, implant loosening, need for implant removal, and death (Zanaty et al. 2015; Honeybul and Ho 2016; Alkhaibary et al. 2020; Dang et al. 2021; Hanko et al. 2021). Early performance of CP may have an enhanced clinical effect but may increase the need for surgical revision (Hanko et al. 2021).
Physicians may offer CP to patients with UWS or MCS following DC. Several publications have reported improvement of consciousness, neurological status, or functional condition following CP in these patients (Liang et al. 2007; Stelling et al. 2011; Dang et al. 2021; Hanko et al. 2021). However, the studies described in these publications did not include control groups, and most of them did not control for the time from brain lesion onset to DC or from DC to CP. The type of DOC before CP and the contribution of time to improvement after CP are therefore uncertain, which casts doubt on the efficacy of CP in these patients. Moreover, a study that controlled for time from the DC to CP did not show improvement (Stelling et al. 2011). The aim of the present study was to assess CP for patients with UWS and MCS, comparing the recovery of communication and consciousness, survival, length of stay (LOS) in Intensive Care and Consciousness Rehabilitation (ICCR), and complications of DOC in patients who underwent CP after DC with the same outcomes of a control group that underwent only DC.
Methods
Patients
Included in the study were patients who were admitted consecutively to the ICCR Department at a rehabilitation medical centre following TBI or NTBI between 2002 and 2018. In ICCR, the patients received medical interventions to prevent and treat complications of the brain injury, and various measures were taken to enable optimal functioning and enhance recovery of awareness (Aidinoff et al. 2018). Patients who had undergone DC were offered CP, usually at least 3 months after the DC, when they were stable and without infections, and after a neurosurgical consultation.
Inclusion criteria were age above 16 years and a clinical diagnosis of UWS or MCS for at least 28 days. The exclusion criterion was lack of evidence of DC performed before the admission to ICCR.
Procedure
Clinical and demographic data were collected from the patients’ medical records. UWS or MCS were diagnosed on the basis of Coma Recovery Scale-Revised (CRS-R) scores (Giacino et al. 2004). The scores were retrieved from the medical records or evaluated retrospectively on the basis of recorded assessments. Additional data included TBI or NTBI aetiology, dates of brain lesion onset, ICCR admission and discharge, DC and CP, and the presence and date of first record of brain injury complications, including hydrocephalus (with or without shunt insertion), infection of shunt, pneumonia, urinary tract infection (UTI), meningitis, epileptic seizures, and complication of CP itself. Recovery of consciousness (e.g. emergence from UWS to MCS or emergence from MCS) was determined on the basis of CRS-R scores at admission and at discharge (Giacino et al. 2004). Level of communicativeness was evaluated using the Loewenstein Communication Scale (LCS), which was found to be reliable and predictive of rehabilitation progress in patients with DOC (Borer-Alafi et al. 2002). Data on mortality before 2023 were retrieved from the Israeli population registry.
Statistical analysis
Separate statistical analyses were conducted for the entire study group and the UWS and MCS groups. Logarithmic transformations were conducted on continuous variables when appropriate. LCS and CRS-R gains were calculated by subtracting the patients’ scores at admission to ICCR from their values at discharge. The significance of the difference in admission and discharge LCS and CRS-R scores between patients who underwent CP and those who did not was assessed by independent samples t-tests. The relationships between undergoing CP and other patient characteristics with LCS gain and LOS were evaluated using t-tests and Pearson correlations, followed by ANCOVA. To evaluate the effect of CP and patient characteristics on consciousness recovery, we used chi-squared tests and t-tests, followed by logistic regression. Survival was analysed using the Kaplan–Meier method and the logrank (Mantel–Cox) test for differences between patients who underwent CP and those who did not. Complication rates were compared using independent samples t-tests, chi-squared tests and logistic regressions to control for LOS. The same tests were used to compare survival, recovery, and complication rates between patients who underwent CP within 120 days after DC (early CP) and those who underwent CP later (late CP). P-values lower than 0.05 were considered significant. Using this level of significance, the sample size in the present study was sufficient for detection of an effect size (risk difference) of 0.25 or more between groups, with a power of 0.84. Statistical analyses were performed using the Statistical Package for the Social Sciences (ver. 28, SPSS, Chicago, IL, USA).
