Depression and anxiety at 1- and 12-months post ischemic stroke: methods for examining individual change over time
Suzanne Barker-Collo A * , Rita Krishnamurthi B , Balakrishnan Nair B , Anna Ranta C , Jeroen Douwes D and Valery Feigin B
A * , Rita Krishnamurthi B , Balakrishnan Nair B , Anna Ranta C , Jeroen Douwes D and Valery Feigin B
A
B
C
D
Abstract
Depression is commonly studied post stroke, while anxiety is less studied. This study presents prevalence of depression and anxiety at 1- and 12-months post ischemic stroke alongside three methods for examining within-subjects change over time.
Participants were ischemic stroke patients of the Auckland Regional Community Stroke Study (ARCOS-V) with Hospital Anxiety and Depression Scale data at 1- (n = 343) and 12-months (n = 307). Change over time was examined using within-subjects repeated measures ANOVA, calculation of the Reliable Change Index, and a Sankey diagram of those meeting cut-off scores (>7) for caseness over time.
Using repeated measures ANOVA, depression scores didn’t change significantly over time, while anxiety symptoms decreased significantly. When reliable change was calculated, 4.2% of individuals had reliable decreases in anxiety symptoms, while 5.7% had reliable decreases in depression symptoms. Those who had a reliable decrease in one tended to have a reliable decrease in the other. In the Sankey, the proportion of those meeting the cut-off score for anxiety did not change over time (12.8 and 12.7% at 1- and 12-months), while those meeting the cut-off for depression increased slightly (3.7–4.5%) and those meeting cut-offs for both decreased from 10.4 to 8.1%.
The three methods produced very different findings. Use of cut-off scores is common but has limitations. Calculation of clinically reliable change is recommended. Further work is needed to ensure depression and anxiety are monitored over time post-stroke, and both should be the subject of intervention efforts in both acute and late stages post-stroke.
Keywords: anxiety, clinically reliable change, depression, ischemic stroke, Sankey diagram, within-subject ANOVA.
Introduction
The study of mood after stroke has tended to focus on depression, as it was thought to be the most common emotional consequence of stroke (Carod-Artal 2006; Paul et al. 2006; Dafer et al. 2008; Turner et al. 2012). One meta-analysis (61 cohorts; N = 24,488 patients) reported that 31% of patients develop depression within 5-years post-stroke (Hackett and Pickles 2014). Other meta-analyses reported similar prevalences (pooled 29%; 43 studies; N = 20,293; (Ayerbe et al. 2013) and 23.8%; six studies; N = 4648 (Wu et al. 2019)). Those with post-stroke depression have more pronounced cognitive deficits, more long-term disability, higher mortality, lower quality of life and higher rates of suicidal ideation as compared to those without depression, indicating that early detection and treatment of depression is important (Wu et al. 2019).
Anxiety is also common post-stroke, but is less studied, with longitudinal studies nearly absent (Angelelli et al. 2004; Barker-Collo 2007). Most studies report prevalence of post-stroke anxiety as similar to or slightly lower than depression (Barker-Collo 2007). However, one large study (N = 4079) reported probable anxiety post-stroke in 29% of patients, compared with a depression rate of 24% (Broomfield et al. 2014). A systematic review (Knapp et al. 2020) of 97 studies (N = 22,262) published between 2009 and 2018 reported the proportion of individuals with any anxiety disorder to be 15.5% (95% CI, 6.3–24.7) within 1-month of stroke, 21.4% (95% CI, 19.2–23.5%) from 1- to 5-months post-stroke, and 31.8% (95% CI, 17.8–45.7%) from 6- to 12-months post-stroke. The frequency of clinically significant symptoms of anxiety assessed using rating scales remained above 20% across all time points up to 12-months, though it did reduce from 25.5% (95% CI, 18.6–32.3) within 1-month to 23.6% (95% CI, 18.9–28.2%) between 1- and 5-months and 21.5% (95% CI, 15.3–27.8%) from 6- to 12-months post-stroke.
We previously explored (Barker-Collo et al. 2017) severity and rate of resolution of post-stroke anxiety and depression in ischemic stroke patients (N = 365) at presentation, 1-, 6- and 12-months post-stroke using the Hospital Anxiety and Depression Scale (HADS). Our study showed that moderate to severe symptoms of anxiety were about twice as likely (range 4.1–10.6%) compared with moderate to severe depression (range 2.5–5.0%) at each assessment. The greatest reduction in anxiety occurred in the first month post-stroke, while the greatest reduction in depression occurred 1–6-months post-stroke.
The literature described above is limited to depicting group-level changes in prevalence or mean performance of samples on common measures of depression and/or anxiety. While such data are informative, it has been noted that without individual participant-level data, it remains unclear whether post-stroke anxiety and/or depression improve in some, while others develop new or worsening anxiety and/or depression over time (Chun et al. 2022). Historically, the most common method of examining within-individual change over time is through repeated-measures within-subjects ANOVA (Grace-Martin 2022). The biggest advantage of this approach is its conceptual simplicity, while its greatest disadvantage is its need for balanced data, meaning that if an individual is missing data at one time-point, that individual is excluded from the analysis. In addition, as statistical analyses identify patterns within samples, a statistically derived indication of a particular trajectory may include patients whose level of change is below a clinically meaningful threshold (e.g. Stanton et al. 2015), making distress trajectories identified by such statistical methods difficult to interpret clinically.
