Northern Australia Quarantine Strategy plant health surveys: over thirty years of a globally unique on- and off-shore solution to island nation biosecurity challenges
Richard I. Davis A B * , Lynne M. Jones A , Harshitsinh A. Vala A , Bradley Pease A , David Cann A , Pere Kokoa C and Francis T. Tsatsia D
A B * , Lynne M. Jones A , Harshitsinh A. Vala A , Bradley Pease A , David Cann A , Pere Kokoa C and Francis T. Tsatsia D
A
B
C
D
Abstract
As the Northern Australia Quarantine Strategy (NAQS) approaches its thirty-fifth year of operations, we outline the Australian Government’s approach to address extraordinary natural and human mediated biosecurity challenges across our sparsely populated northern shores. NAQS is a concept that is unique worldwide but could be equally well applied in many other island nations dealing with similar circumstances. Key to the success of the NAQS has been long collaborations with biosecurity scientists in the neighbouring nations to the north. Some examples of how these relationships have borne fruit as we tackle regionally important plant diseases are illustrated. We also focus on how the plant pathology component of the program developed and evolved from the early 1990s to 2023.
Keywords: biosecurity threats, diagnostics, environmental health, First Nations ranger network, Indonesia, northern Australia, Papua New Guinea, plant based industries, plant health surveys, Solomon Islands, Timor Leste.
Introduction
Australia is surrounded by water, and as such it has a natural geographic barrier that protects it from many exotic pests and pathogens. However, Australia faces unique biosecurity challenges due to its proximity to Indonesia, Timor Leste and Papua New Guinea (PNG), which are countries with many insect pests, plant, fungal and animal diseases and weeds that do not occur in Australia. Natural introduction of exotic biosecurity threats can occur with annual north-west monsoon weather systems including tropical cyclones (Fig. 1).1
Depiction of wind direction and force that prevailed during Tropical Cyclone Niran, 4 March 2021. Image credit: earth.nullschool.net.
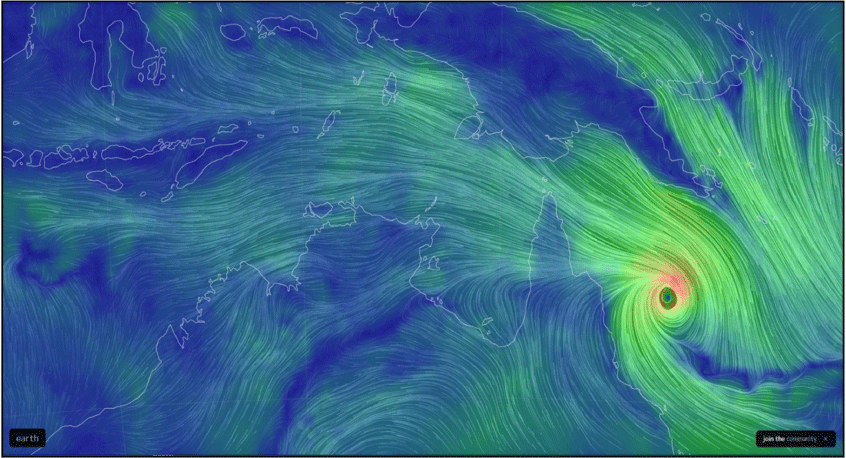
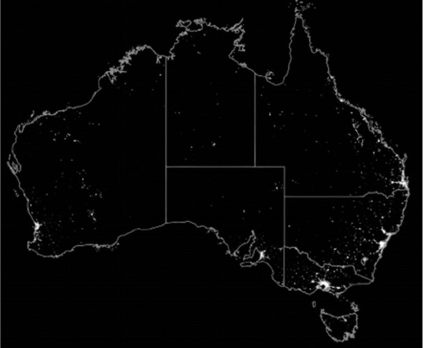
These winds occur in periods of high temperature, relative humidity and cloud cover, making them ideal for long distance transport of insects and fungal spores. There are also human-mediated threats, such as legal and illegal vessel arrivals. The island of New Guinea, shared geographically almost equally by Indonesia in the west and PNG in the east, is at its closest point less than five km from the Australian island of Saibe in Torres Strait. History has shown that exotic pests can use the islands of Torres Strait as ‘stepping stones’ to cross the 150 km of shallow sea that separates PNG from mainland Australia. This is exacerbated by the traditional movements of people and trading of goods, permitted under The Torres Strait Treaty of 1978.2 Early detection of any newly arrived exotic pest provides the best chance of eradication, but this is a difficult task because the northern coastline is sparsely populated and challenging to access (Fig. 2).
Australia at night, an image that highlights the relatively sparce population of the northern coastline compared to the east, south and west. Image and data processing of night-light data by NOAA’s National Geophysical Data Center. Defense Meteorological Satellite Program data collected by the US Air Force Weather Agency. Reproduced from Kamrowski and others (2012).
In 1987, the biosecurity vulnerability of northern Australia was formally addressed when the Quarantine Review Committee headed by Professor David Lindsay published the interim Report on Aerial Littoral Surveillance and Northern Australia Quarantine Strategy (NAQS)3 recommending establishment of a strategy focused on the unique biosecurity risks in northern Australia. There were several triggers for the Lindsay review through the 1970s and 1980s, including detections of exotic fruit flies in both far north Queensland and the Northern Territory. These detections led to the establishment of what was termed the Northern Monitoring Network. From a plant pathology perspective, the most important development in this era was an outbreak of citrus canker disease on Thursday Island in Torres Strait in 1984.4 This disease, caused by the bacterium Xanthomonas citri subsp. citri was then, and remains today, one of the Australian citrus industry’s highest priority biosecurity threats. This was a serendipitous discovery made by David Jones, a plant pathologist who was on the island to work on banana diseases under the Northern Monitoring Network. This incursion, and subsequent successful eradication,5 provided a wakeup call that Australian biosecurity needed. NAQS commenced in 1989 as a collaboration between the federal government, and Queensland, Northern Territory and Western Australian state governments.
