David Albert Cooper 1949–2018
Anthony D. Kelleher A * , Suzanne Crowe B and Anthony Cunningham C
A * , Suzanne Crowe B and Anthony Cunningham C
A
B
C
Abstract
David Cooper was an internationally renowned immunologist and HIV clinician who spearheaded Australia’s world-leading HIV response. Known for advocacy and community engagement, he made several world-first discoveries on HIV pathogenesis and treatment. He was involved in the development of every HIV drug used in Australia and drove the introduction of antiretroviral pre‐exposure prophylaxis (PrEP) in NSW. He established, then led, the Kirby Institute for thirty-two years, remaining at the forefront of communicable disease research in Australia and internationally.
Keywords: AIDS, clinician scientist, HIV, immunology, infectious disease, memoir, pandemic, virology.
Early life and education
David Albert Cooper was born in Sydney on 19 April 1949, the son of Jewish immigrant parents. His manufacturer father, Max Cooper (born Lowicz), had migrated to Australia from Poland in the 1920s and ran a tailor’s shop in Haymarket. His mother, Anita Lazarus (known as Annie), was born in Leeds, UK, to a Lithuanian family and emigrated to Melbourne as a child. They met at a wedding and settled in Sydney’s eastern suburbs.
David and his sister Bettina, who was sixteen years his senior, were brought up in a close, loving family in Dover Heights, Sydney. The family attended the Great Synagogue in central Sydney and David later became a leader of a Jewish student group while at the University of Sydney and subsequently a board member of the newly established Shalom College, a Jewish residential college at the University of New South Wales. Later, David would say it was his exposure to Jewish tradition and growing up in a community where many had first-hand experience of the Holocaust that instilled in him the hatred of discrimination that was to be a guiding principle throughout his life.
David completed high school at Cranbrook School in Sydney, where he was a keen student. He read widely and tangentially, and was fascinated by intellectual challenges. He enjoyed mathematics, science, history, French and Latin, but was especially drawn to biology. Friends recall how he would love to share knowledge with them.
He loved to teach me, he had a blackboard in his room and he said, “It really helps if I can explain it to you, then I know I’ve got it”. – Leonora Abeshouse, childhood friend.1
Very shy and quiet as a child, David was never particularly adept at sport, but instead he managed some of Cranbrook’s football teams and became a scorer at cricket. He was accelerated through school—but as a 1.94 m adult, he always believed he was put in the wrong queue for third class because he was so tall—and started his studies of medicine at the University of Sydney at just fifteen years old. He was socially awkward and shy amid a crowd of older students, so devoted himself to and excelled at his studies.
I first met Coops on the bus to university – he was too young to drive, he was just a kid. He told me he was also studying medicine, and that’s the last time I thought I was better than him. We used to sit there and talk about things. I was interested in passing exams, but not him. – Barry Abeshouse, friend.2
David completed his BSc (Med) with honours in 1969 and, during his medical degree, had a letter on Behcet’s disease published in The Lancet.3 At the end of fifth year medicine, he and his fellow student, David Sonnabend, worked for renowned immunologist Professor Ron Penny at St Vincent’s Hospital (Fig. 1), studying the role of fibrin degradation products (FDP) in thrombo-embolic disease.4 David’s interest in laboratory work was firmly established during this time.
I would read the first two or three paragraphs of a journal article, and he would read the whole thing. He was a polymath, he knew more about everything than anyone else. – Professor David Sonnabend, friend.5
In 1972, having graduated MBBS with honours and still just twenty-three, David embarked on a career in clinical immunology. He worked as a resident medical officer at St Vincent’s Hospital in Sydney and completed fellowships in internal medicine and pathology. He was awarded a doctorate from the University of New South Wales for his thesis ‘Glucocorticosteroid Regulation of Human Lymphocyte Function’ in 1981.6 Professor Ron Penny was his doctoral and clinical supervisor, and was a tremendous inspirational teacher and mentor.
Throughout the seventies and early eighties, David followed a promising career path as a hospital consultant with academic interests in clinical immunology and a steady research output, including a year spent in 1975 as a research fellow in Tucson, Arizona. In 1979, he met a high school teacher, Dorrie Stark, the daughter of Holocaust survivors, who shared his quick wit, dry sense of humour and love of life. They were married in early 1981 and their first daughter, Rebecca, was born later that year. When the baby was six weeks old, the young family left for Boston. David had received an NHMRC fellowship, which included two years as a postdoctoral fellow at the Dana Farber Cancer Institute.