Results
Patient characteristics
A total of 518 patients with UWS or MCS admitted consecutively to the ICCR between 2002 and 2018 were enrolled in the study. Excluded were 312 patients who did not undergo a DC and 62 who failed to meet UWS or MCS criteria. Included in the study were 144 participants whose mean age was 40 years (s.d. = 17), 110 (76%) of whom were males. At admission, 108 of them (75%; mean age = 40 years; s.d. = 17; range: 16–77) had CRS-R scores compatible with UWS: 0–2 for the auditory, motor, and oromotor functions, 0–1 for the visual functions, and 0 for the communication functions. Thirty-six patients (25%; mean age = 42 years; s.d. = 18; range: 19–71) had CRS-R score compatible with MCS criteria: 3–5 for the auditory, motor, and oromotor functions, 2–5 for the visual functions, and 1 for the communication functions (Giacino et al. 2004). Of all the included patients, 116 (81%) had TBI and 28 (19%) had NTBI. Fifty-three patients (37%) had a CP following DC before discharge from ICCR. Of these, seven patients (13%) had a CP before admission to ICCR. The average time from brain lesion onset to admission to the ICCR was 62 days (s.d. = 50; range: 19–397) and to CP was 160 days (s.d. = 103; range: 43–715), which means that all CPs were performed more than 28 days from brain lesion onset. Table 1 presents the characteristics of the patients who underwent CP and those who did not. In patients who underwent only DC vs those who also underwent CP after admission to the ICCR department (87% of all the patients who had CP), admission LCS values were 18 (s.d. = 9) vs 14 (s.d. = 6) (P < 0.05), and admission CRS-R values were 5.8 (s.d. = 4.0) vs 5.3 (s.d. = 3.5) (P > 0.05), respectively.
| DC | DC + CP | P-value | ||
|---|---|---|---|---|
| Number of patients | 91 | 53 | ||
| Age, mean (s.d.) | 41 (17) | 39 (18) | >0.5 | |
| Male sex, n (%) | 72 (79%) | 38 (72%) | >0.3 | |
| Years of education, mean (s.d.) | 12 (3) | 12 (3) | >0.7 | |
| Traumatic aetiology, n (%) | 68 (75%) | 48 (91%) | <0.03 | |
| UWS at admission, n (%) | 64 (70%) | 44 (83%) | >0.05 | |
| Time to admission, days, mean (s.d.) | 56 (36) | 72 (67) | >0.1 | |
| Time to DC, days, mean (s.d.) | 3 (10) | 1 (2) | >0.05 | |
| Time to CP, days, mean (s.d.) | 160 (103) | |||
| Time to discharge, days, mean (s.d.) | 113 (76) | |||
| LCS score at admission, mean (s.d.) | 18 (9) | 15 (6) | <0.05 | |
| CRS-R score at admission, mean (s.d.) | 5.8 (4.1) | 5.4 (3.4) | >0.5 | |
| LOS, days, mean (s.d.) | 125 (109) | 198 (97) | <0.001 |
DC, decompressive craniectomy; CP, cranioplasty; UWS, unresponsive wakefulness syndrome; Time to admission, time from brain lesion onset to admission to ICCR; Time to DC, time from brain lesion onset to decompressive craniectomy; Time to CP, time from brain lesion onset to cranioplasty; Time to discharge, time from cranioplasty to discharge from ICCR; LCS, Loewenstein Communication Scale; CRS-R, Coma Recovery Scale-Revised; ICCR, Intensive Care and Consciousness Rehabilitation; LOS, length of stay in ICCR.
Effect of cranioplasty on communication and consciousness recovery
Admission LCS scores were slightly higher in patients after DC who did not undergo CP before discharge from the ICCR than in those who underwent CP (18 ± 9 and 15 ± 6, respectively, P < 0.05, Table 1). At discharge, the difference between groups was not significant (30 ± 19 and 32 ± 19, respectively, P > 0.6). LCS gain was 12 ± 17 for the patients who did not undergo CP and 16 ± 17 for those who did, but the difference between groups was not significant even after controlling for admission LCS scores, LOS, age, and sex (P < 0.1). When assessed within the UWS and MCS subgroups, CP did not significantly affect LCS scores or gain (Table 2, P > 0.2).
| Subgroup | Admission LCS ± s.d. | Discharge LCS ± s.d. | LCS gain ± s.d. | Recovery (%) | Cumulative survival ± s.e. | LOS ± s.d. | |
|---|---|---|---|---|---|---|---|
| UWS, DC only | 14 ± 5 | 25 ± 19 (n = 48) | 11 ± 17 | 27 (43) | 0.40 ± 0.08 | 145 ± 116 | |
| UWS, with CP | 13.5 ± 5 | 29 ± 20 (n = 42) | 16 ± 19 | 26 (59) | 0.46 ± 0.10 | 204 ± 99 | |
| P-value | >0.4 | >0.2 | >0.2 | >0.1 | >0.4 | <0.001 | |
| MCS, DC only | 25 ± 10 | 40 ± 17 (n = 24) | 15 ± 15 | 18 (69) | 0.71 ± 0.09 | 79 ± 71 | |
| MCS, with CP | 23 ± 6 | 41 ± 10 (n = 9) | 19 ± 7 | 5 (56) | 0.37 ± 0.27 | 167 ± 82 | |
| P-value | >0.5 | >0.7 | >0.4 | >0.7 | >0.8 | <0.01 |
DC, decompressive craniectomy; CP, cranioplasty; LCS, Lowenstein Communication Scale; UWS, unresponsive wakefulness syndrome; MCS, minimally conscious state; LOS, length of stay in ICCR (days); s.d., standard deviation; s.e., standard error; Cumulative survival, survival rate at the end of follow-up.