The operational model for NAQS was based on a Queensland Department of Primary Industries working party report, and consequently the early years were somewhat Queensland focused, with three plant health scientists based in Queensland, two in Western Australia and one in the Northern Territory. But this initial small, dedicated and enthusiastic team of scientific specialists and biosecurity officers went on to forge an enduring legacy. In 1995, again following a key exotic fruit fly incursion in Cairns, a review by Nairn and Muirhead,6 confirmed the value of the NAQS contribution to biosecurity in northern Australia and recommended an increase in activities and resourcing. NAQS transitioned to purely Commonwealth government funding, its area of focus became larger, and more scientific staff and biosecurity officers were employed. Today NAQS is part of the Australian Government’s Department of Agriculture, Fisheries and Forestry’s (DAFF) Science and Surveillance Group (SSG). SSG is tasked with addressing regulated (at the border) and unregulated (through the NAQS) incursion pathways. From that time until today, NAQS has operated across coastal and inland regions and islands that lie between Cairns and Broome, with surveillance frequency dictated by risk modelling (Fig. 3).
NAQS risk zones 2022. Each zone is assigned a plant health survey frequency from annually, once every two years, once every three to five years, to once every five years, depending on perceived risk.
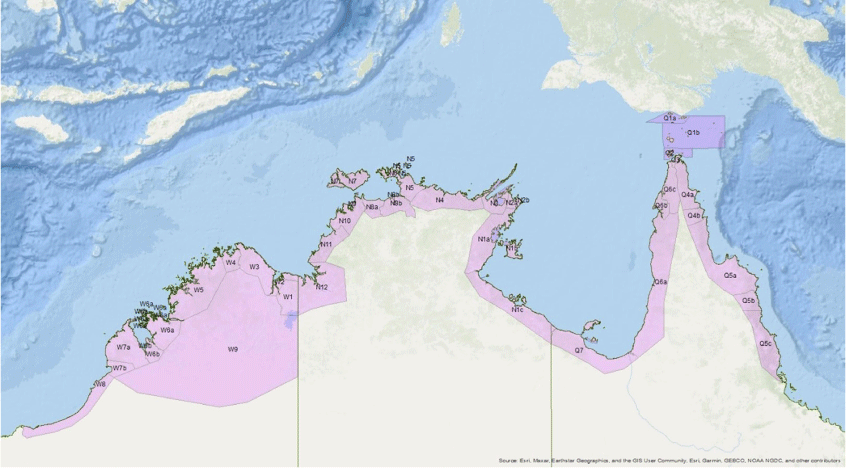
The NAQS plant health approach has been to deploy teams comprising entomologists, botanists and plant pathologists, most with hands-on exotic pest experience gained overseas, to conduct regular surveys, mostly in and around centres of human activity. This surveillance is based on target lists of threats, prioritised by geographic proximity and potential impact, with the highest ranked threats being placed on the ‘A list’. Survey teams collect plant and insect samples and analyse them, initially at NAQS laboratories in Darwin and Cairns, but difficult-to-identify specimens are also forwarded to collaborating laboratories across Australia and overseas. The surveys are normally combined with a public awareness program, encouraging local people and communities to report unusual weeds, insect pests and plant diseases. Remote communities greatly value the plants that they grow and that occur naturally around them, and this facilitates adoption of biosecurity messages.
Essential to NAQS domestic activities has been the ongoing cooperation and goodwill of First Nations communities who today are actively involved in delivering NAQS surveys and other First Nations engagement programs. On the ground, this translates into assistance provided by First Nations biosecurity officers on all inhabited Torres Strait islands and the communities of the Northern Peninsula Area of Cape York Peninsula and local ranger groups and residents providing appropriate permissions to access their properties across the NAQS areas of operation. The interaction with First Nations ranger groups has grown and attracted significant and ongoing departmental funding in recent years to conduct key fee-for-service activities. NAQS domestic surveillance today is a close partnership with rangers (Box 1).
| Box 1.Partnerships between the NAQS and First Nations communities. |
| Rapport between the NAQS and First Nations communities has been built and maintained over decades, initially as informal working relationships and arrangements on a case-by-case basis with Aboriginal and Torres Strait Islander Traditional Owners and ranger groups. These relationships have more recently developed into the Indigenous Ranger Biosecurity Program, a government initiative through which over sixty First Nations ranger groups in northern Australia have fee-for-service contracts to assist the NAQS in the delivery of biosecurity surveillance (Fig. 4). This program leverages the existing work First Nations rangers do in the areas of land conservation and management, native species preservation and caring for country.7 First Nations rangers play two key roles in plant health surveillance. The first being the facilitation and support of NAQS plant health surveys. Rangers disseminate biosecurity messages within their respective communities, provide NAQS with local knowledge about properties with host plants, and accompany NAQS scientists during surveys. The second, emerging, role First Nations rangers play is providing additional plant health surveillance at times when NAQS is not visiting a particular area. Rangers are trained in disease recognition by NAQS plant pathologists, and this is reinforced by on-country engagement and training from department community liaison officers. Groups in high-risk locations then monitor banana and citrus plants in their communities. Key plant diseases include citrus canker, huanglongbing, black Sigatoka and banana freckle. Rangers inspect plants and answer targeted questions using a specialist app developed by the department and are prompted to take photos of particular symptoms. NAQS plant pathologists review photos and request samples if required. This monitoring activity was responsible for the first recorded detection of yellow Sigatoka on Groote Eylandt in Northern Territory by the Anindilyakwa Land and Sea Rangers in 2023. Rangers are also encouraged to report any plant disease symptoms that they find whilst completing their day-to-day work. For example, a report of a misshapen sweet potato by the Garngi Rangers led to a notifiable detection of guava root knot nematode (Meloidogyne enterolobii) on Croker Island in Northern Territory in 2022. While the remit and focus of the NAQS surveillance work has been concerned with hosts of economic importance, the biosecurity work undertaken by First Nations rangers can often be relevant to culturally important native plant species, and therefore is of significant value to the First Nations communities of northern Australia,8 in addition to the Australian agricultural sector. |
Attendees at the 2019 Northern Australia Indigenous Biosecurity Ranger Forum hosted by the Kimberley Land Council, Gurrbalgun on Bardi Country, Western Australia. Image: Kerry Trapnell.