It was an exciting time to be at the Dana Farber. Prior to his arrival, the Institute had discovered the major functional T cell subsets controlling cell mediated immunity, CD4 and CD8 T cells, as well as the T cell receptor. David was one of the Institute’s brilliant young scientists working in the laboratory, studying the characterisation of CD4 and CD8 T cell clones triggered by viral infection7 as well as an early characterisation of the T cell receptor.8
In 1981, the first reports started to come in about a new, highly aggressive immune deficiency syndrome that was affecting mainly gay men and people who injected drugs. ‘It was absolutely fascinating, because there was an immunodeficiency disorder of adults which seemed really quite extraordinary,’ David said later.9 It was clear at the time that CD4 T cells were the key to understanding the virus—in fact, that CD4 was actually the receptor for HIV. David was involved in testing blood samples that had arrived in his laboratory from very unwell gay men in New York, and witnessed the devastation wreaked on the immune system by HIV. As specialists in immunology, infectious diseases, epidemiology, oncology and haematology came together in the United States to understand more about the virus, David correctly predicted that it would reach Australia—and that St Vincent’s Hospital in Darlinghurst, the heart of Sydney’s gay community, would be at the epicentre of the epidemic.
Sydney and the early response to the HIV/AIDS epidemic
David returned to Sydney in mid-1983 to work as a staff specialist in the St Vincent’s immunology department with his mentor, Professor Penny. Penny and his clinical colleagues had diagnosed the first patient with AIDS in Australia in 1982 and he was considered an authority on immunology in Australia.
Australia’s early AIDS response was hampered by stigma and discrimination against gay people.10 Despite inroads towards greater acceptance of homosexuality in the 1970s and early 1980s, which had seen the repeal of sodomy laws in most states, the advent of AIDS resulted in a rapid escalation of fear and discrimination against the gay community. What became known as the ‘gay plague’ became an excuse for violence against gay men, including a spate of bashings and murders in Sydney. Young men who fell sick were often rejected by their families, and there were few hospices or other services willing to cater to their needs. Discrimination also extended to health services, with cleaners, nurses and even surgeons reportedly refusing treatment. HIV/AIDS was being managed by a handful of local general practitioners (GPs) who had a special interest as well as some venereologists and the immunologists at St Vincent’s, including David. Later, these first responders to the virus were to set up the Australasian College of Sexual Health Physicians, now a chapter within the Royal Australasian College of Physicians, with David as an early vice-president.
We were told there was certain death and not to tell anyone, just a couple of close friends. If we told people, the word would get out. We could be bashed up, murders were increasing, I rarely went out at night-time, you could feel this atmosphere down Oxford Street. And then there was David. He was so warm, a really lovely person. The humanity oozed out of him. – David (Polly) Polson, patient and friend.11
David was by now the father of two daughters—Ilana was born a few months after the family returned to Sydney (Fig. 2). When they were young David always read to his girls at night and helped them with their music practice, regardless of his long and stressful day at the hospital. He did not let the national hysteria about AIDS affect his work. He was deeply moved by the plight of the men he met, a rapidly growing number of patients for whom he had few answers or treatments and he rejected the fear and discriminatory attitude held by many of his peers and other members of the healthcare sector. While an understanding of the causes of the disease was increasing, there was very little clinicians could do to prevent the virus from destroying the immune system and causing the cascade of fatal illnesses. David was driven by an intellectual interest in the science of HIV/AIDS but, more than that, by compassion for his patients.
He was distraught that he could not heal them. I remember him coming home in the mid-80s when the ward was overflowing, pacing in the loungeroom with his hand on his forehead and shaking his head from side to side. He said, “I have so many sick patients and I have nothing to give them”. – Dorrie Cooper, wife.12
With daughters Ilana and Bec (dressed as ART) preparing for the Sydney Gay and Lesbian Mardi Gras, 1999.
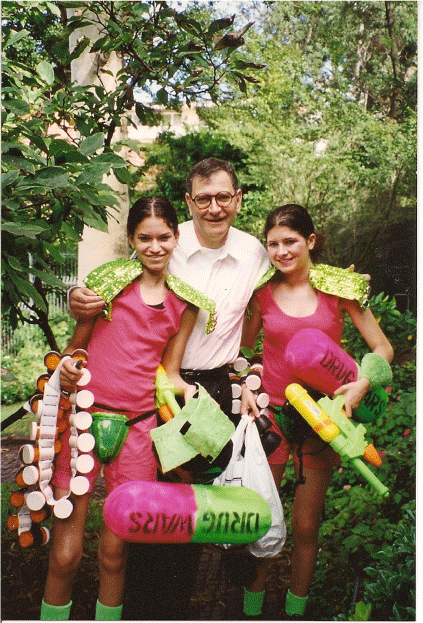
As patient numbers grew to 4500 in the next two years, predominantly gay men, David worked with Professor Penny and a small multidisciplinary team to develop a comprehensive clinical service for patients with HIV at St Vincent’s, encompassing everything from laboratory diagnostics to social and psychological support for both in-patient and out-patient services.
