Health literacy after traumatic brain injury: characterisation and control comparison
Amelia J. Hicks A * , Angelle M. Sander B C , Dean P. McKenzie D E , Sarah Carrier A , Elinor Fraser A , Bronwyn Hall A , Monique R. Pappadis B F and Jennie L. Ponsford A
A * , Angelle M. Sander B C , Dean P. McKenzie D E , Sarah Carrier A , Elinor Fraser A , Bronwyn Hall A , Monique R. Pappadis B F and Jennie L. Ponsford A
A
B
C
D
E
F
Abstract
Little is known about health literacy in traumatic brain injury (TBI) survivors. The aims of this study were to compare health literacy in individuals with TBI with that of a control group; to examine the association between health literacy in individuals with TBI and demographic, injury, and cognitive factors; and compare the relationship between health literacy and physical and mental health outcomes.
A cross-sectional observational study design was used. Adults (≥18 years) were recruited from an outpatient research centre in Victoria, Australia. There were 209 participants with a complicated mild to severe TBI at least 1 year previously (up to 30 years 6 months) and 206 control participants.
Individuals with TBI did not have poorer health literacy than controls (IRR = 1.31, P = 0.102, CI95% [0.947, 1.812]). Further analysis could not be completed due to the highly skewed Health Literacy Assessment Using Talking Touchscreen Technology – Short Form (Health LiTT-SF) data.
Health literacy performance in individuals with TBI was not significantly different to controls. Premorbid education may provide a critical cognitive reserve upon which TBI survivors can draw to aid their health literacy. These findings are specific to the Health LiTT-SF measure only and require replication using more comprehensive health literacy measures in culturally diverse samples.
Keywords: control comparison, health literacy, Health Literacy Assessment Using Talking Touchscreen Technology – Short Form, Health LiTT-SF, long term outcomes, null findings, outcomes, TBI, traumatic brain injury.
Introduction
Traumatic brain injury (TBI) is increasingly recognised as a chronic life-long condition that has significant impact on health outcomes. Changes in functional, physical, and emotional health are common, and survivors often develop medical and psychiatric comorbidities (Ponsford et al. 2014; Hammond et al. 2019). This complex health profile means that individuals with TBI often have considerable contact with healthcare services over the years post injury, requiring them to navigate complicated systems and information. Adequate health literacy is critical to successful health management and optimal health outcomes. Little is known about health literacy in TBI survivors, the factors associated with health literacy, and how this may impact health outcomes.
Health literacy is defined as an individual’s capacity to process, understand, and evaluate health-related information, and the ability to use that information in the management of their own health (Yost et al. 2009; Sørensen et al. 2012). Adequate health literacy can empower individuals in the management of their own health care (Nutbeam 2000; Sørensen et al. 2012). However, navigating healthcare systems requires a certain level of health literacy (Griese et al. 2020). This may be particularly challenging within the modern healthcare system where there is a growing expectation that consumers will adopt a more active role in their health management and participate in shared decision making (Barry and Edgman-Levitan 2012; Levin-Zamir and Bertschi 2018). Indeed, there has been a shift towards policies that promote patient involvement and patient choice for those living with chronic conditions and disability (Department of Health 2001; National Disability Insurance Agency 2021). Although these policies importantly emphasise the needs and preferences of consumers, they may result in health disparities for groups with lower health literacy. For example, individuals may not ask the questions required to make informed decisions and have difficulty evaluating between competing options when given choices (Choudhry et al. 2019).
Low health literacy has been associated with numerous key sociodemographic factors in non-TBI cohorts. These include older age, lower education level, lower household income, poorer cognitive function, and being from a culturally and linguistically diverse background or racial minority group (Barber et al. 2009; Hahn et al. 2017, 2020; Jessup et al. 2017; Simpson et al. 2020). For example, compared to 43% of individuals born in Australia, only 33% of individuals born outside Australia have sufficient or higher health literacy (Ethnic Communities’ Council of Victoria (ECCV) 2012). Notably, there is likely to be an overrepresentation of TBI survivors within these sociodemographic groups, with TBI groups frequently presenting with cognitive impairment, lower educational attainment, and lower household income as a result of inability to work (Ponsford et al. 2014). As such, it seems reasonable to suggest that individuals with TBI may have lower health literacy on average compared to the general community, however; this has not been directly examined in research to-date. Despite the lack of a control comparison, one recent large study (n = 205) did report that 31% (n = 64) of a mostly severe TBI sample had inadequate health literacy (Pappadis et al. 2024).
A growing body of research provides evidence for disparities in health outcomes associated with poor health literacy. Although evidence is mixed across studies, low health literacy has been associated with more hospitalisations, poorer medication adherence, poorer overall medical and emotional health status, and increased mortality (Berkman et al. 2011; Zhang et al. 2014; Beauchamp et al. 2015; Neter and Brainin 2019). There have been two studies in the US examining the associations between health literacy in individuals with mostly severe chronic TBI (at least 1 year and on average approximately 6–8 years post injury) and health outcomes (Hahn et al. 2017; Pappadis et al. 2024). These studies found that higher health literacy was associated with better mobility, less anxiety, better overall health (Hahn et al. 2017), higher perceived physical health, and fewer depressive symptoms (Pappadis et al. 2024).
Given the known consequences of poor health literacy on health outcomes, and the frequent exposure to healthcare systems and information that TBI survivors face over many years, it is critical to characterise health literacy in this group. It is unknown whether adequacy of health literacy differs from that of controls, what factors may predict poorer health literacy after TBI and how health literacy may impact health outcomes across the recovery journey post TBI. To address these gaps in the current evidence, this study set out to examine three key aims.
To compare health literacy between individuals with TBI and a control group
To examine the association between health literacy in individuals with TBI and demographic factors (age, sex, years of education, language spoken at home, country of birth, ethnicity), cognitive function, and injury factors (post-traumatic amnesia (PTA) duration and time since injury)
It was hypothesised that individuals who were older, had less years of education, did not speak English at home, were born outside of Australia, were ethnic minorities, had lower cognitive function, and had longer PTA duration would have lower health literacy scores.
Examination of the associations between sex and time since injury with health literacy were exploratory.
To examine whether health literacy in individuals with TBI was associated with poorer physical and mental health
Methods
All participants provided written informed consent prior to any study procedures in accordance with the Declaration of Helsinki. The study was approved by the Monash University Human Research Ethics Committee (#24465).
Participants
Participants were recruited from a database of participants in an ongoing longitudinal head injury outcome study. This parent study is populated through consecutive admissions to the inpatient rehabilitation program at Epworth HealthCare (Victoria, Australia). The inpatient rehabilitation program involves the following per day (approximately): 1–2 h of physiotherapy, 1 h of occupational therapy, 30 min of speech therapy, and up to 1 h of neuropsychology and 1 h of social work per week.
Eligible participants with TBI for the current study: (1) were aged ≥18 years, (2) had sustained a single complicated mild to severe TBI (Glasgow Coma Scale (GCS) score <13 or PTA duration >24 h or intracranial abnormalities on imaging) (Malec et al. 2007), (3) were time post injury ≥1 year and ≤30 years 6 months, and (4) had been discharged from hospital. Controls were recruited from the general community. Eligible controls: (1) were aged ≥18 years and (2) had no history of TBI, concussion, or any loss of consciousness. All participants were required to have: (1) sufficient English language skills and cognitive function to complete study measures, (2) absence of visual impairment, (3) absence of chronic substance abuse or severe psychiatric disturbances, and (4) absence of other neurological conditions. The TBI and control groups were not matched.