Without a doubt, the key to the success of the NAQS plant health program has been a constant interaction with biosecurity agencies in the countries that lie immediately to the north of the NAQS domestic surveillance region. This has manifested itself over the decades as a long series of on-the-ground plant health surveys overseas. These have been conducted in collaboration with plant health scientists of these countries, with samples and specimens examined in country or returned to Australia for diagnosis under biological material import permit.
The early years of NAQS plant disease surveillance
Initially, the NAQS conducted plant health surveillance in Australia’s extreme north and overseas, in collaboration with the biosecurity agencies of PNG and Indonesia. This resulted in extensive plant disease lists for remote regions of far north Queensland,9 the coastal villages of PNG that lie adjacent to Torres Strait,10 and across the Indonesian half of the island of New Guinea.11 Most records were of plant pathogenic fungi that were preserved as pressed ‘plant’ specimens and identified using morphological features that were visible under the light microscope. Observations of disease symptoms thought to be caused by other pathogen groups on the island of New Guinea were mentioned only in passing. This led to the publication of a letter to the editor of the journal Australasian Plant Pathology protesting that diseases caused by viruses and bacteria deserved equal attention.12 The reason for this lack of focus on diseases caused by non-fungal pathogens may have been the practice that prevailed through earlier years, of returning viral and viral-like disease samples for laboratory analysis as preserved specimens for electron microscopy and fresh material for use in traditional virus-indexing experiments. This meant that moving samples from extremely remote locations within a country or between countries often resulted in the laboratory receiving degraded samples that were not fit for diagnostic testing. The problem was accentuated when the tools of molecular biology rose to the fore in diagnostics from the early 1990s onwards. This was because nucleic acid extractions prepared from degraded plant and fungal materials will contain chemicals that result from oxidative degradation of plant and fungal tissues that interfere with, or completely inhibit downstream PCR reactions.13 NAQS plant pathology sought to address this issue and the key to this approach was the adoption of an in-field sample preservation system in which plant and fungal material is diced finely and rapidly desiccated at 4°C (Box 2).
| Box 2.How a standard laboratory preservation method became a daily tool on NAQS disease surveys. |
| When Richard Davis visited John Thomas’ Queensland government plant virology laboratory in Brisbane in 1993, he was shown their use of a standard virus disease sample preservation technique. This involved finely chopping leaf and shoot tissues and desiccating it over anhydrous calcium chloride in small vials at 4°C, followed by long term storage in a freezer. He was working in the Pacific islands at this time and had been trying to deal with the problem of transporting fresh kava leaf samples from remote island locations to a central diagnostic laboratory in a suitable condition for virus testing using Enzyme Linked Immunosorbent Assay (ELISA). This technique was immediately adopted as a standard field survey method, returning 0.1 g fresh weight leaf samples (enough for several ELISA tests) in 1.5 mL microfuge tubes because it worked well. Upon starting with the NAQS in 1996, the same method was used with quantities of tissue and desiccant multiplied by ten so that more tests were possible and reference material would be left over. This became the standard method adopted by all NAQS plant pathologists. The leaf tissue is separated from the desiccant in the tube by a barrier such as tissue paper or a kimwipe. After twenty four hours the barrier paper and some of the adjacent desiccant will be wet, so they are replaced with dry ones. If the desiccation process occurs entirely at 4°C in a refrigerator, crispy green fragments of tissue result, with no black/brown oxidative reaction products present which can interfere with later molecular diagnostics (Fig. 5). Today, silica gel replaces anhydrous calcium chloride and zip lock plastic bags replace 20 mL collection tubes and the method is used widely by plant pathologists and botanists. |
Early field collection method using anhydrous calcium chloride to desiccate leaf tissue in the field. In this case leaf midribs have been excised to enrich for a phloem limited pathogen. Image: Richard Davis, 1999.

The adoption of this methodology to field work was of course, something not completely new. For example, anhydrous calcium chloride desiccation was used as one of several methods to return plant virus samples from the Solomon Islands to the United Kingdom (UK) in 1985.14 What was unique to the NAQS plant pathology team was the way this method was reached for ‘in the toolbox’ every evening in the field. The result was a stable supply of material for analysis using the tools of serology and molecular biology in NAQS and collaborating laboratories. In this way, numerous viral and phytoplasma records were obtained from remote northern Australia and across both halves of the island of New Guinea.15 This method also proved effective for use with fungal and bacterial diseases for which specific molecular diagnostic tests were available, such as black Sigatoka disease of banana16 and citrus canker.17 Initially, all NAQS collections of rapidly desiccated dried tissue from overseas (including Torres Strait) were first surface-sterilised in a solution of 1% chlorine and blotted dry before chopping finely and desiccating. In the mid-2000s, this import permit condition was replaced with a requirement for gamma irradiation at 25 kGy. Contrary to popular opinion at the time, this had no major impact on diagnostic tests that used PCR to amplify DNA or cDNA from an RNA template, even when the amplimers were large such as the 1800 bp amplicon produced by universal phytoplasma primers.18
The evolution of NAQS plant pathology
The years following the Nairn and Muirhead review were characterised by budgetary constraints for NAQS. A financial boost came in 2006 when NAQS was tasked with delivering foreign fishing vessel response work after a spate of illegal landings on remote coastlines. This was also the time that a network of First Nations ranger groups was first engaged across northern Australia to conduct fee for service activities. From 2014 onwards, the Commonwealth government’s Stronger Biosecurity and Quarantine Initiative allowed NAQS to upgrade its laboratories with newer molecular diagnostic capability. The Commonwealth government’s Agricultural Competitiveness White Paper and Our North, Our Future: White Paper on Developing Northern Australia also resulted in significant funds allocated to NAQS that translated into greater molecular capacity. Most important of all was the ongoing appointment of dedicated molecular biologists in both the Darwin and Cairns NAQS laboratories. In 2021, DAFF released a new strategy paper, called Commonwealth Biosecurity 2030,19 which is a blueprint for the development of a stronger and more sustainable biosecurity system. A key component of this is the Modern Technologies and Diagnostic Tools initiative.20 This will fund the update and modernisation of the departmental plant diagnostics capacity to deal with both regulated and unregulated risk pathways (Box 3).