The hospital was then run by the Sisters of Charity, a religious order who believed that every patient deserved dignity, care and compassion, and that none would be turned away—an ethos David shared. St Vincent’s Hospital, Sydney quickly became the hub of Australia’s early HIV/AIDS response, known for welcoming all patients with compassion and treating them in a non-judgemental way, regardless of their sexual orientation or lifestyle choices. Ward 17 South at St Vincent’s became the first dedicated HIV/AIDS unit in Australia and the healthcare epicentre for patients with the disease. It set the benchmark for the best clinical care available anywhere in the world at the time, and while the ward itself closed in 2007, it is thanks in part to David’s leadership, in partnership with the Sisters of Charity, that St Vincent’s continues to be an internationally recognised centre for excellence in HIV/AIDS clinical care and research today.
The demand for beds rapidly outstripped the capacity of the ward, and clinicians involved in the response worked extremely long, stressful hours to cater to patients’ needs. Associate Professor Anthony Schembri, now chief executive of Northern Sydney Local Health District, described in a speech on World AIDS Day 2019 what it was like to be a social worker on Ward 17 South in the early days of the AIDS response.
I was 22 years old, working on Ward 17 South with other young staff, caring for patients from my community. One recollection is that on a particularly painful day – I was in the nurses’ station having a little cry, and Sr Margaret came and put her arm around me. I said, “Sister, I don’t think I can do this anymore”. And she said, “It’s because it’s hard that we should do it.” If not for the Sisters, Charles Curran, Ron Penny, David Cooper, Phil Cunningham … if not for them, who would do this? … I remember my first patient. He had pneumocystis pneumonia – at the time it was one of the main AIDS-defining illnesses. His family had refused to have anything to do with him. He was under the care of David Cooper and David said, “We won’t let you die alone”. 13 – Associate Professor Anthony Schembri, Chief Executive, Northern Sydney Local Health District.
The US Centers for Disease Control visited St Vincent’s several times in 1983 to consider it as a sentinel site for HIV surveillance. At the same time, David established the Sydney AIDS Study Group with his colleagues Dr Julian Gold, an epidemiologist on the National AIDS Taskforce, and Dr Basil Donovan (now Professor Basil Donovan AO) then a primary care doctor and sexual health physician working with gay men in Darlinghurst.14 David, Donovan and Garrett Prestage, a behavioural researcher with an interest in lesbian and gay men’s health and wellbeing, fronted a fiery community meeting in Paddington Town Hall, attended by hundreds of local community members who were frightened and angry. It was at this meeting that the doctors explained the need for a cohort study to determine the natural history of AIDS and its epidemiology in Australia.
In February the following year, David and his colleagues started recruiting into the Sydney AIDS Prospective Study (SAPS), the first systematic HIV research conducted in Australia. This prospective sero-epidemiological study enrolled more than 1000 gay men who were patients of St Vincent’s Hospital and of local general practices, collecting clinical data and linking these observations with bio-banked samples in the laboratory. When the first blood test for HIV became available in August 1984, the group was able to test the stored blood samples, discovering that 40% were positive.
In 1984, the first Australian data on the incidence of AIDS and the risk factors associated with the development of the disease were published, based on the work of the SAPS network.15 The data provided by SAPS provided crucial early insights into the pathogenesis of AIDS. One of the ‘first 400’ to be diagnosed was Brett Tindall, a medical student who had come to work with Basil Donovan at the Taylor Square Private Clinic (Fig. 3). After a long day seeing patients they met for a drink at a local club off Oxford Street, Kinselas, when Tindall felt suddenly unwell and had to go home. It transpired that he had primary HIV, an acute glandular fever-like illness that occurs within a few weeks of HIV infection.
David and the Sydney AIDS Study Group were the first in the world to describe the seroconversion illness that occurs at the earliest stages of HIV infection, published in The Lancet in 1985 and still used in clinical practice today in identifying the optimum time to intervene.16 Tindall later went on to become a world authority on the HIV seroconversion illness until his death in 1994, while SAPS provided the largest body of prospectively collected data on sexual behaviour in gay men in Australia and was to give rise to similar studies in most capital cities of Australia. Also in 1985, with Dr John Ziegler (now Professor John Ziegler AM), David helped identify that HIV could be transferred to babies in breast milk.17
David provided the clinical leadership that contributed to the internationally respected ‘Australian model’ of AIDS management. He facilitated the partnership model of collaboration and dialogue between patients, clinicians and politicians that resulted in inclusion of at-risk, marginalised population groups in design, development and implementation of the AIDS response. He was practising the principles of the now aspirational goals of ‘codesign’ decades before the term was coined. Innovations in NSW included a medically supervised injecting centre in Sydney for people who use drugs, evaluated in part by David’s team, as well as sustained advocacy to decriminalise sex work and provide health services to sex workers.18
Australia became the second country in the world to adopt the HIV paradox, which took a human rights-based approach to addressing the epidemic. Spearheaded by a bipartisan approach of then Health Minister Dr Neal Blewett AC and Shadow Health Minister Dr Peter Baume AC, Australia relied not on isolation or criminalisation but on reaching out to marginalised groups in order to protect their rights and encourage and sustain behaviour modification.19
These policies resulted in a much lower rate of infection in Australia than in other comparable countries, with David and his team leading the way in engaging with the communities most impacted by HIV/AIDS.