Outcome measures
Injury-related information (PTA duration, GCS score, neuroimaging, date of injury) was obtained from medical records. All outcome data were obtained via phone interview and online survey (Supplementary Table S1). The data collection period was August 2020 to February 2022.
Primary outcome measure
The Health Literacy Assessment Using Talking Touchscreen Technology – Short Form (Health LiTT-SF) (Yost et al. 2009, 2010; Hahn et al. 2011) is a self-administered multimedia touchscreen test used to assess health literacy. The Health LiTT meets psychometric standards for measurement of individual participants (high reliability (≥0.90)), especially in the low to middle range of health literacy (Hahn et al. 2011). A 14-item multiple-choice short form developed using item response theory was used for this study (Pappadis et al. 2024). Each item is displayed on the screen and may be read aloud by clicking on a button. Participants responded to three item types: prose (n = 6), document (n = 6), and quantitative (n = 2) (Hahn et al. 2011). The prose items include a short reading passage and require the participant to complete a reading comprehension task, the document items require participants to identify and interpret information presented in charts or prescription labels, and the quantitative items include either text or an image that requires a calculation to answer questions. For example, the participant may be asked to review a medication dosing schedule and respond with how many tablets are required on a certain day and time. The number of correct responses is summed to create a total score (Hahn et al. 2011). A weighted T-score [mean (M) = 50, standard deviation (s.d.) = 10] is generated for each participant. A T-score of ≥55 was used to indicate adequate health literacy, whereas T-scores <55 were used to indicate inadequate/marginal health literacy, (Slesinger et al. 2020). The Health LiTT is publicly available (www.healthlitt.org).
Secondary outcomes measures
The Brief Test of Adult Cognition (BTACT) (Tun and Lachman 2006; Lachman et al. 2014) is a telephone-administered multidimensional test of cognition. It was originally developed for use in the National Survey of Midlife Development in the United States (MIDUS) (Ryff and Lachman 2009, 2018). The cognitive tasks are based on well-established neuropsychological tests, and measure episodic memory, working memory, reasoning, verbal fluency, processing speed, and executive functioning (Lachman et al. 2014). The BTACT has been validated for use in TBI samples (Nelson et al. 2021; DiBlasio et al. 2021a). Consistent with previous TBI studies (Dams-O’Connor et al. 2018), one BTACT task was omitted (Stop and Go Switch task) as participants reported frustration with this task during piloting of the BTACT.
In line with previous work, we computed three standardised summary scores (M = 0, s.d. = 1) for the BTACT: Composite Score, Episodic Memory Factor, and Executive Function Factor (Dams-O’Connor et al. 2018; DiBlasio et al. 2021a, 2021b). All standardised scores were calculated by age decade, education (< or ≥Bachelor’s degree), and sex based on data from the MIDUS II Cognitive Project sample and MIDUS Refresher (Ryff and Lachman 2009, 2018; Lachman et al. 2014; DiBlasio et al. 2021b).
The Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al. 2001) was used to measure the severity of depressive symptoms. The nine-item self-report measure assesses the frequency of each of the depressive symptoms outlined in the DSM-IV Major Depressive Disorder criteria. For each item, participants must select how often they have been bothered by this problem in the last 2 weeks (e.g. ‘Little interest or pleasure in doing things’), from 0 (not at all) to 3 (nearly every day). Scores are added to create a total score (Range 0–27), with higher scores indicating greater depressive symptoms. The PHQ-9 has satisfactory psychometric properties when used in TBI cohorts (Fann et al. 2005; Dyer et al. 2016; Teymoori et al. 2020). PHQ-9 scores of 5, 10, 15, and 20 represent mild, moderate, moderately severe, and severe depression, respectively (Kroenke et al. 2001).
The Generalized Anxiety Disorder-7 (GAD-7) (Spitzer et al. 2006) was used as a measure of the severity of anxiety symptoms. This seven-item self-report measure assesses the frequency of anxiety symptoms (e.g. ‘feeling nervous, anxious, or on edge’). For each item, participants select how frequently they have been bothered by this problem in the last 2 weeks, from 0 (not at all) to 3 (nearly every day). Scores are added to create a total score (Range 0–21), with higher scores indicating greater anxiety symptoms. The GAD-7 has satisfactory psychometric properties when used in TBI cohorts (Teymoori et al. 2020). Cut points of 5, 10, and 15 represent mild, moderate, and severe levels of anxiety, respectively (Spitzer et al. 2006).
Patient-Reported Outcomes Measurement Information System global health measure
The Patient-Reported Outcomes Measurement Information System (PROMIS) global health measure (Hays et al. 2009) evaluates physical functioning, mental health, social health, pain, fatigue, and perceived quality of life. For each item, participants respond on a Likert scale from 1 to 5 (with the exception of the pain item which is 0–10). The response options for each point on the Likert scale vary between items. For this study, a short form was used that contains eight items. Items were summed to produce two sub-scale scores: Physical Health and Mental Health. A T score was assigned to each sub-scale score (M = 50, s.d. = 10), with higher scores indicating better health. The PROMIS has satisfactory psychometric properties when used in TBI cohorts (Patrick et al. 2022).
Health conditions checklist
A medical and mental health comorbidities interview, from the Traumatic Brain Injury Model Systems national database, was used to inquire about the presence of 26 medical (n = 16; e.g. stroke, diabetes) and mental health (n = 10; e.g. depression, anxiety) comorbidities during an individual’s lifetime, and, if present, whether the diagnosis occurred before or after their TBI. All items used the stem ‘Has a doctor ever told you that you had…’. This interview was adapted from the US National Health and Nutrition Examination Survey (Centers for Disease Control and Prevention n.d.) and National Comorbidity Survey Replication (Kessler and Merikangas 2004).
Procedures
Participants were contacted by e-mail or phone and screened for study eligibility. Eligible participants were invited to enrol in the study. Enrolled participants completed a 30–45 min phone interview with a researcher who had completed or were currently enrolled in postgraduate clinical neuropsychology training. Following the phone interview, all participants independently completed an online survey.
Statistical analysis
Analysis was conducted using SPSS (ver. 26, IBM Corporation, New York 2019) unless otherwise stated. All statistical analyses used a significance level of P <0.05. Appropriate tests for assumptions and missing data were completed. Outliers were identified using visual inspection of box plots (Nuzzo 2016) and were retained where results did not substantively differ when outliers were excluded.