| Box 3.The value of agility in NAQS plant health diagnostics. |
| The diagnostic requirements of the NAQS are ever-changing, encompassing a wide range of plant health scenarios across multiple locations, host plants, climates and stakeholders. The agile diagnostics system employed by the NAQS is more suitable than a traditional diagnostics system to tackle the dynamic challenges posed by plant disease surveillance. The agility of the NAQS diagnostic approach arose from its willingness to seek external expertise whenever necessary. The strategy actively looks for the most suitable experts to assist in each case, whether within Australia or internationally, ensuring that the most relevant and current knowledge is applied. The goodwill of diagnostic collaborators is, however, not endless. NAQS must ensure that samples are effectively triaged to minimise the burden placed on others. To this end, the strategy has adopted a two-step approach for diagnostics. Firstly, NAQS maintains two laboratories in northern Australia with the capability to conduct preliminary testing through bespoke methodologies. The goal of this testing is to generate initial evidence of a pathogen, which can then be used to target the sample to an accredited laboratory for confirmation, if needed. Recently NAQS invested in high throughput sequencing (HTS) workflows, which have proven remarkably effective in improving detection of the range of organisms that are associated with a particular disease. The workflows have highlighted the number of cases involving complex diagnoses and reduced the number of follow-up tests using specific diagnostic assays. |
Over the years, NAQS domestic surveys have resulted in new state, territory and national records of various plant pathogens. Some of these have been formally published, but many have not. A representative selection of these pathogens is listed in Supplementary Table S1. The early focus of NAQS was on the far north Queensland and Torres Strait risk pathway and the only domestic detections of a high-profile pathogen on the NAQS A target list resulted from surveillance in this corridor. These were finds of black Sigatoka disease in back yards in the Northern Peninsula Area of Cape York Peninsula in 199821 and on an inner Torres Strait island in 2023 (L. Jones, NAQS, unpubl. data). However, it is important to note that other regions of the NAQS area of operation are equally vulnerable to major incursions. Two plant disease records stand out because of their extreme national significance and the fact that they were made by NAQS plant pathologists in Darwin who were off duty at the time. Both led to major eradication campaigns that cost millions of dollars. The first was the discovery of grapevine leaf rust, caused by a fungus now named Neophysopella tropicalis in a backyard in suburban Darwin in 2001.22 The second was citrus canker, affecting small plantlets for sale in the garden section of a major hardware chain store in 2018. Both detections were a direct result of personal hands-on experience with these pathogens gained on NAQS surveys overseas. Critically, these detections occurred only because the NAQS staff duty station was the city of Darwin, rather than operating a policy of fly-in-fly-out visits from a major southern capital city.
Another important new record for Australia occurred at the Cairns Botanical Gardens when NAQS conducted a survey there. An aggressive and rapidly lethal wilt disease in ornamental palms, including coconuts, growing in the gardens and later in several adjacent suburbs was discovered. Samples were indexed for phytoplasmas because lethal diseases of palms associated with phytoplasmas have been causing devastating losses elsewhere in the world for more than a century.23 A phytoplasma was indeed found, but it was quite unlike anything ever known before. As these organisms are unculturable, phytoplasma nomenclature is based upon 16S rRNA gene and other sequences and is currently complex. The most closely related phytoplasmas were those associated with palm lethal yellows diseases in Africa that belong to group 16SrXXII (Nigerian coconut lethal decline group). However, the Cairns phytoplasma was sufficiently different for it be assigned to a novel taxon ‘Candidatus Phytoplasma dypsidis’,24 which was later assigned to a new group 16SrXXXIX.25 Deeper investigations into this possibly indigenous phytoplasma are needed, such as determining insect vectors and alternative hosts.
Since the turn of the century, international agricultural trade negotiations have shifted dramatically towards a requirement for evidence of absence of pests rather than being satisfied with an absence of evidence of their presence. Despite surveillance of southern commercial production properties not being part of its mandate, NAQS can make valuable contributions to this by collecting qualitative negative data by examining ‘sentinel host plants’ in very remote areas. These are counts of cultivated garden plants and plants in some commercial crops that were checked for target pests and nothing was observed that raised any suspicion. Such data is also of value for area freedom and delimitation surveillance when incursions do occur. A smaller amount of quantitative negative data, in which samples are collected and indexed negative for target diseases in the laboratory, is also generated.
NAQS surveillance overseas
A key pillar of the NAQS surveillance has been collaborative surveys with the governments of Australia’s northern neighbouring countries, to obtain an ‘over the horizon’ view of plant health threats. These included several surveys in the 1990s of the Indonesian provinces of Papua and West Papua, which were then known as Irian Jaya, by NAQS in collaboration with Indonesia’s Centre for Agricultural Quarantine (CAQ), and later the International Animal Science Research and Development Foundation (INI ANSREDEF). These surveys were critical because the area shares a land border with PNG and it was the focus of an Indonesian government transmigration program that peaked through the 1980s and early 1990s.26 The transmigration program brought about the influx of many thousands of previously landless families from overcrowded western regions of Indonesia, establishing new agricultural enterprises across massive areas of land. Such large-scale movements of plants and animals across Wallace’s line27 poses substantial biosecurity threats. Thus, a change in plant pest status caused by transmigration was expected and indeed, these joint surveys resulted in some important finds on the Indonesian half of the island of New Guinea. These included the first record of blood disease of banana, caused by the bacterium now named Ralstonia syzgii subsp. celebesensis,28 and of ‘tropical’ race 4 of the fungus that causes Panama disease of banana, Fusarium oxysporum f.sp. cubense.29 The end of the 1990s marked the termination of Indonesia’s collaboration with NAQS to undertake surveys of what is today Papua and West Papua Provinces. In contrast, collaborative surveys have been conducted annually in PNG from the early 1990s and in Timor Leste from 1999. Activities in Timor Leste initiated when NAQS was funded by AusAid to deliver a quarantine capacity building project, that included plant health surveys, from 1999 to 2004. Later, DAFF oversaw all surveillance activity, collaborating with the NAQS. For more than fifteen years this has occurred under the auspices of DAFF’s International Plant Health Surveillance Program (IPHSP). DAFF collaboration continues today with PNG’s National Agricultural Quarantine and Inspection Authority (NAQIA) and Timor Leste’s National Directorate for Quarantine and Biosecurity (DNQB). NAQS plant health scientists have also been involved in DAFF activities in the Solomon Islands since 2010. These have been a series of plant and animal biosecurity support projects funded by the Department of Foreign Affairs and Trade (DFAT), working with Biosecurity Solomon Islands (BSI), with surveillance a key component. Key plant disease findings from these overseas surveys that are not discussed individually in this paper are presented in Supplementary Table S2. Timor Leste data is not currently included in this review. Three individual stories that played out across several years are outlined below. Each emphasises the great value of a long-term relationship between Australia and its northern neighbours.