AIDS epidemiology and clinical research
The world-first clinical discoveries and epidemiological insights produced by the Sydney AIDS Study Group provided the necessary impetus and evidence for the government to invest in a research centre to track the disease spread.
In 1986, the year he turned 37, David was serving as director of the St Vincent’s AIDS unit when he was approached by the Australian Government to lead the NHMRC Special Unit in AIDS Epidemiology and Clinical Research, one of three research centres20 established in response to the AIDS epidemic (along with centres for virological and social research).
In its early days, the Special Unit operated upstairs from the Albion St Centre, the first public HIV testing centre (now called The Albion Centre, Australia’s only major interdisciplinary centre with a primary focus on HIV management). The Special Unit pioneered a unique approach to AIDS treatment and prevention, with researchers working alongside patients and their families to find solutions to the issues that affected them.
In 1986, David launched Australia’s first clinical trial of an HIV treatment, using the antivirals zidovudine (AZT) and acyclovir, his first foray into the approach of combination antiviral therapy. Also that year, the Special Unit developed a technique to screen returned syringes for HIV antibodies. When AZT became available for prescription in Australia in 1987, the Special Unit was responsible for monitoring its usage. Over the next three years, it recruited 600 participants for a national study of open-label AZT and showed improvements in survival of patients.21
HIV management turned into a very complicated form of clinical medicine as the virus mutated and became resistant. David would come and give us talks on how to use antivirals. He was the authority, he was the number one on the clinical side that everyone turned to, but we were asking questions no one in the world could answer at that time. – Professor Basil Donovan, primary care doctor.22
Australia’s first National HIV/AIDS Strategy was adopted in 1989, creating a collaborative network with health departments and other health organisations nationally. Reflecting its expanded role under the Strategy, the Special Unit was renamed the National Centre in HIV Epidemiology and Clinical Research (NCHECR). This approach of bringing government, academia, clinicians and the community together to develop contemporary HIV/AIDS strategies continues today, currently under the 8th National HIV/AIDS Strategy.
By this time, NCHECR was coordinating surveillance and epidemiological studies of HIV/AIDS in Australia and collating and analysing data on surveillance and epidemiology collected by the states, territories and Commonwealth. It was also conducting clinical trials as well as undertaking research into epidemiology, natural history and clinical aspects, and providing other centres with assistance in research design, data collection, processing and analysis, and training. An important study in 1989 monitoring the transmission of HIV from mother to child was the first national study of HIV prevalence in babies born 1988–9 and found no cases of HIV infection among 10,000 live births, indicating the success of the public health interventions initiated in Australia.23
From 1990, the National Centre rapidly expanded its participation in clinical trials as well as its international collaborations. The Australian HIV Surveillance Report was first published in 1990 by NCHECR, on behalf of the Australian Government, and the following year NCHECR established collaborations with the Australian Red Cross blood transfusion service, the Australian Defence Force, Departments of Corrections, and with services designed for people who inject drugs. 1992 saw the establishment of the Sydney Men and Sexual Health Study (SMASH) in collaboration with the National Centre in HIV Social Research and the AIDS Council of New South Wales (ACON),24 and in 1993 the first major analysis of AIDS incidence in Australia 1982–92 was published, using data from the National AIDS Registry.
Contributions to science
David made an outstanding contribution to medicine and is considered to have been at the forefront of the global fight against HIV/AIDS, both scientifically and clinically. He achieved a prodigious number of publications, citations, grants and doctoral graduates, but his major contribution to science was the direct impact his research had on people’s lives both in Australia and internationally. Always on top of the data and able to think things through strategically, he had an uncanny ability to distil the main issues into research questions that mattered in the clinic.
As director of the Special AIDS Unit, the NCHECR and from 2011, the Kirby Institute, and Professor of Medicine (appointed in 1994) and then Scientia Professor of Medicine (appointed in 2005, reappointed in 2011 and 2017) at UNSW, David was involved in evaluating every new HIV drug at some level, whether it was in an early phase or phase 3 clinical trial. He worked hard to attract clinical trials to Australia, understanding the appeal for multinational drug companies of conducting clinical trials in Australia, where clinical costs were significantly lower than overseas. These trials often provided the only treatment option for patients who had developed resistance to existing treatments.