Demographic data were compared between groups (TBI vs control) using a t-test, Mann–Whitney U-test, or chi-square test. Group comparisons revealed statistically significant differences between groups for age, sex, and education. To reduce group differences, we conducted coarsened exact matching (CEM) in STATA 17 (Stata Corporation, College Stations, Texas, USA 2021) (Blackwell et al. 2009). This procedure improves the estimation of effects by reducing the imbalance in covariates between strata (Blackwell et al. 2009). To facilitate matching, age was collapsed into three categories (≤35, >35 to ≤55, >55), as was education (<high school, high school, >high school). This created a total of 18 strata: by sex (2), by age (3), by education (3). Participants from the TBI and control groups were assigned to each stratum. Participants were excluded from the matched sample if their stratum did not contain at least one TBI and one control participant. This resulted in the exclusion of 13 participants with TBI (n = 196 individuals with TBI in the ‘Matched’ sample). No control participants were excluded from the matched sample. Because the CEM procedure uses a maximal information approach (i.e. assigns cases to ‘best fitting’ stratum regardless of group), the resulting strata included different numbers of control and TBI participants. To compensate for the different stratum sizes, CEM created a ‘weight’ for each control participant, which reflected the frequency with which the combination of age, sex, and education for that control participant occurs in the TBI group.
For the group comparison analysis (Aim 1), a regression analysis was conducted including the control weights (from the matching procedure) and controlling for group (TBI vs control), age, sex, and years of education. The group comparison analysis was repeated twice: (1) removing five outliers from the TBI group and (2) using the unmatched sample with no weights applied. A negative binomial regression was conducted because the distribution of scores on the Health LiTT-SF was negatively skewed (small number of low scores). As negative binomial regression is generally employed with a positively skewed distribution (small number of high scores), we used a count of the errors made on Health LiTT-SF. Incidence rate ratios (IRR) are presented for negative binomial regressions, allowing us to compare the number of errors between two groups, adjusting for age, sex, and education. In this case the IRR represents the mean error rate for TBI divided by the mean rate for the controls. IRR above one indicates the incidence rate is higher in the TBI group.
The analyses exploring the factors associated with health literacy (Aim 2) and the impact of health literacy on outcomes (Aim 3) could not be conducted due to the very limited variation in Health LiTT-SF scores within the TBI group. This precluded any analyses that attempted to model factors associated with variation in the health literacy score.
Two exploratory analyses are presented. The first analysis examines Health LiTT-SF item-level performance in the TBI and control groups. The second exploratory analysis provides an in-depth descriptive examination of the five individuals with TBI whose poor performance on the Health LiTT-SF suggests they were outliers on this measure (scores were beyond six interquartile ranges).
Results
Participant characteristics
This study included 209 participants with TBI and 206 control participants (Tables 1 and 2).
| Unmatched sample | Matched sample | |||
|---|---|---|---|---|
| Individuals with TBI (n = 209) | Individuals with TBI (n = 196) | Controls (n = 206) | ||
| M (s.d.), Range | M (s.d.), Range | M (s.d.), Range | ||
| Sex (n, %) | ||||
| Male | 145, 69.4% | 133, 67.9% | 76, 36.9% | |
| Female | 64, 30.6% | 63, 32.1% | 130, 63.1% | |
| Current age (years) | 50.23 (15.3), 23.1–84.1 | 49.5 (15.2), 23.1–84.1 | 43.55 (16.8), 18.23–83.1 | |
| Education (years) | 13.5 (2.4), 3–18 | 13.6 (2.5), 3–18 | 14.9 (2.1), 9–20 | |
| Country of birth (n, %) | ||||
| Australia | 178, 85.2% | 167, 85.2% | 129, 62.6% | |
| Outside Australia | 31, 14.8% | 29, 14.8% | 77, 37.4% | |
| Ethnicity (n, %) | ||||
| White | 200, 95.7% | 187, 95.4% | 149, 72.7% | |
| Ethnic minorities | 9, 4.3% | 9, 4.6% | 56, 27.3% | |
| Language spoken at home (n, %) | ||||
| English | 206, 98.6% | 193, 98.5% | 189, 91.8% | |
| Language other than English | 3, 0.5% | 3, 1.5% | 17, 8.3% | |
| Marital status (n, %) | ||||
| Single | 44, 21.1% | 43, 21.9% | 82, 39.8% | |
| Married | 96, 46.0% | 87, 44.4% | 84, 40.8% | |
| Cohabiting | 33, 15.8% | 31, 15.8% | 18, 8.7% | |
| In relationship, not cohabiting | 10, 4.8% | 10, 5.1% | 5, 2.4% | |
| Divorced | 16, 7.7% | 15, 7.7% | 11, 5.3% | |
| Separated | 5, 2.4% | 5, 2.6% | 2, 0.5% | |
| Widowed | 5, 2.4% | 5, 2.6% | 4, 1.9% | |
| Employment (n, %) | ||||
| Employed – full time | 97, 46.6% | 90, 46.2% | 69, 33.5% | |
| Employed – part time/casual | 38, 18.3% | 36, 18.5% | 71, 34.5% | |
| Unemployed | 36, 17.3% | 34, 17.4% | 24, 11.7% | |
| Student | 0 | 0 | 8, 3.9% | |
| Volunteer | 2, 1.0% | 2, 1.0% | 0 | |
| Homemaker | 1, 0.5% | 1, 0.5% | 5, 2.4% | |
| Retired | 34, 16.3% | 32, 16.3% | 29, 3.9% | |
M, mean; s.d., standard deviation. ‘Matched sample’ was matched on sex, age, and years of education. The matched sample excluded 13 participants with TBI. The same control group was used in the Matched and Unmatched sample. Ethnicity was available for 205/206 controls. Employment was available for 208/209 individuals with TBI in the Unmatched sample and 195/196 in the Matched sample. Other ethnicities in the sample were Asian or people who identified as having both white ethnicity as well as Asian or Pacific Islander.
| M (s.d.) | Range | ||
|---|---|---|---|
| Age at injury (years) | 36.0 (15.7) | 14.26–70.63 | |
| Time since injury (years) | 14.2 (7.8) | 1.64–30.41 | |
| Duration of PTA (days) | 25.8 (23.6) | 0–134 | |
| <1 day | 5, 2.4% | ||
| 1–7 days | 45, 22.0% | ||
| >7 days | 155, 75.6% | ||
| GCS | 8.1 (4.3) | 3–15 | |
| 3–8 | 108, 57.8% | ||
| 9–12 | 28, 15.0% | ||
| 13–15 | 51, 27.3% | ||
| CT scan – abnormal (n, %) | 182, 87.1% | ||
| Length of stay (years) | 0.16 (0.16) | 0.01–1.15 | |
| Median; 25th–75th%ile | 0.11 | 0.06–0.18 | |
| Injury mechanism (n, %) | |||
| Motor vehicle accident | 107, 51.2% | ||
| Pedestrian hit by vehicle | 31, 14.8% | ||
| Motorcycle accident | 25, 12.0% | ||
| Bicycle accident | 20, 9.6% | ||
| Fall | 13, 6.2% | ||
| Fall from horse | 3, 1.4% | ||
| Work related injury | 4, 1.9% | ||
| Assault | 2, 1.0% | ||
CT, computed tomography; GCS, Glasgow Coma Scale. PTA was available for 205/209 individuals with TBI. GCS was available for 187/209 individuals with TBI.