Huanglongbing (HLB) disease of citrus on the island of New Guinea
HLB is the most important disease of citrus in the world and in warmer regions is caused by ‘Candidatus Liberibacter asiaticus’, a phloem-limited unculturable bacterium that is vectored by the Asian citrus psyllid (ACP), Diaphorina citri. This disease has no cure and has devastated citrus production in many citrus producing countries outside of Oceania.30 HLB and ACP are both included in DAFF’s National Priority Plant Pest list.31 The experience elsewhere in the world, such as in Florida32 and Brazil,33 has been for the insect vector to establish first, then for the pathogen to arrive a few years later. NAQS collaborative surveillance documented this same phenomenon on the island of New Guinea. With both the disease and the insect known in Indonesia for many years, ACP had been listed as present in the Bird’s Head Peninsula at the northwestern end of Irian Jaya Province.34 Soon after, a joint NAQS/CAQ survey found the insect in the eastern most part of the province, adjacent to PNG (NAQS/CAQ, unpubl. data 1992). Attempts to detect HLB made in 1993 failed because fresh samples deteriorated too much in transit when they were sent to a specialist laboratory in France for DNA hybridisation analysis.35 A survey in 1997 sent DNA extracts prepared in Australia to the same French laboratory and negative results were obtained (NAQS/INI ANSRADEF, unpubl. data). A survey in 1999 returned desiccated material for PCR testing in the NAQS laboratory in Australia, providing the first records of HLB in the same locations where ACP was known in Irian Jaya Province.36 HLB was then detected in the same way across the border in Sandaun Province of PNG in 2002.37 Extensive joint agency surveillance across PNG since then suggests actions taken to mitigate spread have been successful and the insect and disease have remained remarkably restricted to a small area in and around the town of Vanimo.38
Phytoplasmas associated with lethal wilts of banana plants in PNG and Solomon Islands
Collaborative surveillance for banana disease threats to PNG that are present in Indonesia such as blood and Panama disease has been a key feature of the relationship between DAFF and NAQIA. This has been because of their potential to decimate banana production and threaten Musa sp. genetic diversity. Such surveillance led to the discovery of a devastating and previously unheard of worldwide, lethal wilt disease of cooking banana plants associated with phytoplasmas that is also now included in DAFF’s National Priority Plant Pest list.31 The phytoplasma disease was first found in PNG and was named the banana wilt associated phytoplasma (BWAP).39 For over a decade before this, survey teams had been baffled by this disease, cutting down yellow banana plants in many locations in PNG and finding no internal pseudostem symptoms consistent with the key target diseases on the other side of the border. Instead of continuous internal discolouration, which characterises both blood and Panama diseases, a discontinuous patchy necrosis of vascular tissues was always found. It was only when a related disease of coconut palms40 was investigated in the northwest of PNG, where feedback from banana growers, and early PCR testing conducted by PNG’s oil palm research pathologists, pointed toward a phytoplasma etiology for the banana wilt. Today, the disease is known to be widespread across PNG and is believed to have caused thousands of cooking banana plant deaths. It is a new threat to food security in the country because cooking bananas in the highly susceptible Kalapua subgroup are the principle staple crop in many regions. Apparent spread from PNG’s Autonomous Region of Bougainville (AROB) to the adjacent Solomon Islands’ Shortland Islands group was first discovered on a joint agency survey in 2012.41 Molecular analysis of 16S rRNA gene sequences of many collections across the region now indicates that the BWAP phytoplasmas are a diverse group of very closely related phytoplasmas. Those from eastern PNG and Solomon Islands are crucially different to those found further west in PNG that have been assigned to a novel taxon called ‘Candidatus Phytoplasma noviguineense’,42 assigned in the most recent analysis to group 16SrXXXVIII.25 These eastern banana phytoplasmas therefore will likely become another novel ‘Candidatus Phytoplasma’ taxon. In between these variants there is also significant diversity. A sustainable cultural control strategy is desperately needed for this disease and the starting point would be further research to determine the insect vectors and their lifecycles, and alternative hosts.
Cassava bacterial blight in Solomon Islands
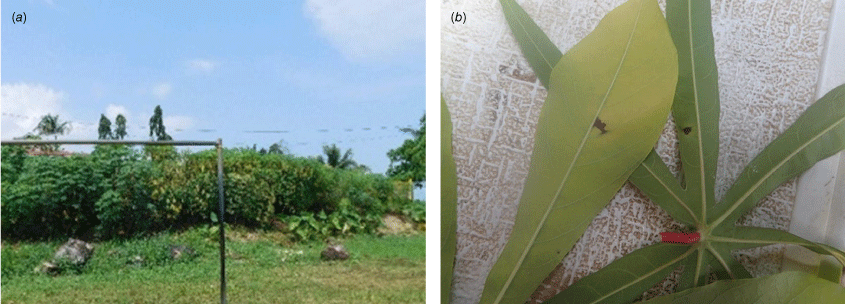
Cassava bacterial blight (CBB) is a major disease of this important staple crop across much of the world caused by Xanthomonas phaseoli pv. manihotis. The disease was found for the first time on any South Pacific Island when it was detected on a joint agency survey of Solomon Islands’ Malaita Province in 2015.43 Widespread damage was observed across numerous cassava plantings, suggesting a recent arrival on the island, previously surveyed and found CBB disease free in 2007 and 2010. Surprisingly, on subsequent joint plant health surveys of several islands in Western and Choiseul Provinces in 2018 and 2023, and again on Malaita in 2019, CBB was present but only at an almost imperceptible incidence and severity. This was despite highly conducive environmental conditions for bacterial disease development. These results strongly suggested that growers had dealt with the problem simply by selecting resistant and tolerant cassava cvs in the past. This process likely occurred several years ago in Western and Choiseul Provinces, and between 2015 and 2019 on Malaita (Fig. 6). It is interesting to note that such a wide range of germplasm was naturally available. There is a range of resistance in cassava, and this is the principle means of bacterial blight control across the world. The Solomon Islands Ministry of Agriculture (MAL) has had a long history of international collaboration with regional projects, making it possible that blight resistant cassava germplasm had been imported and distributed in the past. However, it would be difficult to investigate this as most records were lost when MAL’s principal research facility was destroyed in the ethnic tensions in 2000. An alternative explanation may be variability within the causal bacterium, which exhibits a range of virulence, with some isolates being described as non-pathogenic.44 It is so far not known if this was the case in the west of the country but adoption of resistant germplasm can be the only explanation for the turn around that occurred on Malaita (Box 4).