NCHECR, and David in particular, played a significant role in the international research effort that ultimately led to combination antiretroviral therapy that could induce sustained viral load reduction while avoiding the development of resistance. Initially, working with his colleagues at St Vincent’s, David provided some of the earliest descriptions of the profound immune re-constitution induced by protease inhibitors, new drugs that promised to transform the treatment of HIV. David and colleagues were the first in the world to describe lipodystrophy syndrome associated with protease inhibitors, and that it was caused by off target effects of the protease inhibitors rather than HIV as previously thought.25 The international community was slow to accept the existence of this syndrome. David recounted to his colleague Basil Donovan how he was treated during a talk to some unreceptive clinicians at San Francisco General Hospital. When they told him they did not believe lipodystrophy existed, David told them, ‘Go and have a look in your waiting room.’ Sure enough, the whole room was visibly affected by the condition. A major advance in the treatment of lipodystrophy was to be achieved with the MITOX study in 2002, which demonstrated an evidence-based strategy for reversing lipoatrophy by switching nucleoside reverse transcriptase inhibitors in the treatment program.26
David then collaborated with colleagues in Italy, the Netherlands, Canada and Australia to advocate for one of the first trials of combination antiretroviral therapy, the Delta study. The study showed that survival of those who had not had previous treatment was significantly better with combination therapy, with an estimated reduction in mortality of 38%. The findings provided conclusive evidence that at least two antiretroviral drugs in combination should now be the recommended treatment, and marked a turning point in HIV clinical trials.27
At the 1996 Vancouver International AIDS Conference, there was excitement as Julio Montaner presented the INCA study, a double-blind randomised controlled trial designed by David, Montaner and Professor Joep Lange that used a non-nucleoside rather than a protease inhibitor, showing that a combination of three drugs (nevirapine, didanosine and zidovudine) resulted in a greater decrease in viral load than a combination of two drugs.28 This announcement of highly active antiretroviral therapy (HAART) in 1996 heralded a new era in HIV/AIDS management, ultimately leading to HIV being transformed from a fatal disease to a chronic, treatable condition. ‘It was very exciting to see the field transform before your eyes … We saw the Lazarus effect, the wards just emptied out and that was a huge relief,’ David said later.29 The following year, the fifth year of the SMASH study showed a rapid uptake of treatment by HIV-positive men following the news of the effectiveness of combination therapy.
David continued to lead strategic national and international clinical trials that further refined the approach to treatment and the optimal use of antiretroviral therapy. Pivotal strategy trials included the START trial,30 which provided the evidence for prompt initiation of effective treatment after HIV diagnosis regardless of the particular drug combination used, ultimately reducing HIV spread and contributing to alleviating some of the stigma experienced by people with HIV. He was also involved with the SMART trial,31 that showed therapy should not be interrupted, as well as the ESPRIT and SILCAAT32 that showed the limitations of immunotherapy for HIV and the need for care in the use of surrogate end points in clinical trials. These studies involved mixed-gender cohorts and provided immediately applicable and implementable findings that translated in Australia as well as low- and middle-income countries. Further, the global clinical networks these trials relied on are still functional today, contributing to the global response to COVID-19.
Advocacy and international influence
As all politicians, pharmaceutical company representatives and health administrators who came across David knew, he could be very persuasive. He was able to convince others through the weight of evidence, his reputation, and his lack of ego. Always modest and honest, and without a shred of self-aggrandisement, he worked across the spectrum with clinicians, academics, politicians, activists, and industry to obtain the support he needed to make a difference for the community.
David understood that overcoming stigma would be key to combatting HIV/AIDS, and that he needed to partner with the community both to support research efforts and to understand patients’ needs in order to provide appropriate clinical care. This approach of embedding research in clinical practice and community engagement was to persist throughout his career. David saw himself primarily as a clinician who understood the value of research, and used science to answer real-world problems he encountered among his patients.
Throughout his career, he involved patients in research on methodologies to improve clinical care and treatment regimes. A key contribution was his advocacy, alongside community groups, to achieve equitable access to unapproved new therapies for patients in Australia. In 1990, a review taskforce chaired by Professor Peter McDonald, and with the then-president of AFAO (formerly ACON’s first executive director) Bill Whittaker as member, recommended sweeping changes to the drug approval systems and clinical trials approval processes in Australia, including establishing and promoting Australia as a world leader in HIV/AIDS clinical research and care. The recommendations for the clinical trials approval processes were accepted in full by Federal Minister for Health Brian Howe. Senator for NSW, Dr Peter Baume was appointed to lead a further review into the drug approval processes and shortly after, the subsequent Baume Report of the evaluation of new drugs led to the introduction of the Special Access Scheme, that helped to provide access to clinical trial drugs to HIV positive people failing their treatment regimens or who at very high risk of treatment failure and death. David played an important part in these breakthroughs, working with various community committees and networks to help give those affected a strong voice.