Admission CRS-R scores of the entire examined patient group were 5.6 (s.d. = 3.8), and discharge scores were 12.3 (s.d. = 7.5). Of 142 patients (107 patients admitted with UWS, and 35 with MCS) with complete data on recovery, which was defined as progress from UWS to MCS or from UWS or MCS to full consciousness, 76 (53%) recovered. Twenty-one (15%) patients who had admission CRS-R scores compatible with the UWS criteria achieved CRS-R scores compatible with the MCS criteria at discharge. Thirty-two (22%) patients who had admission CRS-R scores compatible with the UWS criteria regained full consciousness. Twenty-three (16%) patients who had admission CRS-R scores compatible with the MCS criteria regained full consciousness. Altogether, 55 (39%) regained full consciousness: the CRS-R score they achieved was either six for the motor function or two for the communication function (Giacino et al. 2004).
Of the 89 patients with complete recovery data who underwent only DC, 45 (51%) recovered, and of those 53 who also had CP, 31 (58%) recovered (P > 0.2).The differences between the groups were not significant, even after controlling for age, sex, LCS score at admission, and a state of UWS or MCS at admission. When assessed separately, in the UWS or MCS subgroups, there was no significant difference in recovery between patients who had or did not have CP (P > 0.1, Table 2).
Effect of cranioplasty on survival
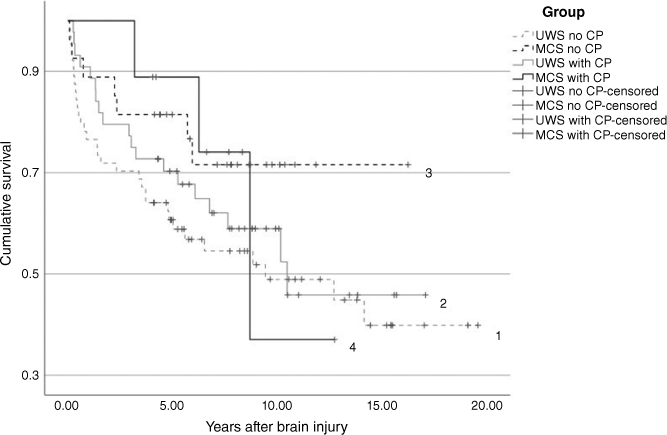
The survival rate at 1 year after injury was 0.80 (s.e. = 0.04) for those who underwent only DC and 0.93 (s.e. = 0.04) for those who also had CP. The 5-year survival rate was 0.67 (s.e. = 0.05) for those who underwent only DC and 0.73 (s.e. = 0.06) for those who also had CP. The corresponding survival rates at the end of the follow-up (2 months to 20 years after injury) were 0.50 (s.e. = 0.06) and 0.48 (s.e. = 0.09) (P > 0.6). When assessed in subgroups, this difference was also non-significant for patients in the UWS or MCS subgroups (Fig. 1, Table 2).
Effect of cranioplasty on LOS
LOS was longer in patients who underwent CP after DC than in those who underwent only DC. The same was true within the UWS and MCS subgroups (P < 0.001, Tables 1 and 2). The effect of CP on LOS was significant even after controlling for admission LCS, although LOS was longer for patients with lower LCS scores at admission for both the entire group (P < 0.04) and the MCS subgroup (P < 0.008).
Cranioplasty and complications
Complications that were found in patients with UWS or MCS following TBI or NTBI are listed in Table 3. Among these are complications that were more plausibly not associated with the CP (DOC complications) and those that were more plausibly associated with the CP itself (CP complications).