Aim 1. Health LiTT-SF performance in individuals with TBI compared to controls
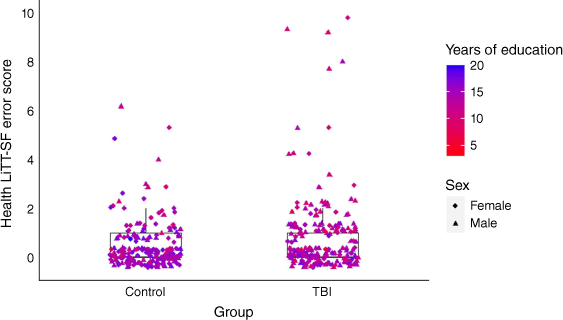
Overall, both groups had highly negatively skewed data, with 94% of individuals with TBI scoring 12–14/14 and 96% of the control group scoring 12–14/14 (Table 3, Fig. 1).
| Unmatched sample | Matched sample | |||
|---|---|---|---|---|
| Individuals with TBI (n = 209) | Individuals with TBI (n = 196) | Controls (n = 206) | ||
| M (s.d.), Range | M (s.d.), Range | M (s.d.), Range | ||
| Health LiTT-SF total score | 13.19 (1.57), 4–14 | 13.18 (1.61), 4–14 | 13.54 (0.94), 8–14 | |
| Health LiTT-SF total errors | 0.81 (1.57), 0–10 | 0.82 (1.6), 0–10 | 0.46 (0.93), 0–6 | |
| Health LiTT-SF T score | 60.64 (5.08), 35.56–63.73 | 60.65 (5.17), 35.56–63.73 | 61.86 (3.52), 44.09–63.73 | |
| Marginal/Inadequate (n, %) | 13, 6.2% | 12, 6.1% | 8, 3.9% | |
| Adequate (n, %) | 196, 93.8% | 184, 93.9% | 198, 96.1% |
‘Matched sample’ was matched on sex, age, and years of education. The matched sample excluded 13 participants with TBI. The same control group was used in the Matched and Unmatched sample. Health LiTT-SF T-scores ≥55 indicate Adequate health literacy, whereas T-scores <55 suggest Inadequate/Marginal health literacy.
Performance on the Health LiTT-SF measure for individuals with TBI and controls. The data points are coloured according to years of education; red denotes less years of education and blue denotes greater years of education. The shape of the data point denotes the participant’s sex; females are represented by a diamond shape and males are represented by a triangle.
In our key analysis, we modelled the number of errors on the Health LiTT-SF as a function of group (TBI vs control), age, sex, and years of education. Contrary to our hypothesis, individuals with TBI did not have a greater number of errors on the Health LiTT-SF than controls (IRR = 1.31, P = 0.102, CI95% [0.947, 1.812]; Table 4). The statistical significance of the group difference was not changed when five potential outliers were removed (Supplementary Table S2) and when the unmatched sample was analysed (Supplementary Table S3). As expected, greater years of education was associated with fewer errors on the Health LiTT-SF (IRR = 0.84, P <0.001, CI95% [0.788, 0.897]). There was a 16% reduction in the mean incident rate of Health LiTT-SF errors with each additional year of education.
| B (s.e.) | 95% CI | P-value | Exp(B) | 95% CI | ||
|---|---|---|---|---|---|---|
| Group | 0.27 (0.17) | −0.054 to 2.67 | 0.102 | 1.31 | 0.95–1.81 | |
| Sex | 0.104 (0.18) | −0.25 to 0.46 | 0.563 | 1.11 | 0.78–1.58 | |
| Age | 0.01 (0.01) | −0.07 to 0.02 | 0.378 | 1.01 | 0.99–1.02 | |
| Education | −0.174 (0.03) | −0.24 to 0.11 | 0.000 | 0.84 | 0.79–0.90 |
CI, confidence interval; s.e., standard error. Exp(B) presents the incidence rate ratios (IRR).
Aim 2. Association between health literacy in individuals with TBI and demographic factors, cognitive function, and injury factors
Analyses for Aim 2 could not be completed due to the very limited variation in Health LiTT-SF scores within the TBI group. This precluded any analyses that attempted to model factors associated with variation in the health literacy score.
Aim 3. Association between health literacy in individuals with TBI and poorer physical and mental health
Analyses for Aim 3 could not be completed due to the very limited variation in Health LiTT-SF scores within the TBI group. This precluded any analyses that attempted to model factors associated with variation in the health literacy score.
Exploratory analysis 1. Item-level Health LiTT-SF performance in individuals with TBI compared to controls
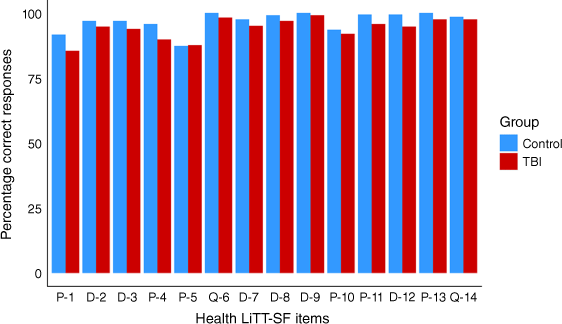
We examined item responses on the Health LiTT-SF items for individuals with TBI and controls (Table 5, Fig. 2). Both groups had a higher frequency of incorrect responses for the prose items compared with the document and quantitative items. However, there was variation in performance on the prose items (% incorrect responses: TBI group, 2.4–14.4%; control group, 0–12.6%). The two quantitative items had a very low frequency of incorrect responses from both groups.
The TBI group had a greater frequency of incorrect responses on all items except prose item 5 (% incorrect responses: TBI group, 12.4%; control group, 12.6%). Although the TBI group had a higher frequency of incorrect responses, the pattern of incorrect responses across items was generally consistent between groups.
| Item number | Item type | Individuals with TBI (n = 209) | Controls (n = 206) | |
|---|---|---|---|---|
| Item 1 | Prose | Correct: 179, 85.6% | Correct: 189, 91.7% | |
| Incorrect: 30, 14.4% | Incorrect: 17, 8.3% | |||
| Item 2 | Document | Correct: 198, 94.7% | Correct: 200, 97.1% | |
| Incorrect: 11, 5.3% | Incorrect: 6, 2.9% | |||
| Item 3 | Document | Correct: 196, 93.8% | Correct: 200, 97.1% | |
| Incorrect: 13, 6.2% | Incorrect: 6, 2.9% | |||
| Item 4 | Prose | Correct: 188, 90% | Correct: 197, 95.6% | |
| Incorrect: 21, 10% | Incorrect: 9, 4.4% | |||
| Item 5 | Prose | Correct: 183, 87.6% | Correct: 180, 87.4% | |
| Incorrect: 26, 12.4% | Incorrect: 26, 12.6% | |||
| Item 6 | Quantitative | Correct: 205, 98.1% | Correct: 206, 100% | |
| Incorrect: 4, 1.9% | Incorrect: 0, 0% | |||
| Item 7 | Document | Correct: 199, 95.2% | Correct: 201, 97.6% | |
| Incorrect: 10, 4.8% | Incorrect: 5, 2.4% | |||
| Item 8 | Document | Correct: 203, 97.1% | Correct: 204, 99% | |
| Incorrect: 6, 2.9% | Incorrect: 2, 1.0% | |||
| Item 9 | Document | Correct: 207, 99% | Correct: 206, 100% | |
| Incorrect: 2, 1% | Incorrect: 0, 0% | |||
| Item 10 | Prose | Correct: 192, 91.9% | Correct: 193, 93.7% | |
| Incorrect: 17, 8.1% | Incorrect: 13, 6.3% | |||
| Item 11 | Prose | Correct: 200, 95.7% | Correct: 205, 99.5% | |
| Incorrect: 9, 4.3% | Incorrect: 1, 0.5% | |||
| Item 12 | Document | Correct: 198, 94.7% | Correct: 199, 96.6% | |
| Incorrect: 11, 5.3% | Incorrect: 7, 3.4% | |||
| Item 13 | Prose | Correct: 204, 97.6% | Correct: 206, 100% | |
| Incorrect: 5, 2.4% | Incorrect: 0, 0% | |||
| Item 14 | Quantitative | Correct: 204, 97.6% | Correct: 203, 98.5% | |
| Incorrect: 5, 2.4% | Incorrect: 3, 1.5% |
There are three item types: prose (reading comprehension), document (identify and interpret information presented in charts, graphs, or tables), and quantitative (perform arithmetic operations).