David set the benchmark for how the medical and research sectors should engage with affected communities. He understood, from very early on, that to deliver an impactful HIV response, you needed community engagement to be able make an impact. He understood this in a truly meaningful way, and through the whole journey, that didn’t change. He maintained and elevated principles around codesign and collaboration, and saw his work as not being separate from the community, but very much a part of it. – Nicolas Parkhill, CEO of ACON.33
Following this overhaul of the drug evaluation process, NCHECR’s clinical trials activity expanded to include antiretroviral therapy, opportunistic infections, HIV-associated malignancies, immune-based therapies, vaccines and laboratory developments.
The NCHECR also started to expand and prioritise research into other blood-borne viruses. It built world-leading expertise in epidemiology and surveillance, biostatistical analysis, Aboriginal and Torres Strait Islander health, sexual health and justice health, as well as a laboratory program of immunology and virology. Under David’s oversight, the NCHECR, which became the Kirby Institute in 2011, completed the world’s largest study of the treatment and natural history of acute hepatitis C infection among people who inject drugs (ATAHC). Through the Aboriginal and Torres Strait Islander Health Program, it undertook a study called STRIVE—STIs in remote communities: improved and enhanced primary health care. This cluster randomised study took place in sixty-eight primary care clinics,34 and was designed to support primary healthcare services to achieve best practice in sexually transmitted infection care. David’s leadership led to several large, back-to-back NHMRC-funded program grants from 2004 onwards in partnership with academics from other universities including the Australian National University and the Universities of Sydney, Melbourne, Adelaide and Western Australia to address the urgent global health priorities concerning the development of vaccines and better treatments for HIV, hepatitis C, and influenza.
David also advocated for access to therapies in lower income countries, and was among the first academic leaders in the world to show that clinical research could be done well in countries with limited resources, providing there was adequate support and mentoring. In Australia and internationally, he took the time to train local investigators and nurture them into leadership roles. Rather than dictating what should be studied, he was always sure to listen to local problems and work with research partners to work out how to fix them in the local population.
He designed clinical trials to make the most of available resources, with the aim of providing pragmatic answers to questions that underpinned the provision of excellent care no matter where someone lived in the world. Three significant studies were ENCORE1, SECOND-LINE and D2EFT. ENCORE1 showed the equal effectiveness of a lower dosage of efavirenz in people starting antiretroviral therapy for the first time—a finding that was adopted in WHO treatment guidelines, reduced the costs of antiretroviral therapy in a lower-resource setting, and allowed many more people to access treatment.35 The SECOND-LINE study compared an alternate HIV treatment regimen combining two new classes of drug with a WHO-recommended regimen for people for whom the standard, recommended HIV treatments did not work. It found that the alternate treatments were safe, well tolerated and effective.36 The recently completed D2EFT study established that two simplified combinations of antiretroviral drugs (dolutegravir with ritonavir boosted darunavir and dolutegravir with tenofovir and lamivudine or emtricitabine) are as safe and effective as the currently-recommended World Health Organization standard of care second-line regimen.
Among David’s proudest achievements was his success in the Asia-Pacific region. In 1995, David met with HIV research centre directors Professor Praphan Phanuphak from Bangkok and Professor Lange from Amsterdam to discuss the ongoing HIV epidemic in Thailand. They highlighted the need for more affordable treatments and discussed how to overcome pharmaceutical companies’ reluctance to conduct HIV clinical research in areas where HIV was most prevalent. In response, the group established HIV-NAT (HIV Netherlands, Australia, Thailand collaboration), a multicentre clinical research organisation in Thailand that conducted its first study in 1996. Since its inception, HIV-NAT has completed studies that have influenced Thai and international HIV treatment guidelines, convened the annual Bangkok International Symposium on HIV Medicine—the largest meeting of its kind in southeast Asia—and provided HIV care services to thousands of people living with HIV throughout Thailand. HIV-NAT is now the premier place to conduct HIV and blood-borne virus clinical trial research in southeast Asia. Sadly, David’s close friend Joep Lange, Director of the Amsterdam Institute of Global Health & Development, was one of the victims of the MH17 Malaysia Airlines flight that was shot down over Ukraine in 2014.37
David was President of the International AIDS Society (IAS) when the decision was made to take the 2000 meeting to South Africa, which at the time had the highest HIV prevalence rates in the world without access to the array of drugs available in developed countries. The 13th International AIDS Conference in Durban was the first to be held in a low- or middle-income country and the first to be held on the African continent, and ensured that the people most affected by HIV would have a voice. The meeting confronted treatment inequity and AIDS denialism, and culminated in more than 5000 delegates signing the Durban Declaration affirming that AIDS was caused by HIV. The conference is widely regarded as having created the momentum to change the approach to global public health.