Bacterial blight of cassava damage observed on Malaita Island, Solomon Islands in 2015 (a) and in 2019 (b) after growers presumably switched to resistant germplasm naturally available on the island. Images: Richard Davis.
| Box 4.Revelations and resolutions. |
| Sometimes what appears to be a new discovery turns out to not be new at all, the matter was just awaiting technological developments and the re-examination of historical specimens. |
| Passiflora virus Y |
| A major impediment to passionfruit (Passiflora foetida) production worldwide, including Australia, is woodiness disease, caused by passionfruit woodiness virus (PWV, genus Potyvirus). In the late 1990s, strong viral leaf symptoms on the related weedy vine Passiflora foetida were found on NAQS surveys on Torres Strait islands, Cape York Peninsula and in the adjacent Papua Province of Indonesia. Samples were sent to a collaborating laboratory in what is today the Queensland Department of Agriculture and Fisheries (QDAF), where initial immunosorbent electron microscopy indicated something different from PWV. Subsequent molecular analysis indicated that a unique potyvirus was present, given the name passiflora virus Y.45 A diagnostic was developed which revealed that this virus was already well established and widespread in Queensland and NSW passionfruit production. It had not been noticed before because it was usually present as a mixed infection with PWV. The virions look the same under the electron microscope and both cause symptoms in the common indicator used at that time, Phaseolus vulgaris. |
| Phytophthora tropicalis |
| During 2020 when regular NAQS plant health surveys were cancelled because of the COVID-19 pandemic, NAQS plant pathologists engaged in surveillance closer to home. A leaf blight of a common ornamental arrowhead vine (Syngonium podophyllum) was discovered in the backyard of a Cairns NAQS entomologist and a Phytophthora sp. was isolated. This isolate was morphologically similar to P. tropicalis, a species considered exotic to Australia and serious on black pepper, macadamia, cacao and breadfruit. Molecular diagnostics conducted by QDAF identified the specimen as an undescribed Phytophthora sp. in Clade 2. In this study, a historical Phytophthora sp. collected in 2019 from south-east Queensland from a different host (Tristaniopsis laurina) was found to be identical to the arrowhead vine phytophthora in Cairns. Further molecular analysis by plant pathologists in the Western Australia Department of Primary Industries and Regional Development (WADPIRD) showed that three isolates from Queensland’s collection from Mandevilla sp. in the Cairns region in 1997 were in fact, the true P. tropicalis. This phytophthora, first considered a damaging exotic threat, was already well established in far north Queensland, and this would not have been known until a slight variant appeared. |
| Citrus scab in far north Queensland |
| Citrus scab disease is common in Australia’s tropics and historically has been attributed to the fungus Elsinoë fawcettii. Two pathotypes of E. fawcettii are recognised and the disease is found mostly on lemons, less so on limes and sometimes on mandarins under exceptional environmental circumstances. On a survey in Weipa, Queensland, in 2022, abnormal looking citrus scab symptoms were found on a back yard lemon tree. Molecular analyses conducted by QDAF demonstrated that it was a newly named species, E. citricola.46 An earlier molecular re-examination of historical specimens in the Queensland collection had revealed that this species had been collected before. The first find was from a NAQS survey in Cooktown in 2000, then four more records from southeast Queensland. |
Conclusions
In the paper, we have described and characterised the approach of the Australian Federal Government to the unique biosecurity situation the country faces in its extreme north. We have demonstrated the outstanding success of the NAQS program by referring to actual journeys of plant disease discovery both on- and off-shore, that have played out over nearly thirty-five years. We have also indicated that serendipity has played an important role in ‘off-duty’ discoveries of plant pathogens.
Data availability
The research data associated with this article is not publicly available. It remains the property of the relevant Australian and international jurisdictions.
Acknowledgements
The authors would like to gratefully acknowledge the support and professionalism over the years of many staff, too numerous to mention individually, of what is today the Australian Government Department of Agriculture Fisheries and Forestry’s Northern Australia Quarantine Strategy. Within NAQS, this is the plant health team members from other disciplines, biosecurity officers, community liaison officers, support staff, and management. Beyond NAQS, the professional wisdom, understanding and wise perception of Bart Rossel and Chris Dale who led the department’s International Plant Health Surveillance Program (IPHSP) since its inception should be highlighted. They orchestrated the involvement of NAQS personnel in the long series of international plant health surveys and oversaw a natural synergy that produced valuable returns to both Australia and our partner countries to the north. NAQS staff built long-term, hands-on experience with key target organisms whilst counterpart agencies overseas benefitted from close collegiate relationships and ready access to Australian and international diagnostic networks when needed. Barbara Waterhouse, the longest serving NAQS plant health scientist, Andrew Geering, ex Australasian Plant Pathology Society President, and Bernadette Curnuck, NAQS senior communications officer are all gratefully acknowledged for editorial comment. NAQS Operations Officer, Tim Kerlin, is thanked for assistance with figures.
References
Anonymous (1985) Treaty Between Australia and the Independent State of Papua New Guinea Concerning Sovereignty and Maritime Boundaries in the Area Between the Two Countries, Including the Area Known as Torres Strait, and Related Matters, Australian Treaty Series 1985 No 4, Department of Foreign Affairs, Canberra. Australian Government Publishing Service, Canberra.