| DC, No CP (n = 91) | CP (n = 53) | Before CP | After CP | Total (n = 144) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | ||
| DOC complications | |||||||||||
| HCP | 60 | 66 | 42 | 79 | 41 | 77 | 1 | 2 | 102 | 71 | |
| Pneumonia | 45 | 49 | 29 | 55 | 22 | 42 | 7 | 13 | 74 | 51 | |
| UTI | 24 | 26 | 19 | 36 | 11 | 21 | 8 | 15 | 43 | 30 | |
| Seizures | 14 | 15 | 14 | 26 | 7 | 13 | 7 | 13 | 28 | 19 | |
| Meningitis | 18 | 20 | 9 | 17 | 7 | 13 | 2 | 4 | 27 | 19 | |
| Pressure ulcer | 13 | 14 | 11 | 21 | 9 | 17 | 2 | 4 | 24 | 17 | |
| Shunt infection | 13 | 14 | 4 | 8 | 1 | 2 | 3 | 6 | 17 | 12 | |
| Sepsis A | 10 | 11 | 10 | 7 | |||||||
| Intracranial infection (brain infection or abscess) A | 7 | 8 | 1 | 2 | 1 | 2 | 8 | 6 | |||
| PNBF | 3 | 3 | 4 | 8 | 1 | 2 | 3 | 6 | 7 | 5 | |
| DVT | 5 | 5 | 5 | 3 | |||||||
| Gastrointestinal bleeding | 1 | 1 | 1 | 0.7 | |||||||
| Panhypopituitarism | 1 | 2 | 1 | 2 | 1 | 0.7 | |||||
| SIADH | 1 | 2 | 1 | 2 | 1 | 0.7 | |||||
| Extracranial infection (DC wound) A | 1 | 1 | 1 | 0.7 | |||||||
| Intracranial bleeding (of brain) A | 1 | 2 | 1 | 2 | 1 | 0.7 | |||||
| CP complications | |||||||||||
| Extracranial infection (CP wound infection) | 6 | 11 | 6 | 11 | 6 | 4 | |||||
| Intracranial infection (brain or epidural infection or abscess) | 5 | 9 | 5 | 9 | 5 | 3 | |||||
| Intracranial bleeding (epidural hematoma) | 3 | 6 | 3 | 6 | 3 | 2 | |||||
| Extracranial bleeding (CP wound or other hematoma of the head) | 2 | 4 | 2 | 4 | 2 | 1 | |||||
| Sepsis | 2 | 4 | 2 | 4 | 2 | 1 | |||||
| Uninfected CP site epidural collection | 1 | 2 | 1 | 2 | 1 | 0.7 | |||||
DC, decompressive craniectomy; CP, cranioplasty; DOC complications, complications that were found in this study in the patients with UWS or MCS after TBI or NTBI and most probably were not related to CP; CP complications, complications that most probably were related to CP itself; HCP, hydrocephalus; UTI, urinary tract infection; PNBF, periarticular new bone formation; DVT, deep vein thrombosis; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Of the total group of 144 examined patients, 137 (95%) had at least one DOC or CP complication. The average rate of all complications (DOC + CP complications) was 12% (s.d. = 18%) for the entire patient population, 13% (s.d. = 20%) for the CP group, and 11% (s.d. = 17%) for the DC-only group (P > 0.6, Table 3). The average number of all complications per patient was 2.5 (s.d. = 1.4) for the entire patient population, 2.9 (s.d. = 1.3) for the CP group and 2.3 (s.d. = 1.3) for the DC-only group (P = 0.047).
A total of 136 patients (94%) from the entire group of examined patients had at least one DOC complication, and 19 (13%) had at least one CP complication. The average rate of DOC complications was higher than that of DOC + CP complications, because the lower rate of the CP complications reduced the average. The average rate of DOC complications was 15% (s.d. = 20%) for the entire patient population, 16% (s.d. = 23%) for the CP group, and 15% (s.d. = 18%) for the DC-only group (P > 0.7, Table 3). The average number of DOC complications per patient was 2.4 (s.d. = 1.3) for the entire patient population, 2.5 (s.d. = 1.2) for the CP group, and 2.3 (s.d. = 1.3) for the DC-only group (P > 0.6). The rates of the most frequent DOC complications in the entire patient population were 71% for hydrocephalus (of whom 85% were given a ventriculoperitoneal shunt), 51% for pneumonia, 30% for UTI, 19% for seizures, 19% for meningitis, 17% for pressure sores, and 12% for shunt infection (Table 3). In the DC-only group, 84 patients (92%) had DOC complications, and in the CP group 52 patients (98%) had DOC complications (P > 0.5). Of these complications, hydrocephalus appeared in 42 patients (79% of the patients in this group), and all of them but one were diagnosed before the CP. In 3 of these 42 patients, no shunt was inserted. Of the remaining 39 patients who had both shunt insertion and CP, 5 (13%) had CP before shunt insertion (in one of them, hydrocephalus was diagnosed after the CP), 10 (26%) had the two procedures performed together, and 24 (61%) had shunt insertion first. In seven patients with hydrocephalus diagnosed before CP, no shunt was inserted before the CP. In one of them, the hydrocephalus resolved following CP.
CP complications appeared in 19 patients (36%) in the CP group. The rate of these complications (intracranial or extracranial bleeding, infection, or non-infected fluid collection) was 2–11% (6% on average) in this group, whereas the rate of similar complications in the DC-only group was 0–11% (3.3% on average, P > 0.2, Table 3).