Professor Linda-Gail Bekker, IAS Past President, now Director of the Desmond Tutu HIV Centre and Chief Executive Officer of the Desmond Tutu Health Foundation, remembers the challenges of navigating burgeoning HIV/AIDS numbers, a denialist government in South Africa and a strong anti-science lobby.
We were in the throes of a catastrophe. David was one of the key individuals who were instrumental in looking beyond what was happening in North America and Europe and paying attention to Africa and Asia. When he brought the IAS [Conference] to Africa, it was a huge risk and a big step, but it was absolutely pivotal at the time. – Professor Linda-Gail Bekker, Director of the Desmond Tutu HIV Centre and Chief Executive Officer of the Desmond Tutu Health Foundation.38
After the conference, in 2003 the US President’s Emergency Plan for AIDS Relief (PEPFAR) was formed, heralding a major investment in the global HIV/AIDS response and improved access to treatments in low- and middle-income countries.
David held numerous professional positions, including member of the Meeting on AIDS Drug and Vaccine Supply (Geneva 1991), member of the WHO-IFPMA Joint Working Groups on the development, utilisation and supply of drugs and vaccines for HIV Infection and HIV-related disease (Geneva 1991), member of the Trial Management Committee, PETRA study on perinatal HIV transmission in Africa (1995–2002), Chairman and member of the WHO-UNAIDS HIV Vaccine Advisory Committee (2004–12), member of the WHO Strategic and Technical Advisory Committee for HIV/AIDS (STAC-HIV, from 2006), member of the WHO Guidelines Committee (2014), Member of the Pacific Friends of the Global Fund Advisory Council (2015) and Atlantic Philanthropies Chair in Infection and Immunity (2016).
He was also on several government advisory bodies, including the National AIDS Forum (1988–90) and the NSW Ministerial Advisory Committee on AIDS Strategy (1997–2000), as well as President of the International AIDS Society (1994–8).
He has provided international leadership over three decades within the medical academic community, including his past-presidency of the International AIDS Society, and advisory roles to WHO/UNAIDS in strategic direction, ART guidelines, and HIV vaccines. – Dr Anthony S Fauci, former director of the US National Institute of Allergy and Infectious Diseases.39
David continued to give a voice to members of the Australian community throughout his life. By 2011, global attention was turning to HIV/AIDS prevention. David and the Kirby Institute, in consultation with ACON, worked with NSW Health to advocate for the EPIC-NSW trial, the premise of which was that rapid, at scale, roll out of antiretroviral pre‐exposure prophylaxis (PrEP), the HIV prevention medication, targeting men at highest risk of HIV would impact on transmission at a population level. The EPIC-NSW trial rapidly recruited 10,000 at-risk gay men in NSW by 2016. This trial was one of the world’s largest community-wide implementation studies of PrEP, was associated with an eventual 30% decrease in HIV incidence and provided the basis40 for it being approved by the Therapeutic Goods Administration then listed in the Australian Pharmaceutical Benefits Scheme in 2018.
The Kirby Institute
In 2011, the NCHECR—which had operated for twenty-five years on the precinct of St Vincent’s, amid the community most affected by HIV—was renamed the Kirby Institute, reflecting its new focus on behavioural-related infectious diseases affecting marginalised, disempowered and other at-risk communities. Its name was taken in honour of the Hon. Michael Kirby AC CMG, Australia’s longest-serving judge and a passionate advocate for health and human rights. With David as Director, the Institute moved to a new, purpose-built facility on UNSW’s Kensington campus, funded with a philanthropic gift through Atlantic Philanthropies. True to form, David consulted closely with the HIV community before he agreed to the move.
Over his thirty-two years as leader of the Kirby Institute, David helped transform Australia’s research landscape. He created a national clinical trials network that enabled Australia to participate in international clinical trials in HIV and many other conditions, and brought rapid access to new treatments for Australian patients and attracted major large-scale multicentre trials to this country.
The Kirby Institute grew into an internationally recognised infectious disease research institute which conducted its work across the full spectrum of medical research disciplines, from the laboratory, mathematical modelling, clinical trials, public health interventions, public health surveillance, and behavioural research. The Kirby Institute’s HIV research expanded into co-infection with hepatitis viruses as well as human papillomavirus (HPV), as well as the management of the cardiovascular and other chronic disease manifestations of HIV, and the most efficient way to use PrEP.
Since 1997, the Kirby Institute has been responsible for collecting and analysing national data on HIV, STIs, and viral hepatitis, on behalf of the Australian Government. It conducts national surveys on the impact of Australia’s world-leading needle syringe program on HIV and hepatitis C prevalence, and led a world-first study to evaluate curative hepatitis C treatments as a means of preventing its spread within prisons. It has led and coordinated a global clinical trials network, first established to optimise HIV treatment, and more recently, utilised to respond to pandemic health threats. During the COVID-19 pandemic, Kirby Institute research uncovered the immune profile for long COVID and rapidly responded to the pandemic by swiftly adapting its containment laboratories to analyse the virus and its variants. The Institute is widely recognised for conducting highly collaborative research, bringing together multiple sectors to achieve a common goal, and centring the communities and people most impacted by infectious disease; the ethos that David established from the beginning.