An alternative approach is the calculation of minimum clinically reliable change in an outcome. The concept of clinically reliable change acknowledges that a statistically non-significant outcome does not mean that a treatment has not been clinically effective, and conversely a statistically significant change might not reflect a clinically relevant change. This is particularly important as small sample sizes and measurement variability in this area of research can influence statistical results (Batterham and Hopkins 2006). It has also been noted (Page 2014) that the absence of significant group-level change in clinical trials may reflect that the intervention was effective, but only in a sub-sample of those in a treatment group. Clinical interpretation of research using calculation of clinically reliable or clinically important change to describe treatment outcomes is important because it more directly translates into clinical decision-making, leading to the recommendation that authors provide results relative to clinically reliable change (Page 2014). However, as with ANOVA, one disadvantage is its exclusion of individuals who have missing data.
A further way to examine change over time without the exclusion of those with missing data is through data visualisation. Sankey diagrams are a data visualisation technique or flow diagram that emphasise flow/movement/change from one state to another and/or from one time to another, in which the width of the arrows is proportional to the flow rate of the depicted property (Otto et al. 2022). This technique is commonly used in physics and engineering to display energy flow, and in economics and business to examine complex multi-step processes (Yu and Silva 2017), but remains limited in use in medical literature. In a review of the medical literature (Otto et al. 2022) conducted to identify references to Sankey diagrams, only 13 articles were identified in which Sankey diagrams were used to visualise flow/transitions of medical conditions or symptoms over time, flow/transitions to specific events, and to demonstrate associations. Longitudinal studies that group patients into diagnostic/distress trajectories using clinical cut-offs across two time points typically result in four clinically meaningful trajectories of distress: (1) non-cases (not meeting clinical criteria at either time point), (2) recovered (meeting cut-off at the first timepoint only), (3) persistent (meeting cut-off at both time points), and (4) emerging (meeting cut-off at the second timepoint only). While missing data can affect the overall Sankey display, removal of those with missing data is only recommended if this affects a very small (i.e. <5%) proportion of the data (Otto et al. 2022), allowing visualisation of the entire cohort at each display point and adding clinically meaningful information.
One potential disadvantage of Sankey diagrams in examining diagnostic/distress trajectories, is the use of categorical data. Reporting continuous data allows examination of an outcome free of any established structures or expectations, while reporting categorically puts information in context based on existing priorities. For example, when using screening tools such as the Hospital Anxiety and Depression Scale (HADS), examining continuous data gives an indication of overall severity of anxiety or depression symptoms across the full range of scores (0–21), while the cut-off scores for ‘probably depression or anxiety’ (>7) (Zigmond and Snaith 1983), are commonly used in clinical practice to identify those whose scores place them in a range most likely to require further assessment. One disadvantage of data that has been categorised based on cut-off scores is that it is possible for an individual changing by a single point to switch from above to below cut-offs. Despite changing categories, such a small change would not seem clinically meaningful and could reflect measurement error. Thus, while acknowledging this limitation, cut-off scores for screening tools such as the HADS do retain utility for the identification of those requiring more in-depth assessment in clinical settings.
The purpose of this study was to investigate changes in depression and anxiety across the first year following ischemic stroke using three methods for examining within-subject changes over time. First, within-subjects ANOVA are presented as an indication of whether within-subject changes across the group using continuous data were statistically significant. Second, clinically reliable change in depression and anxiety were calculated to determine the proportion of individuals within the sample whose change in depression or anxiety met criteria for being a clinically reliable increase or decrease. Finally, a Sankey diagram is presented which allows visualisation of the proportion of individuals who met clinical cut-off sores for depression, anxiety, both, neither or who had missing data at 1- and/or 12-months post-stroke.
Methods
This study was approved by the New Zealand Health and Disability Ethics Committee (ref#19/NTA/177) and conducted in accordance with the STROBE guideline for observational studies.
Participants
Patients with ischemic stroke enrolled in the ARCOS-V study were eligible for inclusion. ARCOS-V is the latest in a series of prospective population-based studies of stroke incidence, prevalence and outcomes in Auckland, New Zealand (Krishnamurthi et al. 2014). Each study included all incident strokes in Auckland Region residents aged ≥16 years. Complete case ascertainment from 1 March 2020 to 31 August 2021 (March–August 2020 during COVID, and the remaining non-COVID incidence used here) was assured by multiple overlapping sources of information on all new hospitalised or non-hospitalised cases (‘hot pursuit’ method). Daily searches were made of admissions data for all public hospitals, with weekly checks of hospital discharge and outpatient clinic data. Regular checks of private hospitals, rest homes and community health services were also conducted. New Zealand Ministry of Health data on all fatal and nonfatal stroke cases in the study population (‘cold-pursuit’ methods) were examined. Residents meeting inclusion criteria but with stroke that occurred outside the Auckland Region were also included.
Stroke was defined using WHO classification (Aho et al. 1980), and a diagnostic review committee composed of neurologists confirmed the diagnosis and classification using medical history, hospital discharge summaries, clinical and laboratory findings (including vascular and cardiac imaging), or necropsy results when available. Cases without imaging or pathological necropsy confirmation of subtype were classified as ‘undetermined’.
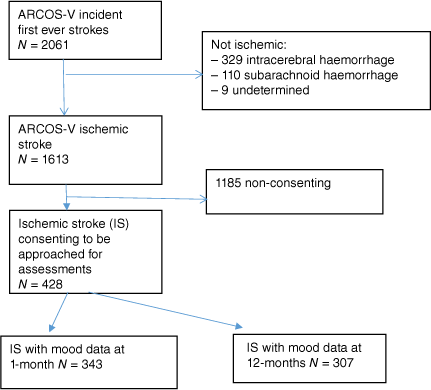
The analyses described here included all ARCOS-V cases who: (1) had an ischemic stroke; (2) provided written informed consent; and (3) had HADS outcome data. Fig. 1 shows selection of cases for the presented analyses.
Each assessment took 1 h to complete and was conducted via telephone. Assessment data used here occurred 1- and 12-months post-stroke (±2 weeks).