The rate of most of the complications was not significantly different between the DC-only and the CP groups in patients with UWS as well as in patients with MCS (P > 0.2, Table 4). However, shunt infections were more common in the DC-only group, for all the patients combined, and in the UWS group (P < 0.05, Table 4). In the MCS subgroup, significantly more seizures were diagnosed in patients who underwent CP (Table 3), but these patients had more seizures before CP as well (P < 0.04, Table 4).
| Consciousness state | UWS | MCS | All | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CP | No CP | P-value | CP | No CP | P-value | CP | No CP | P-value | ||
| Number of patients | 44 | 64 | 9 | 27 | 53 | 91 | ||||
| Seizures, n (%) | 6 (14%) | 11 (17%) | >0.8 | 8 (89%) | 3 (11%) | 0.003 | 14 (26%) | 14 (15%) | 0.063 | |
| Pneumonia, n (%) | 26 (59%) | 36 (56%) | >0.6 | 3 (33%) | 9 (33%) | >0.9 | 29 (55%) | 45 (49%) | >0.5 | |
| Hydrocephalus, n (%) | 35 (79.5%) | 46 (72%) | >0.7 | 7 (78%) | 14 (52%) | >0.6 | 42 (79%) | 60 (66%) | >0.4 | |
| Shunt infection, n (%) | 2 (5%) | 10 (15%) | 0.035 | 2 (22%) | 3 (11%) | >0.8 | 4 (8%) | 13 (14%) | 0.049 | |
| Urinary infection, n (%) | 16 (36%) | 18 (28%) | >0.4 | 3 (33%) | 6 (22%) | >0.9 | 19 (36%) | 24 (26%) | >0.4 | |
| Meningitis, n (%) | 7 (16%) | 12 (19%) | >0.7 | 2 (22%) | 6 (22%) | >0.7 | 9 (17%) | 18 (20%) | >0.6 | |
| Pressure ulcer, n (%) | 8 (18%) | 10 (16%) | >0.6 | 3 (33%) | 3 (11%) | >0.2 | 11 (21%) | 13 (14%) | >0.2 | |
| Sepsis, n (%) | 8 (18%) | 2 (4.5%) | >0.3 | 2 (22%) | 0 | >0.9 | 2 (4%) | 10 (11%) | >0.3 | |
Consciousness state, consciousness state at admission; UWS, unresponsive wakefulness syndrome; MCS, minimally conscious state; CP, cranioplasty.
Effects of early vs late CP
Most of the effects of early CP, performed within 120 days of DC in 43% of the patients, were non-significantly different from those of late CP, performed after 120 days in 57% of patients. For the early and late CP groups, LCS gain values were 14 (s.d. = 16) and 20 (s.d. = 18) (P > 0.2), consciousness recovery rates were 61% and 52% (P > 0.3), the estimated 5-year survival rates were 0.703 (s.e. = 0.103) and 0.713 (s.e. = 0.086), and the estimated survival rates at the end of follow-up were 0.703 (s.e. = 0.103) and 0.207 (s.e. = 0.121) (P > 0.1), respectively. The rates of pneumonia were 57% and 54% (P > 0.5), of seizures 24% and 32% (P > 0.3), of hydrocephalus 91% and 75% (>0.1), of shunt infections 6% and 15% (P > 0.4), of meningitis 14% and 21% (P > 0.5), and of pressure ulcers 23% and 21% (P > 0.9), respectively. The rates of complications of CP itself, such as infections and epidural collections, were 42.1% and 30.7%, respectively (P > 0.3). Only UTI rates were significantly higher after late CP: they occurred in 19% of the patients after early CP and in 50% after late CP (P < 0.05).
Discussion
This is the first study that compared outcomes of patients with DOC who underwent CP after DC with those in a control group of patients who underwent only DC. Other studies that assessed outcomes after CP in these patients did not include control groups (Liang et al. 2007; Stelling et al. 2011; Dang et al. 2021; Hanko et al. 2021). The present study assessed the effect of CP on communication, consciousness recovery, and survival, and its association with complications after UWS or MCS, and with LOS.
Main findings and their implications
The average improvement in scores for communicative performance and the rate of consciousness recovery were higher in the group of patients with DOC who underwent CP after DC than in the group that did not. One- and 5-year survival rates of patients who underwent CP were somewhat higher than those of patients who had only DC before or during ICCR rehabilitation. These differences between groups did not reach statistical significance, however, and survival rates at the end of the follow-up were similar for the two groups.