Exploratory analysis 2. Qualitative description of five individuals with TBI with poor performance on Health LiTT-SF
There were five individuals with TBI who demonstrated markedly poorer performance on the Health LiTT-SF as compared to the broader TBI group (Tables 6 and 7).
| Variable | M (s.d.); Range | |
|---|---|---|
| BTACT – Episodic Memory Factor | −0.17 (−0.95); −2.77 to 3.26 | |
| BTACT – Executive Function Factor | 0.93 (0.93); −1.19 to 3.72 | |
| BTACT composite score | 0.57 (0.91); −2.18 to 3.31 | |
| PHQ-9 | 5.2 (4.5); 0–23 | |
| 0–4: minimal depression | 112, 53.8% | |
| 5–9: mild depression | 59, 28.4% | |
| 10–14: moderate depression | 30, 14.4% | |
| 15–19: moderately severe | 4, 1.9% | |
| Depression ≥20: severe depression | 3, 1.4% | |
| GAD-7 | 5.2(4.7); 0–21 | |
| 0–4: minimal anxiety | 111, 53.1% | |
| 5–9: mild anxiety | 61, 29.2% | |
| 10–14: moderate anxiety | 28, 13.4% | |
| ≥15: severe anxiety | 9, 4.3% | |
| PROMIS physical health T score | 50.2 (8.2); 26.70–67.0 | |
| PROMIS mental health T score | 48.7 (8.03); 21.2–67.6 | |
| Health conditions – medical health | 1.7 (1.9); 1.0; 0–11 | |
| No medical conditions | 69, 33% | |
| ≥1 medical condition | 140, 67% | |
| Health conditions – mental health | 101 (1.3); 0.5; 0–6 | |
| No mental health conditions | 104, 50% | |
| ≥1 mental health condition | 104, 50% |
BTACT composite score and Episodic Memory Factor were available for 207/209 individuals with TBI. BTACT Executive Function Factor was available for 208/209 individuals with TBI. PHQ_Total was available for 208/209 individuals with TBI. Mental health conditions was available for 208/209 individuals with TBI. The median has also been provided for medical and mental health conditions due to the skewed distribution of this data.
| Case 1 – 75 | Case 2 – 140 | Case 3 – 151 | Case 4 – 208 | Case 5 – 221 | Individuals with TBI (n = 209) | ||
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Sex | Male | Male | Male | Female | Male | Male: 145, 69.4% | |
| Female: 64, 30.6% | |||||||
| Current age A (years) | 30–40 | 60–70 | 50–60 | 40–50 | 30–40 | M = 50.23 (15.3); R = 23.1–84.1 | |
| Education (years) | 11 | 9 | 15 | 12 | 10 | M = 13.5 (2.4); R = 3–18 | |
| Country of birth | Australia | Australia | Australia | Australia | Australia | Australia: 178, 85.2% | |
| Outside Australia: 31, 14.8% | |||||||
| Ethnicity | White | White | White | White | White | White: 200, 95.7% | |
| Other Ethnicities: 9, 4.3% | |||||||
| Language spoken at home | English | English | English | English | English | English: 206, 98.6% | |
| Language other than English: 3, 0.5% | |||||||
| Injury characteristics | |||||||
| Age at injury (years) | 15 | 54 | 24 | 41 | 23 | M = 36.0 (15.7); R = 14.26–70.63 | |
| Time since injury (years) | 14 | 12 | 26 | 7 | 13 | M = 14.2 (7.8); R = 1.64–30.41 | |
| Duration of PTA (days) | 6 | 43 | 35 | 19 | 28 | M = 25.8 (23.6); R = 0–134 | |
| GCS | 13 | 3 | 3 | 10 | 3 | M = 8.1 (4.3); R = 3–15 | |
| Health LiTT-SF | |||||||
| Health LiTT total score | 6 | 5 | 6 | 4 | 5 | M = 13.19 (1.57); R = 4–14 | |
| Health LiTT total errors | 8 | 9 | 8 | 10 | 9 | M = 0.81 (1.57); R = 0–10 | |
| Health LiTT T score | 39.75; Marginal/Inadequate | 37.67; Marginal/Inadequate | 39.75; Marginal/Inadequate | 35.56; Marginal/Inadequate | 37.67; Marginal/Inadequate | M = 60.64 (5.08); R = 35.56–63.73 | |
| Marginal/Inadequate: 13, 6.2% | |||||||
| Adequate: 196, 93.8% | |||||||
| Cognition, medical, and emotional health | |||||||
| BTACT – Episodic Memory Factor | −0.71 | −1.48 | −2.77 B | −2.05 | −1.47 | M = −0.17 (−0.95); R = −2.77 to 3.26 | |
| BTACT – Executive Function Factor | −0.03 | 1.14 | −1.10 B | −0.11 | 0.57 | M = 0.93 (0.93); R = −1.19 to 3.72 | |
| BTACT composite score | −0.37 | 0.10 | −2.18 B | −1.12 | −0.32 | M = 0.57 (0.91); R = −2.18 to 3.31 | |
| PHQ-9 | 22 C | 0 | 23 C | 12 | 11 | M = 5.2 (4.5); R = 0–23 | |
| GAD-7 | 12 | 0 | 21 C | 10 | 8 | M = 5.2 (4.7); R = 0–21 | |
| PROMIS physical health T score | 26.7 B | 54.1 | 32.4 B | 34.9 | 50.8 | M = 50.2 (8.2); R = 26.70–67.0 | |
| PROMIS mental health T score | 28.4 B | 59.0 | 21.2 B | 45.8 | 53.3 | M = 48.7 (8.03); R = 21.2–67.6 | |
| Health conditions – medical health | 1 | 2 | 0 | 2 | 2 | M = 1.7 (1.9); Md = 1.0; R = 0–11 | |
| Health conditions – mental health | 1 | 0 | 3 | 2 | 4 C | M = 1.01 (1.3); Md = 0.5; R = 0–6 | |
The majority of participants were male (n = 4/5). All five participants were born in Australia, of White ethnicity, and spoke English at home. Across the five cases, a pattern emerged whereby participants had one or more of: low education (less than high school), older age (>60 years), severe TBI, and cognitive impairment on the BTACT. Most notably, Case 3 was in the lowest 2% of the TBI group for his BTACT composite score, Episodic Memory Factor score and Executive Function Factor score. Case 4 also had significant cognitive impairment, performing in the lowest 2% for his BTACT Episodic Memory Factor score in the TBI group and almost 2 s.d.s below the mean BTACT composite score for the TBI group.