Assessments
The Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith 1983) was used to assess the likelihood of problematic depression and anxiety. The HADS (Zigmond and Snaith 1983; Snaith 2003) is a brief, cost-effective screening tool for anxiety and depression. Designed for use in medical populations, the HADS omits items regarding somatic symptoms so that these do not interfere with or conflate reported levels of anxiety and depression. It is the most commonly used screening tool in medically ill patients (Meader et al. 2011). The HADS depression and anxiety subscales have been validated for use in stroke patients without communication problems (Aben et al. 2002; Healey et al. 2008). The HADS contains 14 statements (e.g. ‘I have lost interest in things’). Half of these relate to anxiety and the remaining half to depression. Prior to completing the scale, patients are asked to complete it to reflect how they ‘have been feeling during the past week’ (Zigmond and Snaith 1983). For each statement, the participant chooses one of four responses (e.g. ‘definitely as much’, ‘not quite as much’, ‘only a little’, ‘hardly at all’). Scores for each subscale (anxiety and depression) range from 0 to 21. Scores can be categorised based on severity as follows: normal, 0–7; mild, 8–10; and moderate to severe, 11 and over (Zigmond and Snaith 1983). Alternatively, a cut-off score may be employed to identify those likely to have problematic depression or anxiety. In the present study, cut-off scores were used rather than categorisations based on severity due to small sample size, which would have been further reduced by division into multiple categories, limiting interpretability.
While past studies in stroke have suggested an ideal cut-off for the HADS total score of 11, this does not differentiate anxiety from depression (Aben et al. 2002; Sagen et al. 2009). A recent study which examined depression screening tools such as the HADS post-stroke (Turner et al. 2012) reported that an ideal cut-off for the HADS depression score, in terms of both sensitivity and specificity, was >5. Unfortunately, no similar cut-offs for the HADS anxiety score was presented, and the sample was heterogeneous, including those in post-acute inpatient and outpatient services, with any number or type of stroke, and with time since stroke ranging from 3-weeks to 540-months. Given the above, in this study a cut-off score of >7 (that provided by the test author, Zigmond and Snaith 1983) was used for identifying symptoms reporting outside the normal range (i.e. whether someone was classified positive for depression or anxiety caseness), as this is clinically the most commonly used cut-off and a systematic review identified this as having good specificity and sensitivity for both depression (0.79 and 0.83, respectively) and anxiety (0.78 and 0.90) (Bjelland et al. 2002).
Analyses
Demographic information is presented for the ARCOS-V ischemic stroke cohort who did not consent to follow-up assessments and those who consented and had HADS data at 1- or 12-months. Parametric and non-parametric tests were used as appropriate to compare these groups to determine potential sources of bias and reduced generalisability.
Performance across HADS item and total scores at each time point are presented as means and standard deviations. Within subject change from 1- to 12-months post-stroke for each HADS item and anxiety and depression total scores were examined using within-subjects repeated-measures ANOVA.
Clinically reliable change in anxiety and depression was then calculated for each participant in the sample using an updated Jacobson and Truax’s (1991) Reliable Change Index formula. Change was examined from 1- to 12-months post-stroke, calculated as:
where X1 and X2 are the individual’s observed 1- and 12-month scores, M1 and M2 are the group mean scores at 1- and 12-months, and SDD is the standard deviation of the difference between 1- and 12-month scores. Here, the SDD was calculated as the standard deviation of the individual difference scores for the present sample.
These were calculated separately for anxiety and depression for each participant in the sample. The correction for practice effects is the addition of the constant that is based on group level average change (Sveen et al. 2010). Participant scores that are ±1.96 indicate reliable improvement and reliable decline as a z-score of ±1.96 corresponds to the 97.5th and 2.5th percentile of a normal distribution; in other words, 95% of all z-standardised values in a normal distribution are smaller or equal to ±1.96. Following the convention of using 5% as the threshold for statistical significance, a Reliable Change Index score of greater than ±1.96 is considered clinically significant. The proportion of individuals who met criteria for a significant increase, significant reduction, or no significant change on the HADS depression and/or anxiety scores is presented.
The proportion meeting and not meeting the cut-off (a score >7) on the HADS anxiety and/or depression subscales was also presented as a Sankey diagram, which is a technique used to visualise the flow of cases from one time to another, including those with missing data at a single timepoint, and in which the width of the arrows is proportional to the flow rate.
This study was conducted in accordance with the Helsinki Declaration.
Results
The total pool of people with first-ever stroke enrolled in the ARCOS-V study was 2061 (Fig. 1). Of these, 1613 (78.3%) had experienced ischemic stroke. Of those who experienced ischemic stroke, 428 (26.5%) individuals gave informed consent at 1-month and at that point agreed to be approached for follow-up assessments. Of these, 343 had mood data at 1-month and 307 had mood data at the 12-month follow-up.
The cause of ischemic stroke among consenting individuals at 1-month who had HADS data comprised 46 (13.7%) due to large artery atherosclerosis, 76 (22.7%) due to cardioembolism, 157 (46.9%) due to small vessel occlusion, 14 (4.2%) with ischemic stroke of other known etiology and 42 (12.5%) with ischemic stroke of unknown etiology. For those who participated and provided mood data at 1 month, 41.4% had facial weakness, 61.5% were experiencing limb weakness, 55% were experiencing sensory deficits, and 51.7% were experiencing difficulties with speech when first presenting to hospital.