Thus, we did not demonstrate the contribution of CP to brain lesion recovery or to survival in patients with DOC. Not being able to show the contribution of CP suggests that CP did not improve recovery or survival in these patients, but it may also reflect the influence of factors that affect assignment to CP and differences in the effect of characteristics of the CP and DC-only groups on outcomes. Patient assignment to CP was not random. According to its policy, and consistent with customary considerations for CP (Kim et al. 2023), the ICCR department referred for CP every patient 3 months or more after DC provided the patient was stable and free of infections. But many of the patients did not undergo CP after DC during their stay in ICCR for several reasons: (a) the neurosurgeons considered some additional procedure before CP; (b) the availability of the neurosurgical facility was limited for non-urgent procedures, and the patients had infection or displayed medical instability when the neurosurgical facility was available; or (c) the ICCR department postponed CP to allow earlier use of the available neurosurgical facility for patients experiencing limited functional recovery.
Therefore, the DC-only group included patients with medical conditions that were less severe than those of the DC + CP group as well as patients with more severe medical conditions. The admission LCS, which was higher for the DC-only group, may indicate that the overall data were biased against the DC + CP group. We addressed this potential bias by controlling for admission LCS when comparing recovery in patients who underwent DC only and those who also underwent CP. The CP effect on recovery remained, however, non-significant. We also found a non-significant difference in admission CRS-R scores between patients who underwent DC only and those who also underwent CP after admission to the ICCR department. This reduced the probability that differences in brain lesion severity between the above groups limited the CP effect, if they were evident at admission.
Not being able to demonstrate the contribution of CP may also reflect the relatively small sample available for this study. The power calculation indicated that with the available sample, analyses had a high probability of identifying risk differences only if they were 0.25 or higher. This means that analyses could have missed small contributions of CP to recovery or survival, but also that if there was a CP contribution to these, it was rather small. In any case, CP did not reduce the odds of recovery or survival, but we cannot rule out its contribution to these.
In addition, it seems that CP did not substantially increase the complication rate in the examined population. The majority of the patients included in the study contracted at least one DOC or CP complication. The number of DOC + CP complications per patient and the average rate of all these complications were somewhat higher in the CP group, but the rate difference was not statistically significant. The differences in DOC complication rates and in the rates of most of the complications in both patients with UWS and patients with MCS were not statistically significant either.
CP complications, which were the complications that CP itself added, were relatively frequent: they appeared in 36% of the patients who underwent CP. These complications were more frequent in the present study than in several previous ones (Morton et al. 2018; Singh et al. 2019; Dang et al. 2021; Mee et al. 2022) and less frequent than in another study (Hanko et al. 2021). The difference between the rate of CP complications and that of similar complications in the DC-only group was not significant, however. Moreover, the rate of most DOC complications in the CP group was higher before the CP than after it. This may explain the marginal contribution of CP to the overall complication rate after DOC, despite the fact that the operation itself added complications.
Hydrocephalus, which was the most common DOC complication we found, was diagnosed and treated after CP in only one patient. Of seven patients with hydrocephalus diagnosed in the CP group before CP who underwent no shunt insertion before the CP, in only one was hydrocephalus resolved following CP. It appears, therefore, that on one hand, CP did not increase the risk for most DOC complications, and on the other, it did not resolve hydrocephalus in most of the relevant patients.
The longer LOS in the DC + CP group may be attributed to various factors, including the waiting time for neurosurgical services, the time required for recovery from the surgical intervention, discontinuity of the rehabilitation procedures, and specific complications of the CP itself. But it is also possible to attribute it mainly to the more severe brain lesions in this group, as indicated by lower admission LCS scores in the patients who underwent CP and the association found between LOS and lower LCS scores. A previous study also attributed longer LOS in DOC to more severe brain lesions (Katz et al. 2009).
Early vs late CP
Because offering CP to patients after DC is customary (Kim et al. 2023), most of the patients in the DC-only group were actually assigned to late CP. Some of the patients who underwent CP during ICCR, which was associated with a longer LOS in this group, also had late CP. Comparing patients who underwent early vs late CP during ICCR showed no significant differences in outcomes, in the majority of DOC complications, and in the complications of CP itself. These findings are consistent with those of some other publications (Stelling et al. 2011), although some studies found early CP to be associated with an enhanced effect on neurological recovery (Malcolm et al. 2018; Dang et al. 2021) or with a greater need for revision due to complications (Hanko et al. 2021). Our findings suggest that early performance does not compromise the effect of CP on recovery, survival, and complications, although postponing CP up to ~9 months after DC (Table 1) may not compromise the effect of CP either.