Anonymous (2019) ‘National priority plant pests’, https://www.agriculture.gov.au/biosecurity-trade/pests-diseases-weeds/plant/national-priority-plant-pests-2019, viewed 9 April 2024
Anonymous (2023a) ‘Commonwealth Biosecurity 2030’, https://www.agriculture.gov.au/biosecurity-trade/policy/commonwealth-biosecurity-2030, viewed 9 May 2024.
Anonymous (2023b) ‘Modern technologies and diagnostic tools’, https://www.agriculture.gov.au/sites/default/files/documents/QID108443_Modern%20Technologies%20and%20Diagnostic%20Tools.pdf, viewed 9 April 2024.
Aubert, B. (1990) ‘Integrated activities for the control of huanglungbin-greening and its vector Diaphorina citri Kuwayama in Asia’ in Proceedings of the 4th International Asia Pacific Conference on Citrus Rehabilitation, eds B. Aubert, S. Tontyaporn, D. Buangsuwoon, Chiang Mai, Thailand, 4–10th February 1990, pp. 133–144.
Bove, J. M. (2006) Huanglongbing: a destructive, newly-emerging, century-old disease of citrus, Journal of Plant Pathology, 88, 7-37.
| Google Scholar |
Davis, R. I., Grice, K. R. E., Jacobson, S. C., Gunua, T. G., and Rahamma, S. (2000a) Surveillance for black Sigatoka disease of banana in and near the Torres Strait, Australasian Plant Pathology, 29, 225.
| Crossref | Google Scholar |
Davis, R. I., Fegan, M., Tjahjono, B., and Rahamma, S. (2000b) An outbreak of blood disease of banana in Irian Jaya, Indonesia, Australasian Plant Pathology, 29, 152.
| Google Scholar |
Davis, R. I., Moore, N. Y., Bentley, S., Gunua, T. G., and Rahamma, S. (2000c) Further records of Fusarium oxysporum f.sp. cubense from New Guinea, Australasian Plant Pathology, 29, 224.
| Crossref | Google Scholar |
Davis, R. I., Jacobson, S. C., Rahamma, S., and Gunua, T. G. (2000d) Surveillance for citrus huanglongbing (greening) disease in New Guinea and north Queensland, Australasian Plant Pathology, 29, 226.
| Google Scholar |
Davis, R. I., Thomas, J. E., McMichael, L. A., Dietzgen, R. G., Callaghan, B., James, A. P., Gunua, T. G., and Rahamma, S. (2002) Plant virus disease surveys on the island of New Guinea and adjacent regions of northern Australia, Australasian Plant Pathology, 31, 385-390.
| Crossref | Google Scholar |
Davis, R. I., Jacobson, S. C., De La Rue, S. J., Tran-Nguyen, L., Gunua, T. G., and Rahamma, S. (2003) Phytoplasma disease surveys in the extreme north of Queensland, Australia, and the island of New Guinea, Australasian Plant Pathology, 32, 269-277.
| Crossref | Google Scholar |
Davis, R. I., Kokoa, P., Jones, L. M., Mackie, J., Constable, F. E., Rodoni, B. C., Gunua, T. G., and Rossel, J. B. (2012) A new banana wilt disease associated with phytoplasmas in Papua New Guinea, Australasian Plant Disease Notes, 7, 91-97.
| Crossref | Google Scholar |
Davis, R. I., Henderson, J., Jones, L. M., McTaggart, A. R., O’Dwyer, C., Tsatsia, F. T., Fanai, C., and Rossel, J. B. (2015) First record of a wilt disease of banana plants associated with phytoplasmas in Solomon Islands, Australasian Plant Disease Notes, 10, 14.
| Crossref | Google Scholar |
Davis, R. I., Jones, L. M., Pease, B., Perkins, S. L., Vala, H. R., Kokoa, P., Apa, M., and Dale, C. J. (2021) Plant virus and virus-like disease threats to Australia’s north targeted by the northern Australia quarantine strategy, Plants, 10, 2175.
| Crossref | Google Scholar | PubMed |
Eagles, D., Deveson, T., Walker, P. J., and Zalucki, M. P. (2012) Evaluation of long-distance dispersal of Culicoides midges into northern Australia using a migration model, Medical and Veterinary Entomology, 26, 334-340.
| Crossref | Google Scholar | PubMed |
EFSA Panel on Plant Health (PLH), Jeger, M., Bragard, C., Candresse, T., Chatzivassiliou, E., Dehnen‐Schmutz, K., Gilioli, G., Gregoire, J.-C., Jaques Miret, J. A., MacLeod, A., Navarro, M. N., Niere, B., Parnell, S., Potting, R., Rafoss, T., Rossi, V., Urek, G., Van Bruggen, A., Van der Werf, W., West, J., Winter, S., Dickinson, M., Marzachi, C., Hollo, G., and Caffier, D. (2017) Scientific opinion on pest categorisation of palm lethal yellowing phytoplasmas, EFSA Journal, 15(5028), 27.
| Crossref | Google Scholar |
Fan, X. L., Barreto, R. W., Groenewald, J. Z., Bezerra, J. D. P., Pereira, O. L., Cheewangkoon, R., Mostert, L. C. M., Tian, C. M., and Crous, P. W. (2017) Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes), Studies in Mycology, 87, 1-41.
| Crossref | Google Scholar | PubMed |
Fearnside, P. M. (1997) Transmigration in Indonesia: lessons from its environmental and social impacts, Environmental Management, 21, 553-570.
| Crossref | Google Scholar |
Gonzalez, C., Restrepo, S., Tohme, J., and Verdier, V. (2002) Characterization of pathogenic and nonpathogenic strains of Xanthomonas axonopodis pv. manihotis by PCR-based DNA fingerprinting techniques, FEMS Microbiology Letters, 215, 23-31.
| Crossref | Google Scholar | PubMed |
Hyde, K. D., and Alcorn, J. L. (1993) Some disease associated microorganisms on plants of Cape York Peninsula and Torres Strait islands, Australasian Plant Pathology, 22, 73-83.