Emotional and physical health problems were also prominent across the five cases. Most notably, Case 3 scored 2 s.d.s below the average PROMIS Physical Health T score, and almost 3.5 s.d.s below the average PROMIS Mental Health T score compared to the broader TBI group. He also reported ‘Severe’ depression (PHQ-9) and ‘Severe’ anxiety (GAD-7). Similarly, Case 1 scored almost 3 s.d.s below the average PROMIS Physical Health T score, and 2.5 s.d.s below the average PROMIS Mental Health T score compared to the TBI group. He also reported ‘Severe’ depression (PHQ-9) and ‘Moderate’ anxiety (GAD-7).
Discussion
This study examined health literacy within a large Australian sample of complicated mild to severe TBI survivors from 1 to 30 years post injury. Contrary to our hypotheses in Aim 1, individuals with TBI did not show significantly lower health literacy. Rather, both groups performed very well on the Health LiTT-SF assessment, producing skewed distributions of high scores. Due to the skewed distribution of the Health LiTT-SF scores and the associated lack of variation in the data, further planned analyses for Aims 2 and 3 could not be conducted. Item-level analysis in Exploratory Analysis 1 revealed a similar response pattern between groups with the relative frequency of incorrect responses on each item being consistent. The frequency of incorrect responses was highest for prose items, although there was variation. Exploratory Analysis 2 identified five individuals with TBI who showed markedly poorer performance on the Health LiTT-SF. These individuals were notable for poor cognitive performance, and problems with physical and emotional health. Overall, these findings are positive for the TBI community, as adequate health literacy can empower individuals in the management of this chronic and dynamic health condition. However, further work is required to explore higher levels of health literacy using more comprehensive health literacy measures.
There were no group differences in health literacy between the TBI and control groups. This null effect may have been partly due to the measure of health literacy used in the study. The Health LiTT-SF was normed on a primary care sample consisting mostly of low-education and low-income patients, and was specifically designed to capture the low to middle range of health literacy (Hahn et al. 2011). As a result, the Health LiTT-SF was not able to distinguish between those with high and very high health literacy (Hahn et al. 2011). However, it is notable that this measure was used in a recently published study from the US, with n = 205 mostly severe TBI survivors, which found 31% of the sample had Marginal/Inadequate health literacy (Pappadis et al. 2024). This is far higher than our finding of 6.2% with Marginal/Inadequate health literacy. It is possible this is due to sample differences in key sociodemographic factors known to influence health literacy. Direct comparison of the samples with respect to age and education is difficult as only frequencies have been reported in Pappadis et al. 2024. However, years of education does not appear higher in our sample (M 13.5 s.d. 2.4 vs 60% greater than high school education); nor does our sample appear to be younger (M 50.23 s.d. 15.3 vs 70% younger than 45 years). There was greater representation of ethnic minorities in Pappadis et al. (2024) (69% non-Hispanic white vs 95.7% in our sample). Discrepancies in findings may have also been introduced by the comprehensive inpatient rehabilitation provided to our Australian sample.
All individuals with TBI in this study experienced comprehensive inpatient rehabilitation, with an average length of stay of approximately 2 months, and up to 1.2 years. This may have provided a critical foundation of knowledge regarding health and healthcare systems following TBI that was maintained into the future. Lending some support to this, a recent qualitative study conducted with participants from the same longitudinal head injury outcome study, at an average of 22.5 years post injury, reported that many still felt that early rehabilitation services had an impact on their current lives (Lefkovits et al. 2021). Participants in this study also have access to ongoing outpatient rehabilitation as part of a no-fault accident insurance scheme in Victoria, Australia. However, we do not have data on specific services received and duration of outpatient rehabilitation for this cohort. Pre-morbid education may also provide critical cognitive reserve that contributes to better health literacy post injury. Indeed, we found that greater education was associated with better health literacy even in the context of severe injuries and cognitive impairments.
Although the TBI group performed very well overall on the Health LiTT-SF, there were five key outliers with markedly poorer performance. These participants did show a pattern of sociodemographic factors and health outcomes that are consistent with previous research. Across the five cases there was representation from those with lower education (less than high school), older age (>65 years), severe TBI, and greater cognitive impairment on the BTACT. However, it is important to note that other participants in the sample also had one or more of these characteristics and yet had adequate health literacy. The most notable characteristic across cases was cognitive impairment, with one of the five cases having the greatest cognitive impairment in the TBI group. It is also of interest to note that all five cases reported poor physical and/or mental health, which is consistent with previous work showing poorer health outcomes for TBI survivors with poor health literacy (Hahn et al. 2017). These findings must be interpreted with caution due to the very small sample size.
Limitations
The fundamental limitation of this study was that the skewed distribution of Health LiTT-SF scores precluded much of our planned analyses. Despite this, we were able to complete the key group analyses and provide interesting insights from the descriptive analysis of five individuals with distinctly poor health literacy. The recruited samples were not matched with respect to age, sex, and education. However, we conducted a comprehensive matching procedure that eliminated the statistically significant differences in categorised age, sex, and education. Bias may have been present in our sample as participants were required to have internet access and requisite computer skills to complete the Health LiTT-SF independently. However, previous work shows that computer-naïve and older participants have not had difficulty competing the Health LiTT (Yost et al. 2010). Further, it has been suggested that the independent online administration could actually identify more individuals with low health literacy by minimising the stigma that participants may feel when completing a health literacy measure with a researcher (Yost et al. 2009, 2010). Our sample did lack sociocultural diversity, which may have contributed to the ceiling effects seen. Accessing and including these communities in studies is challenging but should be a focus of future research. In addition, future work should recruit individuals with TBI from around Australia with less or no access to the comprehensive inpatient and outpatient rehabilitation provided to the current sample. Continued research examining health literacy after TBI is of particular importance in light of the recent COVID-19 pandemic, which can amplify the impacts of poorer health literacy and may place individuals at greater risk of harm from the virus (McCaffery et al. 2020). Indeed, a recent Australian study with over 4000 participants showed that people with inadequate health literacy had poorer understanding of COVID-19 symptoms, were less able to identify behaviours to prevent infection, and held more misinformation beliefs about COVID-19 and vaccination (McCaffery et al. 2020). These disparities may disproportionally impact individuals from culturally and linguistically diverse backgrounds; with one British study highlighting that there is a concerning lack of translated and graphics-based COVID-19 information online (Khan et al. 2021).