Clinical inferences
Although outcomes tended to be better after CP, our findings do not provide sufficient support for performing CP on patients with DOC to increase the odds of improvement in communication, consciousness recovery, or survival. However, the fact that we cannot rule out the contribution of CP to recovery or survival in patients with DOC after DC and that we did not find a significant increase in complications after CP favours the continuation of offering CP to these patients. The fact that early CP did not increase the risk of CP complications supports offering early CP. These facts support performing CP for other purposes, such as restoring cosmesis, improving cerebrospinal fluid dynamics, providing protection to the brain, and preventing sinking skin flap syndrome (Iaccarino et al. 2020). CP can be carried out for such purposes, bearing in mind the possibility of the contribution of CP to brain recovery and survival, without concern for its negative effects.
Limitations
Non-random patient assignment to CP, which could bias the effect of CP, and the limited number of patients with DOC, which may have restricted the ability of the findings to reach statistical significance, limit the inferences of this study. They do not negate support, however, for performing CP after DC.
Conclusion
The effect of CP on communication, consciousness recovery, and survival may be positive, but it did not prove to be significant, nor was the association of CP with complications after UWS or MCS in patients with DOC significant. These findings support performing CP on patients with DOC as it may contribute to brain recovery and survival, and achieve additional goals.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
The research was supported by a Loewenstein Rehabilitation Medical Center fund [grant number KM600010301].
Ethics standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The institutional review board (IRB) of the rehabilitation medical center approved the study before its commencement (ID: 0022-15-LOE). The requirement for informed consent from the study subjects was waived by the IRB due to the retrospective study design.
Acknowledgements
The authors would like to thank Uri Bireman for assistance in data collection and Fabian Poznanski for assisting with the literature review.
Author contributions
E. A., A. C. & A. O. contributed to conceptualisation and design of the study. Data were collected by L. F., A. K., H. L. & I. D. and analysed by L. F. and I. G., E. A., H. L., A. C., A. K. & L. F., interpreted data, drafted the manuscript and conducted its preparation. I. G., I. D., and A. O. revised it critically. All the authors agree to be accountable for all aspects of the work.
References
Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM (2006) Outcome following decompressive craniectomy for malignant swelling due to severe head injury. Journal of Neurosurgery 104(4), 469-479.
| Crossref | Google Scholar | PubMed |
Agner C, Dujovny M, Gaviria M (2002) Neurocognitive assessment before and after cranioplasty. Acta Neurochirurgica 144(10), 1033-1040.
| Crossref | Google Scholar | PubMed |
Aidinoff E, Groswasser Z, Bierman U, Gelernter I, Catz A, Gur-Pollack R (2018) Vegetative state outcomes improved over the last two decades. Brain Injury 32(3), 297-302.
| Crossref | Google Scholar | PubMed |
Alkhaibary A, Alharbi A, Alnefaie N, Oqalaa Almubarak A, Aloraidi A, Khairy S (2020) Cranioplasty: A Comprehensive Review of the History, Materials, Surgical Aspects, and Complications. World Neurosurgery 139, 445-452.
| Crossref | Google Scholar | PubMed |
Beez T, Munoz-Bendix C, Steiger HJ, Beseoglu K (2019) Decompressive craniectomy for acute ischemic stroke. Critical Care (London, England) 23(1), 209.
| Crossref | Google Scholar | PubMed |
Borer-Alafi N, Gil M, Sazbon L, Korn C (2002) Loewenstein communication scale for the minimally responsive patient. Brain Injury 16(7), 593-609.
| Crossref | Google Scholar | PubMed |
Dang Y, Ping J, Guo Y, Yang Y, Xia X, Huang R, Zhang J, He J (2021) Cranioplasty for patients with disorders of consciousness. Annals of Palliative Medicine 10(8), 8889-8899.
| Crossref | Google Scholar | PubMed |
Di Stefano C, Sturiale C, Trentini P, Bonora R, Rossi D, Cervigni G, Piperno R (2012) Unexpected neuropsychological improvement after cranioplasty: a case series study. British Journal of Neurosurgery 26(6), 827-831.
| Crossref | Google Scholar | PubMed |
Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, Kelly JP, Rosenberg JH, Whyte J, Zafonte RD, Zasler ND (2002) The minimally conscious state: definition and diagnostic criteria. Neurology 58(3), 349-353.
| Crossref | Google Scholar | PubMed |
Giacino JT, Kalmar K, Whyte J (2004) The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Archives of Physical Medicine and Rehabilitation 85(12), 2020-2029.
| Crossref | Google Scholar | PubMed |
Hanko M, Cmarkova K, Hanzel R, Snopko P, Opsenak R, Kolarovszki B (2021) Analysis of clinical efficiency and early postoperative complications after cranioplasty. Bratislavske Lekarske Listy 122(7), 461-468.
| Crossref | Google Scholar | PubMed |
Honeybul S, Ho KM (2016) Cranioplasty: morbidity and failure. British Journal of Neurosurgery 30(5), 523-528.