| Crossref | Google Scholar |
Hyde, K. D., and Philemon, E. (1994) Some disease-associated microorganisms on plants in the Western Province of Papua New Guinea, Australasian Plant Pathology, 23, 69-76.
| Crossref | Google Scholar |
Jones, D. R. (1991) Successful eradication of citrus canker from Thursday Island, Australasian Plant Pathology, 20, 89-91.
| Crossref | Google Scholar |
Jones, D. R., Moffett, M. L., and Navaratnam, S. J. (1984) Citrus canker on Thursday Island, Australasian Plant Pathology, 13, 64-65.
| Crossref | Google Scholar |
Jones, L. M., Grice, K. R. E., and Davis, R. I. (2010) Gamma irradiation of dried plant material: implications for the identification of plant pathogens employing molecular techniques, Australasian Plant Pathology, 39, 358-362.
| Crossref | Google Scholar |
Jones, L. M., Pease, B., Perkins, S. J., Constable, F. E., Kinoti, W., Warmington, D., Allgood, B., Powell, S., Taylor, P., Pearce, C., and Davis, R. I. (2021) ‘Candidatus Phytoplasma dypsidis’, a novel taxon associated with a lethal wilt disease of palms in Australia, International Journal of Systematic and Evolutionary Microbiology, 71, 004818.
| Crossref | Google Scholar | PubMed |
Kamrowski, R. L., Limpus, C., Moloney, J., and Hamann, M. (2012) Coastal light pollution and marine turtles: assessing the magnitude of the problem, Endangered Species Research, 19(1), 85-98.
| Crossref | Google Scholar |
Kelly, P. L., Reeder, R., Kokoa, P., Arocha, Y., Nixon, T., and Fox, A. (2011) First report of a phytoplasmas identified in coconut palms (Cocos nucifera) with lethal yellowing-like symptoms in Papua New Guinea, New Disease Reports, 23, 9.
| Crossref | Google Scholar |
Maclean, K., Robinson, C., Bock, E., and Rist, P. (2022) Reconciling risk and responsibility on Indigenous country: bridging the boundaries to guide knowledge sharing for cross-cultural biosecurity risk management in northern Australia, Journal of Cultural Geography, 39, 32-54.
| Crossref | Google Scholar |
Miyazaki, A., Shigaki, T., Koinuma, H., Iwabuchi, N., Rauka, G. B., Kembu, A., Saul, J., Watanabe, K., Nijo, T., Maejima, K., Yamaji, Y., and Namba, S. (2018) ‘Candidatus Phytoplasma noviguineense’, a novel taxon associated with Bogia coconut syndrome and banana wilt disease on the island of New Guinea, International Journal of Systematic and Evolutionary Microbiology, 68, 170-175.
| Crossref | Google Scholar | PubMed |
Parry, J. N., Davis, R. I., and Thomas, J. E. (2004) Passiflora virus Y, a novel virus infecting Passiflora spp. in Australia and the Indonesian Province of Papua, Australasian Plant Pathology, 33, 423-427.
| Crossref | Google Scholar |
Ray, J. D., Taylor, R. K., Griffin, R. L., James, R. S., Dale, C., Ximenes, A., and Jones, L. M. (2017) Confirmation of Xanthomonas citri subsp. citri causing citrus canker in Timor-Leste, Australasian Plant Disease Notes, 12, 44.
| Crossref | Google Scholar |
Reed, G., Brunet, N. D., Longboat, S., and Natcher, D. C. (2021) Indigenous guardians as an emerging approach to indigenous environmental governance, Conservation Biology, 35, 179-189.
| Crossref | Google Scholar | PubMed |
Schrader, C., Schielke, A., Ellerbroek, L., and Johne, R. (2012) PCR inhibitors–occurrence, properties and removal, Journal of Applied Microbiology, 113, 1014-1026.
| Crossref | Google Scholar | PubMed |
Shivas, R. G., Suyoko, S., Raga, N., and Hyde, K. D. (1996) Some disease-associated microorganisms on plants in Irian Jaya, Indonesia, Australasian Plant Pathology, 25, 36-49.
| Crossref | Google Scholar |
Taylor, R. K., Griffin, R. L., Jones, L. M., Pease, B., Tsatsia, F., Fanai, C., Macfarlane, B., Dale, C. J., and Davis, R. I. (2017) First record of Xanthomonas axonopodis pv. manihotis in Solomon Islands, Australasian Plant Disease Notes, 12, 49.
| Crossref | Google Scholar |
Teakle, D. S. (1996) Diagnosis of virus and bacterial diseases—does accuracy matter? Australasian Plant Pathology, 25, 147.
| Crossref | Google Scholar |
Teixeira, D. C., Danet, J. L., Eveillard, S., Martins, E. C., Junior, W. C. J., Yamamoto, P. T., Lopes, S. A., Bassanezi, R. B., Ayres, A. J., Saillard, C., and Bove, J. M. (2005) Citrus huanglongbing in Sao Paulo state, Brazil: PCR detection of the ‘Candidatus’ Liberibacter species associated with the disease, Molecular and Cellular Probes, 19, 173-179.
| Crossref | Google Scholar |
Wei, W., and Zhao, Y. (2022) Phytoplasma taxonomy: nomenclature, classification, and identification, Biology, 11, 1119.
| Crossref | Google Scholar | PubMed |
Weinert, M. P., Shivas, R. G., Pitkethley, R. N., and Daly, A. M. (2003) First record of grapevine leaf rust in the Northern Territory, Australia, Australasian Plant Pathology, 32, 117-118.
| Crossref | Google Scholar |
Weinert, M. P., Jacobson, S. C., Grimshaw, J. F., Bellis, G. A., Stephens, P. M., Gunua, T. G., Kame, M. F., and Davis, R. I. (2004) Detection of Huanglongbing (citrus greening disease) in Timor-Leste (East Timor) and in Papua New Guinea, Australasian Plant Pathology, 33, 135-136.
| Crossref | Google Scholar |
Footnotes
5 Jones (1991).
12 Teakle (1996).
14 Brunt (1987).
26 Fearnside (1997).
27 George (1981).
30 Bove (2006).
31 Anonymous (2019).
32 Halbert (2005).
34 Aubert (1990).