To determine generalisability of the data to the wider population of those with ischemic stroke, demographic and stroke characteristics of non-consenting ischemic stroke (IS) patients and those with IS who consented and provided data for these analyses at 1- and 12-months are presented in Table 1.
| Continuous variable | Did not consent | Consented and had HADS data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-month | 12-months | |||||||||||
| N = 1185 | N = 343 | t-test P value | N = 307 | t-test P value | ||||||||
| N | Mean | s.d. | N | Mean | s.d. | N | Mean | s.d. | ||||
| Age (years) | 1185 | 72.0 | 15.0 | 335 | 68.1 | 13.2 | <0.0001 | 307 | 67.2 | 13.9 | <0.0001 | |
| Categorical variables | N | % | N | % | Chi square P-value | N | % | Chi square P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | 0.0001 | 0.0016 | |||||||
| Female | 590 | 49.7 | 127 | 37.9 | 126 | 39.3 | |||
| Male | 595 | 50.2 | 208 | 62.1 | 195 | 60.7 | |||
| Ethnicity | <0.0001 | 0.0007 | |||||||
| Non-Caucasian | 519 | 43.8 | 96 | 28.7 | 101 | 31.5 | |||
| Caucasian | 666 | 56.2 | 239 | 71.3 | 220 | 68.5 | |||
| Current marital status | 0.0003 | 0.0011 | |||||||
| Married, civil union, defacto | 628 | 53.0 | 226 | 67.5 | 204 | 63.6 | |||
| Single | 85 | 7.1 | 23 | 6.9 | 33 | 10.3 | |||
| Separated, divorced, widowed | 398 | 33.6 | 70 | 20.9 | 69 | 21.5 | |||
| Missing | 74 | 6.2 | 16 | 4.8 | 15 | 4.7 | |||
| TOAST criteria | 0.0265 | 0.0097 | |||||||
| Large-artery atherosclerosis | 160 | 13.5 | 46 | 13.7 | 40 | 12.5 | |||
| Cardioembolism | 339 | 28.6 | 76 | 22.7 | 66 | 20.6 | |||
| Small-vessel occlusion | 456 | 38.5 | 157 | 46.9 | 154 | 48 | |||
| Other determined aetiology | 38 | 3.2 | 14 | 4.2 | 10 | 3.1 | |||
| Undetermined etiology | 192 | 16.2 | 42 | 12.5 | 51 | 15.9 |
Those who provided data at each time of assessment were significantly younger than those who did not consent; they were also more likely to be male, Caucasian, married, and to have experienced small vessel occlusion (Table 1). Given the lower-than-expected rates of anxiety and depression, and missing data at 12-months, those who provided data at only 1-month were compared to those who had data at both 1- and 12-months to determine if they differed in their 1-month HADS scores. There was no significant difference in either 1-month anxiety or depression scores (both P > 0.05).
Anxiety and depression scores over time
Using within-subjects repeated-measures ANOVA, overall depression scores did not change significantly. Three depression items (which reflect feeling slowed down, feeling cheerful and enjoying things one used to enjoy) did improve between 1- and 12-month assessments (Table 2). The anxiety overall score improved significantly between assessments, with the most pronounced improvement seen for items reflecting feeling tense/wound up and having frightened feelings.
| HADS item | 1-month | 12-months | Within-subject change (DF1 = 1, DF2 = 208) | |
|---|---|---|---|---|
| N = 343 | N = 307 | |||
| Mean (s.d.) | Mean (s.d.) | |||
| No interest in appearance | 0.17 (0.51) | 0.24 (0.62) | F = 1.052, P = 0.306 | |
| Look forward/enjoyment | 0.28 (0.55) | 0.37 (0.67) | F = 0.00 P = 1.000 | |
| Enjoy things I used to | 0.56 (0.72) | 0.52 (0.75) | F = 4.378, P = 0.038 | |
| Laugh/see funny side | 0.18 (0.48) | 0.21 (0.49) | F = 0.016, P = 0.900 | |
| I feel cheerful | 0.41 (0.65) | 0.34 (0.61) | F = 4.229, P = 0.041 | |
| Feel slowed down | 1.46 (0.97) | 1.33 (0.93) | F = 5.721, P = 0.018 | |
| Enjoy good book | 0.18 (0.51) | 0.22 (0.60) | F = 1.332, P = 0.250 | |
| Depression total | 3.24 (2.8) | 3.23 (2.95) | F = 2.935, P = 0.088 | |
| Butterflies in stomach | 0.35 (0.59) | 0.40 (0.70) | F = 0.123, P = 0.727 | |
| Feel restless | 0.71 (0.86) | 0.60 (0.79) | F = 3.416, P = 0.066 | |
| Sudden panic | 0.36 (0.62) | 0.41 (0.68) | F = 0.209 P = 0.648 | |
| Feel tense/wound up | 0.68 (0.78) | 0.65 (0.81) | F = 8.294, P = 0.004 | |
| Frightened feeling | 0.63 (0.80) | 0.54 (0.75) | F = 7.301, P = 0.007 | |
| Worrying thoughts | 0.61 (0.84) | 0.60 (0.85) | F = 0.509, P = 0.476 | |
| Sit at ease/relaxed | 0.39 (0.66) | 0.39 (0.70) | F = 2.197, P = 0.140 | |
| Anxiety total | 3.74 (3.54) | 3.56 (3.71) | F = 7.352, P = 0.007 |
Note: individual item ratings range from 0 to 3; anxiety and depression totals range from 0 to 21. Higher scores indicate poorer outcomes. Bold text indicates items for which within-subject change was statistically significant.
While group means and SDs are informative, of greater clinical interest is the proportion of individuals who met clinical cut-offs/criteria for being a case of probable depression and/or anxiety, as well as those whose change in anxiety and/or depression was clinically reliable.