| Crossref | Google Scholar | PubMed |
Honeybul S, Janzen C, Kruger K, Ho KM (2013) The impact of cranioplasty on neurological function. British Journal of Neurosurgery 27(5), 636-641.
| Crossref | Google Scholar | PubMed |
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, et al. (2016) Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. The New England Journal of Medicine 375(12), 1119-1130.
| Crossref | Google Scholar | PubMed |
Iaccarino C, Kolias AG, Roumy LG, Fountas K, Adeleye AO (2020) Cranioplasty Following Decompressive Craniectomy. Frontiers in Neurology 10, 1357.
| Crossref | Google Scholar | PubMed |
Katz DI, Polyak M, Coughlan D, Nichols M, Roche A (2009) Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1-4 year follow-up. Progress in Brain Research 177, 73-88.
| Crossref | Google Scholar | PubMed |
Kim JH, Choo YH, Jeong H, Kim M, Ha EJ, Oh J, Lee S (2023) Recent Updates on Controversies in Decompressive Craniectomy and Cranioplasty: Physiological Effect, Indication, Complication, and Management. Korean Journal of Neurotrauma 19(2), 128-148.
| Crossref | Google Scholar | PubMed |
Liang W, Xiaofeng Y, Weiguo L, Gang S, Xuesheng Z, Fei C, Gu L (2007) Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. The Journal of Craniofacial Surgery 18(3), 526-532.
| Crossref | Google Scholar | PubMed |
Malcolm JG, Rindler RS, Chu JK, Chokshi F, Grossberg JA, Pradilla G, Ahmad FU (2018) Early Cranioplasty is Associated with Greater Neurological Improvement: A Systematic Review and Meta-Analysis. Neurosurgery 82(3), 278-288.
| Crossref | Google Scholar | PubMed |
Mee H, Anwar F, Timofeev I, Owens N, Grieve K, Whiting G, Alexander K, Kendrick K, Helmy A, Hutchinson P, Kolias A (2022) Cranioplasty: A Multidisciplinary Approach. Frontiers in Surgery 9, 864385.
| Crossref | Google Scholar | PubMed |
Morton RP, Abecassis IJ, Hanson JF, Barber JK, Chen M, Kelly CM, Nerva JD, Emerson SN, Ene CI, Levitt MR, Chowdhary MM, Ko AL, Chesnut RM (2018) Timing of cranioplasty: a 10.75-year single-center analysis of 754 patients. Journal of Neurosurgery 128(6), 1648-1652.
| Crossref | Google Scholar | PubMed |
Pisa FE, Biasutti E, Drigo D, Barbone F (2014) The prevalence of vegetative and minimally conscious states: a systematic review and methodological appraisal. The Journal of Head Trauma Rehabilitation 29(4), E23-E30.
| Crossref | Google Scholar | PubMed |
Singh S, Singh R, Jain K, Walia B (2019) Cranioplasty following decompressive craniectomy – analysis of complication rates and neurological outcomes: a single center study. Surgical Neurology International 10, 142.
| Crossref | Google Scholar | PubMed |
Stelling H, Graham L, Mitchell P (2011) Does cranioplasty following decompressive craniectomy improve consciousness? British Journal of Neurosurgery 25(3), 407-409.
| Crossref | Google Scholar | PubMed |
Stiver SI, Wintermark M, Manley GT (2008) Reversible monoparesis following decompressive hemicraniectomy for traumatic brain injury. Journal of Neurosurgery 109(2), 245-254.
| Crossref | Google Scholar | PubMed |
Whitfield PC, Patel H, Hutchinson PJ, Czosnyka M, Parry D, Menon D, Pickard JD, Kirkpatrick PJ (2001) Bifrontal decompressive craniectomy in the management of posttraumatic intracranial hypertension. British Journal of Neurosurgery 15(6), 500-507.
| Crossref | Google Scholar | PubMed |
Yamaura A, Makino H (1977) Neurological deficits in the presence of the sinking skin flap following decompressive craniectomy. Neurologia Medico-Chirurgica 17(1 Pt 1), 43-53.
| Crossref | Google Scholar | PubMed |
Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M, Schwartz E, Kunkel ES, Efthimiadis-Budike AS, Jabbour P, Dalyai R, Rosenwasser RH, Tjoumakaris SI (2015) Complications following cranioplasty: incidence and predictors in 348 cases. Journal of Neurosurgery 123(1), 182-188.
| Crossref | Google Scholar | PubMed |
Zhang D, Xue Q, Chen J, Dong Y, Hou L, Jiang Y, Wang J (2017) Decompressive craniectomy in the management of intracranial hypertension after traumatic brain injury: a systematic review and meta-analysis. Scientific Reports 7(1), 8800.
| Crossref | Google Scholar | PubMed |