Conclusions
Adequate health literacy was identified in a large sample of Australian TBI survivors using the Health LiTT-SF measure. These findings suggest that even in the context of severe TBI and significant cognitive impairment, some individuals can understand and interpret health information necessary to inform healthcare decisions. Comprehensive inpatient rehabilitation and education may be two key factors that lay an important foundation for health literacy after TBI. Ongoing access to rehabilitation care may provide important knowledge of health and health care, which are vital for self-management of chronic TBI. Given the association between poor health literacy and negative health outcomes, these findings are positive for the TBI community. However, these findings are specific to the Health LiTT-SF measure only and future work should examine whether they are replicated using a more comprehensive health literacy measure and in a more culturally diverse sample without access to comprehensive rehabilitation.
Declaration of funding
This work was funded by Monash-Epworth Rehabilitation Research Centre. The contents of this publication were developed under grants from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant numbers 90DPTB0025 [PIs: Sander, Juengst] and 90DPTB0016 [PIs: Sherer, Sander]), the National Institute on Aging (NIA grant number K01AG065492 [PI: Pappadis]), and the National Institute on Minority Health and Health Disparities (NIMHD contract number L60MD009326L [PI: Pappadis]). The contents of this publication are the responsibility of solely the authors and do not necessarily represent the policy or official views of NIDILRR, NIA, or NIMHD, and you should not assume endorsement by the Federal Government.
Ethics standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Acknowledgements
The authors would like to acknowledge Ms Pavika Thevar for her assistance with data collection.
References
Barber MN, Staples M, Osborne RH, Clerehan R, Elder C, Buchbinder R (2009) Up to a quarter of the Australian population may have suboptimal health literacy depending upon the measurement tool: results from a population-based survey. Health Promotion International 24(3), 252-261.
| Crossref | Google Scholar | PubMed |
Barry MJ, Edgman-Levitan S (2012) Shared decision making—the pinnacle of patient-centered care. The New England Journal of Medicine 366(9), 780-781.
| Crossref | Google Scholar | PubMed |
Beauchamp A, Buchbinder R, Dodson S, Batterham RW, Elsworth GR, McPhee C, Sparkes L, Hawkins M, Osborne RH (2015) Distribution of health literacy strengths and weaknesses across socio-demographic groups: a cross-sectional survey using the Health Literacy Questionnaire (HLQ). BMC Public Health 15, 678.
| Crossref | Google Scholar | PubMed |
Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K (2011) Low health literacy and health outcomes: an updated systematic review. Annals of Internal Medicine 155(2), 97-107.
| Crossref | Google Scholar | PubMed |
Blackwell M, Iacus S, King G, Porro G (2009) Cem: coarsened exact matching in Stata. The Stata Journal 9(4), 524-546.
| Crossref | Google Scholar |
Centers for Disease Control and Prevention (n.d.) ‘National Health and Nutrition Examination Survey (NHANES).’ (Center for Disease Control and Prevention, National Center for Health Statistics) Available at https://www.cdc.gov/nchs/nhanes/index.htm
Choudhry FR, Ming LC, Munawar K, Zaidi STR, Patel RP, Khan TM, Elmer S (2019) Health Literacy Studies Conducted in Australia: A Scoping Review. International Journal of Environmental Research and Public Health 16(7), 1112.
| Crossref | Google Scholar | PubMed |
Dams-O’Connor K, Sy KTL, Landau A, Bodien Y, Dikmen S, Felix ER, Giacino JT, Gibbons L, Hammond FM, Hart T, Johnson-Greene D, Lengenfelder J, Lequerica A, Newman J, Novack T, O’Neil-Pirozzi TM, Whiteneck G (2018) The Feasibility of Telephone-Administered Cognitive Testing in Individuals 1 and 2 Years after Inpatient Rehabilitation for Traumatic Brain Injury. Journal of Neurotrauma 35(10), 1138-1145.
| Crossref | Google Scholar | PubMed |
DiBlasio CA, Novack TA, Cook III EW, Dams-O’Connor K, Kennedy RE (2021a) Convergent Validity of In-Person Assessment of Inpatients With Traumatic Brain Injury Using the Brief Test of Adult Cognition by Telephone (BTACT). The Journal of Head Trauma Rehabilitation 36(4), E226-E232.
| Crossref | Google Scholar | PubMed |
DiBlasio CA, Sima A, Kumar RG, Kennedy RE, Retnam R, Lachman ME, Novack TA, Dams-O’Connor K (2021b) Research Letter: Performance of the Brief Test of Adult Cognition by Telephone in a National Sample. The Journal of Head Trauma Rehabilitation 36(4), E233-E239.
| Crossref | Google Scholar | PubMed |
Dyer JR, Williams R, Bombardier CH, Vannoy S, Fann JR (2016) Evaluating the Psychometric Properties of 3 Depression Measures in a Sample of Persons With Traumatic Brain Injury and Major Depressive Disorder. The Journal of Head Trauma Rehabilitation 31(3), 225-232.
| Crossref | Google Scholar | PubMed |
Fann JR, Bombardier CH, Dikmen S, Esselman P, Warms CA, Pelzer E, Rau H, Temkin N (2005) Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. The Journal of Head Trauma Rehabilitation 20(6), 501-511.
| Crossref | Google Scholar | PubMed |
Griese L, Berens E-M, Nowak P, Pelikan JM, Schaeffer D (2020) Challenges in Navigating the Health Care System: Development of an Instrument Measuring Navigation Health Literacy. International Journal of Environmental Research and Public Health 17(16), 5731.
| Crossref | Google Scholar | PubMed |
Hahn EA, Choi SW, Griffith JW, Yost KJ, Baker DW (2011) Health Literacy Assessment Using Talking Touchscreen Technology (Health LiTT): a new item response theory-based measure of health literacy. Journal of Health Communication 16(Suppl 3), 150-162.
| Crossref | Google Scholar | PubMed |
Hahn EA, Magasi SR, Carlozzi NE, Tulsky DS, Wong A, Garcia SF, Lai J-S, Hammel J, Miskovic A, Jerousek S, Goldsmith A, Nitsch K, Heinemann AW (2017) Health and Functional Literacy in Physical Rehabilitation Patients. Health Literacy Research and Practice 1(2), e71-e85.
| Crossref | Google Scholar | PubMed |
Hahn EA, Boileau NR, Hanks RA, Sander AM, Miner JA, Carlozzi NE (2020) Health literacy, health outcomes, and the caregiver role in traumatic brain injury. Rehabilitation Psychology 65, 401-408.
| Crossref | Google Scholar | PubMed |
Hammond FM, Corrigan JD, Ketchum JM, Malec JF, Dams-OʼConnor K, Hart T, Novack TA, Bogner J, Dahdah MN, Whiteneck GG (2019) Prevalence of Medical and Psychiatric Comorbidities Following Traumatic Brain Injury. The Journal of Head Trauma Rehabilitation 34(4), 1.
| Crossref | Google Scholar | PubMed |
Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D (2009) Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Quality of Life Research 18(7), 873-880.
| Crossref | Google Scholar | PubMed |
Jessup RL, Osborne RH, Beauchamp A, Bourne A, Buchbinder R (2017) Health literacy of recently hospitalised patients: a cross-sectional survey using the Health Literacy Questionnaire (HLQ). BMC Health Services Research 17(1), 52.
| Crossref | Google Scholar | PubMed |
Kessler RC, Merikangas KR (2004) The National Comorbidity Survey Replication (NCS-R): background and aims. International Journal of Methods in Psychiatric Research 13(2), 60-68.