A reliable change index was calculated for anxiety and depression for all those who had data at both 1- and 12-months. In this sample, using Jacobson and Truax’s formula, the degree of change in HADS score required to meet criteria for a reliable change on the depression scale was 5.3697 for a reliable increase and −4.9837 for a reliable decrease. For the anxiety scale, change of 6.3984 or more reflected a reliable increase, while change of −6.5784 or less reflected a reliable decrease. For anxiety, 9 (4.2%) individuals had a reliable decrease in symptoms, only 2 (0.9%) had a reliable increase and the remaining 200 (94.9%) had no reliable change. For depression, 12 (5.7%) had a reliable decrease in symptoms, 2 (0.9%) had a reliable increase, and the remaining 197 (93.3%) had no reliable change.
Of the 12 individuals who had a reliable decrease in depression symptoms, seven also had a reliable decrease in anxiety symptoms, while the remainder had no reliable change. Similarly, of the nine who had significant decreases in anxiety, six also had significant reductions in depressive symptoms. For the two individuals who had clinically reliable increases in depressive symptoms, there was no reliable change in anxiety. Similarly, for the two individuals who had a reliable increase in anxiety symptoms, there was no reliable change in depression.
In regards to the proportion of individuals who met clinical cut-offs for probable depression and/or anxiety, Fig. 2 presents the number of individuals who met cut-off scores for depression only, anxiety only, both depression and anxiety, neither depression nor anxiety and those missing data at 1- and 12-months post-stroke. At 1-month, 12.8, 3.7 and 10.4% of participants expressed symptoms that met criteria for anxiety only, depression only or comorbid anxiety and depression. At 12-months, these figures were 12.7, 4.5 and 8.1%, respectively. Most individuals did not experience depression or anxiety at either assessment. Of those with anxiety or depression at 1-month, the majority had neither depression nor anxiety at 12-months (n = 9), followed closely by those who still had both at 12-months (n = 8). Only one individual who had both anxiety and depression at 1-month had depression at 12-months, while two had anxiety at 12-months.
Sankey diagram showing flow of cases from 1- to 12-months for depression and/or anxiety post ischemic stroke.
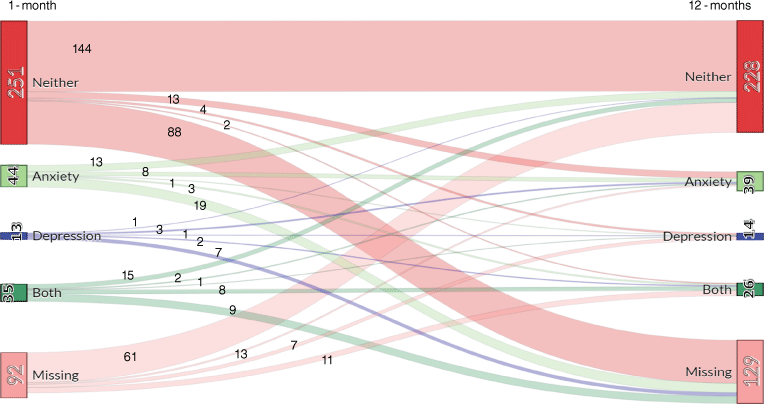
Fig. 2 shows that the largest proportion of those who had depression at 12-months either had no 1-month data or had neither depression nor anxiety at 1-month. Only one person who met the cut-off for depression at 1-month still met that cut-off at 12-months, while eight who met criteria for both depression and anxiety continued to do so over time. As with depression, the Sankey diagram tells a different story for anxiety, with the greatest number of individuals with anxiety at 1-month either not having data or having neither depression nor anxiety at 12-months. The next greatest proportion of those with anxiety at 1-month had anxiety at 12-months. Conversely, the largest proportion of those with anxiety at 12-months had either missing data or neither depression nor anxiety at 1-month.
Discussion
This study found that post-stroke anxiety occurred three times more frequently than post-stroke depression at 1-month and more than twice as often at 12-months after stroke onset. This is in contrast to our previous study of similar methodology (Barker-Collo et al. 2017) where anxiety was about twice as likely as depression across the first year post ischemic stroke. Age, ethnicity and education levels of those who provided mood data in both studies were similar; however, the present sample had more males (62.1 and 60.7% at 1- and 12-months, compared to 53.2%). It is therefore possible that reduced reporting of depressive symptoms in the present sample was due to known sex differences in depression, with women being almost twice as likely to have depression as men across the lifespan (Brody et al. 2018). It is also possible that the increase in those meeting cut-offs for anxiety reflects the impact of the COVID-19 pandemic. Initially scheduled to begin in March 2020, incidence and outcomes data collection was delayed until late 2021 due to the COVID-19 pandemic and associated lockdowns. An increase in anxiety scores and in the proportion of individuals showing anxiety levels equal to or greater than cut-off scores for anxiety have been reported in older adult populations in a number of countries during the pandemic (Parlapani et al. 2020; Qiu et al. 2020; Gosselin et al. 2022).
Prevalence of caseness within this sample (anxiety, depression and both were 12.8, 3.7 and 10.4% at 1-month and 12.7, 4.5 and 8.1% at 12-months) are comparable to those of our previous study (anxiety range 4.1–10.6%; depression range 2.5–5.0%). These are in contrast to the literature, where much higher rates of anxiety and depression (29% anxiety and 21–24% depression) are reported (Broomfield et al. 2014), though these were a mixed stroke and Transient Ishemic Attack (TIA) sample. It is possible that the lower rates found here could reflect bias in the sample in which those who consented and provided mood data were more likely to be male, Caucasian, and to be married compared to those who did not consent. Broomfield et al. (2014) in particular found that females report more depression and anxiety symptoms.
Perhaps the greatest contribution of the findings is the differing picture offered by the three methods of examining change. Within-subjects ANOVA revealed that improvement over time was significant for anxiety but not depression. This is consistent with a recent meta-analysis which showed that stroke survivors with early-onset depression (within 3-months post-stroke) make up two-thirds of those who are depressed 1-year post-stroke (Liu et al. 2023). However, this contrasts with examination of the proportion of individuals who experienced clinically reliable change, with slightly fewer individuals having reliable decreases in anxiety symptoms compared to depressive symptoms (4.2% versus 5.7%). Those who experienced a reliable decrease in one tended to also experience a reliable decrease in the other. The discrepancy between the two methods suggests that while more people in the sample may have experienced reduced symptoms of anxiety (contributing to statistical significance), the degree of change experienced did not as frequently meet criteria to be considered a clinically reliable change. An additional method to consider is the calculation of minimal clinically important difference (MCID), which, unlike clinically reliable change, reflects the smallest difference in a score that a patient perceives as meaningful/beneficial. The calculation of MCID has been criticised on two fronts. First, it is noted that MCID ‘sets a low bar’, seeking the minimal amount of change required (Rossi et al. 2023). Second, it has been noted that there is no standard way in which to calculate these, and the various methods of doing so can yield varying results (Hajiro and Nishimura 2002; Cook 2008). Further work is needed to standardise such methods.
The Sankey diagram presents yet another picture, at least in part due to its retention of those who had missing data at either 1- or 12-months. The Sankey suggests that monitoring for anxiety occur in both those with and without initial anxiety, as the greatest number of those with anxiety at 12-months had neither anxiety nor depression at 1-month. The visualisation also suggests that those with comorbid anxiety and depression or just anxiety at 1-month were the most likely to still meet cut-offs for caseness at 12-months and should therefore be the focus of intervention efforts. The literature suggests that interventions for both anxiety and depression can be effective at both acute (Hoffmann et al. 2015) and post-acute (Fang et al. 2017) stages of stroke recovery.
The greatest limitation of this study was sample size. While the initial incidence sample of first-ever strokes was large, very few consented to participate in the study and provided HADS data during the follow-ups, leaving a small sample with mood data. While this is a relatively large sample when compared to other published studies in this area, those included in the analyses differed significantly from those excluded. Thus, any generalisation of the findings must be cautious. This is particularly true for mood data given that characteristics of the sample (e.g. gender) are associated with fewer mood symptoms, particularly in relation to depression. Therefore, these data might represent an underestimate.
A related issue is the small number of individuals who met criteria for caseness for depression and/or anxiety at each time point, reducing confidence in generalising the findings. In addition, the amount of missing data for the 12-month assessment further reduced sample size and could impact generalisability. The possibility of imputation of missing 12-month data was considered. Imputation works well for data missing in the independent variable, and works best applied when the purpose is prediction, and standard error needs to be reduced. The focus of this paper was on change in the dependent variables (depression and anxiety) over time, rather than prediction. Thus, rather than using imputation, those who provided data at only 1-month were compared to those who had data at both 1- and 12-months to determine if their 1-month HADS scores differed. There was no significant difference in either 1-month anxiety or depression scores (both P > 0.05).
Further, depression and anxiety were assessed using the self-report HADS. The greatest pitfall of self-report measures is their reliance on accurate reporting, which can be negatively impacted by factors such as participant understanding of the items, ability to introspect, image management, and response bias. The primary reason for selecting a self-report measure of depression and anxiety for this study was practicality. With an incidence sample of >2000 first-ever strokes with assessment potentially occurring twice for each individual, it was not possible to fund administration of structured diagnostic interviews. Also considered was potential burden of completing a 14-item questionnaire when compared to a lengthy structured interview. However, the measure has good indications for both reliability and validity which increases confidence in their use. Also relevant here is that while the most frequently used self-report measure of depression and anxiety in medical populations is the HADS, there has been debate regarding optimal cut-off values (Annunziata et al. 2020; Turner et al. 2012), with some indication that lower cut-offs should be applied, at least in the case of post-stroke depression (Turner et al. 2012). This highlights the need for foundational research to establish valid cut-offs specific to stroke for both depression and anxiety.
Finally, the decision was taken in this manuscript not to employ imputation of missing values. HADS scores did not differ between those with complete and incomplete data at 1-month, suggesting imputation was an option due to the likely missing-at-random nature of the HADS data. Indeed, Little’s missing completely at random (MCAR) test using each of the HADS items from 1- and 12-months was not significant (X2(26) = 12.231, P = 0.509), suggesting data were indeed missing at random. Table 1 demonstrates demographic differences between those with and without 12-month data, suggesting there was bias in the study results. Multiple imputation has been used in longitudinal cohorts with self-reported data (e.g. Biering et al. 2015; Mainzer et al. 2021) Thus, the decision not to conduct imputation but to retain missing data in the analyses is a limitation.
Conclusions
The three methods of examining change over time in anxiety and depression over time post ischemic stroke yielded very different pictures for this sample. Within-subjects ANOVA findings indicated that depression did not change, while anxiety scores improved significantly. This contrasts with examination of the proportion of individuals who experienced clinically reliable change, with 4.2% having reliable decreases in anxiety symptoms and 5.7% having reliable decreases in depressive symptoms. Furthermore, those who experienced a reliable decrease in one tended to also experience a reliable decrease in the other. These are both in contrast to the visualisation of cut-off scores, wherein the vast majority of individuals did not meet cut-offs at either time point. For those meeting cut-offs, anxiety was about three times more common than depression at both time points, with comorbid depression and anxiety 1-month post-stroke tending to remain as comorbid depression and anxiety or shifting to only anxiety at 12-months.
The findings suggest that further work needs to be done to ensure that both depression and anxiety are monitored over time post-stroke, with a high likelihood of these being comorbid. Furthermore, the proportion of individuals meeting criteria for possible caseness didn’t change substantially over time, suggesting that there is a need for psychological interventions for depression and anxiety both in acute and late stages post-stroke.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This project was funded by the New Zealand Health Research Council (ref# 19/691).