| Crossref | Google Scholar | PubMed |
Khan S, Asif A, Jaffery AE (2021) Language in a Time of COVID-19: Literacy Bias Ethnic Minorities Face During COVID-19 from Online Information in the UK. Journal of Racial and Ethnic Health Disparities 8(5), 1242-1248.
| Crossref | Google Scholar | PubMed |
Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 16(9), 606-613.
| Crossref | Google Scholar | PubMed |
Lachman ME, Agrigoroaei S, Tun PA, Weaver SL (2014) Monitoring cognitive functioning: psychometric properties of the brief test of adult cognition by telephone. Assessment 21(4), 404-417.
| Crossref | Google Scholar | PubMed |
Lefkovits AM, Hicks AJ, Downing M, Ponsford J (2021) Surviving the “silent epidemic”: a qualitative exploration of the long-term journey after traumatic brain injury. Neuropsychological Rehabilitation 31(10), 1582-1606.
| Crossref | Google Scholar | PubMed |
Levin-Zamir D, Bertschi I (2018) Media Health Literacy, eHealth Literacy, and the Role of the Social Environment in Context. International Journal of Environmental Research and Public Health 15(8), 1643.
| Crossref | Google Scholar | PubMed |
Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN, Perkins PK (2007) The mayo classification system for traumatic brain injury severity. Journal of Neurotrauma 24(9), 1417-1424.
| Crossref | Google Scholar | PubMed |
McCaffery KJ, Dodd RH, Cvejic E, Ayre J, Batcup C, Isautier JMJ, Copp T, Bonner C, Pickles K, Nickel B, Dakin T, Cornell S, Wolf MS (2020) Health literacy and disparities in COVID-19–related knowledge, attitudes, beliefs and behaviours in Australia. Public Health Research & Practice 30(4), e30342012.
| Crossref | Google Scholar |
National Disability Insurance Agency (2021) ‘What principles do we follow to create your plan?’ (NDIS) Available at https://ourguidelines.ndis.gov.au/how-ndis-supports-work-menu/what-principles-do-we-follow-create-your-plan
Nelson LD, Barber JK, Temkin NR, Dams-O’Connor K, Dikmen S, Giacino JT, Kramer MD, Levin HS, McCrea MA, Whyte J, Bodien YG, Yue JK, Manley GT, British Neurosurgical Trainee Research Collaborative (BNTRC) (2021) Validity of the Brief Test of Adult Cognition by Telephone in Level 1 Trauma Center Patients Six Months Post-Traumatic Brain Injury: A TRACK-TBI Study. Journal of Neurotrauma 38(8), 1048-1059.
| Crossref | Google Scholar | PubMed |
Neter E, Brainin E (2019) Association Between Health Literacy, eHealth Literacy, and Health Outcomes Among Patients With Long-Term Conditions. European Psychologist 24(1), 68-81.
| Crossref | Google Scholar |
Nutbeam D (2000) Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promotion International 15(3), 259-267.
| Crossref | Google Scholar |
Nuzzo RL (2016) The Box Plots Alternative for Visualizing Quantitative Data. PM & R: The Journal of Injury, Function, and Rehabilitation 8(3), 268-272.
| Crossref | Google Scholar | PubMed |
Pappadis MR, Sander AM, Juengst SB, Leon-Novelo L, Ngan E, Bell KR, Corrigan JD, Driver S, Dreer LE, Lequerica AH (2024) The Relationship of Health Literacy to Health Outcomes Among Individuals With Traumatic Brain Injury: A Traumatic Brain Injury Model Systems Study. The Journal of Head Trauma Rehabilitation 39(2), 103-114.
| Crossref | Google Scholar | PubMed |
Patrick SD, Sanders G, Boulton AJ, Tulsky DS (2022) Measurement invariance of physical, mental, and social health PROMIS measures across individuals with spinal cord injury and traumatic brain injury. Quality of Life Research 31(7), 2223-2233.
| Crossref | Google Scholar | PubMed |
Ponsford JL, Downing MG, Olver J, Ponsford M, Acher R, Carty M, Spitz G (2014) Longitudinal follow-up of patients with traumatic brain injury: outcome at two, five, and ten years post-injury. Journal of Neurotrauma 31(1), 64-77.
| Crossref | Google Scholar | PubMed |
Ryff CD, Lachman ME (2009) Midlife in the United States (MIDUS 2): Cognitive Project, 2004-2006 [Dataset]. Inter-university Consortium for Political and Social Research [distributor]. 10.3886/ICPSR25281.v6
Ryff CD, Lachman ME (2018) Midlife in the United States (MIDUS Refresher 1): Cognitive Project, 2011-2014 [Dataset]. Inter-university Consortium for Political and Social Research [distributor]. 10.3886/ICPSR37081.v2
Simpson RM, Knowles E, O’Cathain A (2020) Health literacy levels of British adults: a cross-sectional survey using two domains of the Health Literacy Questionnaire (HLQ). BMC Public Health 20(1), 1819.
| Crossref | Google Scholar | PubMed |
Slesinger NC, Yost KJ, Choi SW, Hahn EA (2020) Validation of a Short Form for Health Literacy Assessment Using Talking Touchscreen Technology. Health Literacy Research and Practice 4(4), e200-e207.
| Crossref | Google Scholar | PubMed |
Sørensen K, Van den Broucke S, Fullam J, Doyle G, Pelikan J, Slonska Z, Brand H, (HLS-EU) Consortium Health Literacy Project European (2012) Health literacy and public health: a systematic review and integration of definitions and models. BMC Public Health 12, 80.
| Crossref | Google Scholar | PubMed |
Spitzer RL, Kroenke K, Williams JBW, Löwe B (2006) A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine 166(10), 1092-1097.
| Crossref | Google Scholar | PubMed |
Teymoori A, Real R, Gorbunova A, Haghish EF, Andelic N, Wilson L, Asendorf T, Menon D, von Steinbüchel N (2020) Measurement invariance of assessments of depression (PHQ-9) and anxiety (GAD-7) across sex, strata and linguistic backgrounds in a European-wide sample of patients after Traumatic Brain Injury. Journal of Affective Disorders 262, 278-285.
| Crossref | Google Scholar | PubMed |
Tun PA, Lachman ME (2006) Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age and Ageing 35(6), 629-632.
| Crossref | Google Scholar | PubMed |
Yost KJ, Webster K, Baker DW, Choi SW, Bode RK, Hahn EA (2009) Bilingual health literacy assessment using the Talking Touchscreen/la Pantalla Parlanchina: Development and pilot testing. Patient Education and Counseling 75(3), 295-301.
| Crossref | Google Scholar | PubMed |
Yost KJ, Webster K, Baker DW, Jacobs EA, Anderson A, Hahn EA (2010) Acceptability of the talking touchscreen for health literacy assessment. Journal of Health Communication 15(Suppl 2), 80-92.
| Crossref | Google Scholar | PubMed |
Zhang NJ, Terry A, McHorney CA (2014) Impact of health literacy on medication adherence: a systematic review and meta-analysis. The Annals of Pharmacotherapy 48(6), 741-751.
| Crossref | Google Scholar | PubMed |