Informed consent
Written informed consent was obtained from the patient(s) for anonymised information to be published.
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Ethical approval was obtained from the New Zealand Health and Disability Ethics Committee (19/NTA/177) and Auckland University of Technology Ethics Committee (20/71).
Author contributions
All authors are ARCOS-V steering committee members, contributing to study design and implementation. SBC produced the initial draft manuscript and conducted the main analyses. All named authors contributed through critical feedback on and approved the final version of the manuscript.
References
Aben I, Verhey F, Lousberg R, Lodder J, Honig A (2002) Validity of the Beck Depression Inventory, Hospital Anxiety and Depression scale, SCL-90, and Hamilton Depression Rating Scale as screening instruments for depression in stroke patients. Psychosomatics 43, 386-393.
| Crossref | Google Scholar | PubMed |
Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T (1980) Cerebrovascular disease in the community: Results of a WHO collaborative study. Bulletin of the World Health Organisation 58, 113-130.
| Google Scholar | PubMed |
Angelelli P, Paolucci S, Bivona U, Piccardi L, Ciurli P, Cantagallo A (2004) Development of neuopsychiatric symptoms in post-stroke patients: A cross-sectional study. Acta Psychiatrica Scandinavica 110, 55-63.
| Crossref | Google Scholar | PubMed |
Annunziata MA, Muzzatti B, Bidoli E, Flaiban C, Bomben F, Piccinin M, Gipponi KM, Mariutti G, Busato S, Mella S (2020) Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Supportive Care in Cancer 28, 3921-3926.
| Crossref | Google Scholar | PubMed |
Ayerbe L, Ayis S, Wolfe C (2013) Natural history, predictors, and outcomes of depression after stroke: A systematic review and meta-analysis. British Journal of Psychiatry 202, 14-21.
| Crossref | Google Scholar | PubMed |
Barker-Collo SL (2007) Depression and anxiety 3 months post stroke: Prevalence and correlates. Archives of Clinical Neuropsychology 22, 519-531.
| Crossref | Google Scholar | PubMed |
Barker-Collo S, Krishnamurthi R, Witt E, Theadom A, Starkey N, Barber A, Bennett D, Rush E, Aroll B, Feigin V (2017) Depression and Anxiety Across the First Year After Ischemic Stroke: Findings from a Population-Based New Zealand ARCOS-IV Study. Brain Impairment 18(3), 265-276.
| Google Scholar |
Batterham A, Hopkins W (2006) Making meaningful inferences about magnitudes. International Journal of Sports Physiology and Performance 1(1), 50-57.
| Google Scholar | PubMed |
Biering K, Hjollund NH, Frydenberg M (2015) Using multiple imputation to deal with missing data and attrition in longitudinal studies with repeated measures of patient-reported outcomes. Clinical Epidemiology 7, 91-106.
| Crossref | Google Scholar | PubMed |
Bjelland I, Dahl A, Haug T, Neckelmann D (2002) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research 52(2), 69-77.
| Crossref | Google Scholar | PubMed |
Broomfield N, Quinn T, Abdul-Rahim A, Walters M, Evans J (2014) Depression and anxiety symptoms post-stroke/TIA: prevalence and associations in cross-sectional data from a regional stroke registry. BMC Neurology 14, 198-206.
| Crossref | Google Scholar | PubMed |
Carod-Artal F (2006) Depresión postictus (I). Epidemiología, criterios diagnosticos y factores de riesgo. Revista de Neurologia 42, 169-175 [In Spanish].
| Google Scholar | PubMed |
Chun H, Ford A, Kutlubaev MA, Almeida OP, Mead GE (2022) Depression, Anxiety, and Suicide After Stroke: A Narrative Review of the Best Available Evidence. Stroke 53(4), 1402-1410.
| Crossref | Google Scholar | PubMed |
Cook CE (2008) Clinometrics corner: The minimal clinically important change score (MCID): A necessary pretence. Journal of Manual and Manipulative Therapy 16(4), 82E-83E.
| Crossref | Google Scholar |
Dafer R, Rao S, Shareef A, Sharma A (2008) Poststroke depression. Topics in Stroke Rehabilitation 15, 13-21.
| Crossref | Google Scholar | PubMed |
Fang Y, Mpofu E, Athanasou J (2017) Reducing depressive or anxiety symptoms in post-stroke patients: Pilot trial of a constructive integrative psychosocial intervention. International Journal of Health Science 11(4), 53-58.
| Google Scholar | PubMed |
Gosselin P, Castonguay C, Goyette M, Lambert R, Brisson M, Landreville P, Grenier S (2022) Anxiety among older adults during the COVID-19 pandemic. Journal of Anxiety Disorders 92, 102633.
| Crossref | Google Scholar | PubMed |
Grace-Martin K (2022) Approaches to repeated measures data: repeated measures anova, marginal, and mixed models. The Analysis factor LCC. Available at https://www.theanalysisfactor.com/repeated-measures-approaches/ [retrieved 20 June 2024]
Hackett M, Pickles K (2014) Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. International Journal of Stroke 9, 1017-1025.
| Crossref | Google Scholar | PubMed |
Hajiro T, Nishimura K (2002) Minimal clinically significant difference in health status: the thorny path of heath status measures? European Respiratory Journal 19, 390-391.
| Crossref | Google Scholar | PubMed |
Healey AK, Kneebone II, Carroll M, Anderson SJ (2008) A preliminary investigation of the reliability and validity of the brief assessment schedule depression cards and the Beck Depression Inventory-Fast Screen to screen for depression in older stroke survivors. International Journal of Geriatric Psychiatry 23, 531-536.
| Crossref | Google Scholar | PubMed |
Hoffmann T, Ownsworth T, Eames S, Shum D (2015) Evaluation of brief interventions for managing depression and anxiety symptoms during early discharge period after stroke: a pilot randomized controlled trial. Topics in Stroke Rehabilitation 22(2), 116-126.
| Crossref | Google Scholar | PubMed |
Jacobson N, Truax P (1991) Clinical significance: a statistical approach to defining meaningful change in psychotherapy-research. Journal of Consulting and Clinical Psychology 59, 12-19.
| Crossref | Google Scholar | PubMed |
Knapp P, Dunn-Roberts A, Sahib N, Cook L, Astin F, Kontou E, Thomas SA (2020) Frequency of anxiety after stroke: an updated systematic review and meta-analysis of observational studies. International Journal of Stroke 15, 244-255.
| Crossref | Google Scholar | PubMed |
Krishnamurthi R, Jones A, Barber P, Barker-Collo S, McPherson K, Bennett D, et al. (2014) Methodology of a population-based stroke and TIA incidence and outcomes study: the Auckland Regional Community Stroke Study (ARCOS IV) 2011-2012. International Journal of Stroke 9(1), 140-147.
| Crossref | Google Scholar | PubMed |
Liu L, Xu M, Marshall IA, Wolfe CDA, Wang Y, O’Connell MDL (2023) Prevalence and natural history of depression after stroke: A systematic review and meta-analysis of observational studies. PLoS Medicine 20(3), e1004200.
| Crossref | Google Scholar | PubMed |
Mainzer R, Apajee J, Nguyen CD, Carlin JB, Lee KJ (2021) A comparison of multiple imputation strategies for handling missing data in multi‐item scales: guidance for longitudinal studies. Statistics in Medicine 40, 4660-4674.
| Crossref | Google Scholar | PubMed |
Meader N, Mitchell AJ, Chew-Graham C, Goldberg D, Rizzo M, Bird V, Kessler D, Packham J, Haddad M, Pilling S (2011) Case identification of depression in patients with chronic physical health problems: a diagnostic accuracy meta-analysis of 113 studies. British Journal of General Practice 61, e808-e820.
| Crossref | Google Scholar | PubMed |
Otto E, Culakova E, Meng S, Zhang Z, Xu H, Mohile S, Flannery MAM (2022) Overview of Sankey Flow Diagrams: Focusing on Symptom Trajectories in Older Adults with Advanced Cancer. Journal of Geriatric Oncology 13(5), 742-746.
| Crossref | Google Scholar | PubMed |
Page P (2014) Beyond statistical significance: clinical interpretation of rehabilitation research literature. International Journal of Sports Physical Therapy 9(5), 726-36.
| Google Scholar | PubMed |
Parlapani E, Holeva V, Nikopoulou VN, Sereslis K, Athanasiadou M, Godosidis A, Diakogiannis I (2020) Intolerance of uncertainty and loneliness in older adults during the COVID-19 pandemic. Frontiers in Psychiatry 11, 842.
| Crossref | Google Scholar | PubMed |
Paul S, Dewey H, Sturm J, Macdonell R, Thrift A (2006) Prevalence of depression and use of antidepressant medication at 5 years post-stroke in the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 37, 2854-2855.
| Crossref | Google Scholar | PubMed |
Qiu J, Shen B, Zhao M, Wang Z, Xie B, Xu Y (2020) A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. General Psychiatry 33, e100213.
| Crossref | Google Scholar | PubMed |
Rossi MJ, Brand JC, Lubowitz JH (2023) Minimally Clinically Important Difference (MCID) is a low bar. Arthroscopy 39(2), 139-141.
| Crossref | Google Scholar | PubMed |
Sagen U, Vik TG, Moum T, Mørland T, Finset A, Dammen T (2009) Screening for anxiety and depression after stroke: Comparison of the Hospital Anxiety and Depression Scale and the Montgomery and Asberg Depression Rating Scale. Journal of Psychosomatic Research 67(4), 325-332.
| Crossref | Google Scholar | PubMed |
Snaith R (2003) The Hospital Anxiety and Depression Scale. Health & Quality of Life Outcomes 1, 29.
| Crossref | Google Scholar | PubMed |
Stanton AL, Wiley JF, Krull JL, Crespi CM, Hammen C, Allen JJ, Barrón M, Jorge A, Weihs KL (2015) Depressive episodes, symptoms, and trajectories in women recently diagnosed with breast cancer. Breast Cancer Research and Treatment 154, 105-115.
| Crossref | Google Scholar | PubMed |
Sveen U, Bautz-Holter E, Sandvik L, Alvsåker K, Røe C (2010) Relationship between competency in activities, injury severity, and post-concussion symptoms after traumatic brain injury. Scandinavian Journal of Occupational Therapy 17, 225-232.
| Crossref | Google Scholar | PubMed |
Turner A, Hambridge J, White J, Carter G, Clover K, Nelson L, Hackett M (2012) Depression Screening in Stroke: A Comparison of Alternative Measures With the Structured Diagnostic Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Major Depressive Episode) as Criterion Standard. Stroke 43, 1000-1005.
| Crossref | Google Scholar | PubMed |
Wu QE, Ai-min Z, Han YP, Liu YM, Yang Y, Wang XM, Shi X (2019) Poststroke depression and risk of recurrent stroke: a meta-analysis of prospective studies. Medicine (Baltimore) 98, e17235.
| Crossref | Google Scholar | PubMed |
Yu B, Silva CT (2017) VisFlow - web-based visualization framework for tabular data with a subset flow model. IEEE Transactions on Visualization and Computer Graphics 23, 251-260.
| Crossref | Google Scholar | PubMed |
Zigmond A, Snaith R (1983) The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 67(6), 361-370.
| Crossref | Google Scholar | PubMed |