David was highly respected at the Kirby Institute for creating an environment that supported collaborative research and career development. He fostered leadership and encouraged and supported his colleagues, who came from a wide range of research disciplines, trusting them with full responsibility to turn ideas into reality. He was known as a mediator who respected others’ opinions; he was always the last to speak, listening to others around the table and then quietly finding a way forward that left everyone feeling valued. He was also recognised as an insightful mentor of the next generation, supervising dozens of doctoral candidates and clinician scientists, and creating an environment that fostered innovation and leadership. He put complete faith in those he trusted, handing over responsibility for projects while suggesting new ways of tackling problems or forming collaborations. Most of the current leaders of the Kirby Institute were mentored by David, as were numerous HIV researchers, clinicians and industry leaders in Australia and internationally.
David facilitated the Institute’s work with Aboriginal and Torres Strait Islander populations in Australia, as well as marginalised populations throughout Australia and the region, including in Papua New Guinea, Indonesia, the Philippines, Cambodia, Lao PDR, and the Pacific Islands. His focus was on implementation—from how to improve prevention of HIV, test and treat hepatitis C in people who inject drugs or STIs in pregnant women, or how to get health messages to people in isolated communities where risk factors are still highly stigmatised.
As part of his determination to make a difference in the region, in 2017, he established the Myanmar-Australia Research Collaboration for Health (MARCH) with the University of Medicine 2 (UM2) in Myanmar to build research capacity in infectious diseases in Myanmar, with a particular emphasis on HIV, tuberculosis and malaria. However, his efforts and those of his Australian and Myanmar colleagues which were rapidly bearing fruit were stymied by the military coup of early 2021 and subsequent civil war.
Honours and awards
David received numerous honours and awards throughout his career, including Fellowship of the Australian Academy of Sciences and the Australian Academy of Health and Medical Sciences.
He was appointed Officer of the Order of Australia (AO) in 2003 for ‘service to medicine as a clinician, researcher and leading contributor in the field of HIV/AIDS research’. He was awarded the Sir William Upjohn Medal in 2009 by the University of Melbourne for distinguished services to medicine, and the James Cook Medal by the Royal Society of South Wales in 2016 for his research on the understanding and treatment of HIV/AIDS.
In 2018, David was posthumously appointed Companion of the Order of Australia (AC) for ‘eminent service to medicine, particularly in HIV/AIDS research, as a clinician, scientist and administrator, to the development of therapies and to health programs in Southeast Asia and the Pacific’.
Personal life and legacy
In his too-short life, David developed from a lanky, shy, bookish student to become one of Australia’s most distinguished academic leaders with an international reputation for groundbreaking, collaborative infectious disease research that has saved countless lives globally.
David’s family and friends describe him as someone with immense modesty and humility, with an innate humanity and sense of compassion. He relished the stimulation of research and his international collaborations, but most of all he loved his patients. He continued to see the survivors from his original HIV cohort throughout his career and was widely known for being a reassuring and trusted doctor. He found the gay community colourful and welcoming; they would confide in him things they would not tell other people. He became extremely fond of his patients. Amidst the sadness there was fun. One owned a vineyard and gifted David boxes of wine, then the next week rabbits for the girls. Dinners for international collaborators were organised in the Coopers’ home and it was not unusual for an employee to dress in drag, dance around the backyard and sit on David’s lap.
David treated me physically but also mentally. He’d greet me with “Hello sweetheart, how are you?” He would give me a hug and say, “Are you OK?” It was just that comfort and that embrace when you were petrified. When I was very sick in ICU, he came in and held my hand and spoke to me, he gave me such confidence and comfort and empathy. – Jason Starling, patient.
David was very good at getting things done, even when a problem seemed insurmountable. He had an ability to break a problem down into individual steps. He would often work from dawn until midnight, attending international teleconferences late into the night and falling asleep with the phone on his chest, according to his wife Dorrie. Exhausted by the workload and his constant travel, he would catch up on reading medical journals during plane journeys. His passion for learning was insatiable.
He detested discrimination. During the 1980s, some colleagues refused to shake his hand; he was furious when some surgeons refused to operate on patients who were HIV-positive. David eventually won those debates, despite the entrenched stigma against people living with HIV. He worked tirelessly to advocate for people living with HIV/AIDS and led a NCHECR/Kirby Institute float in the Sydney Gay and Lesbian Mardis Gras parade on two occasions. The first, in 1999, he attended with his daughters, dressed as HIV antiretroviral pills (Fig. 2).
David loved his social life, friends and family. He enjoyed fine food and wine, and cooked like a scientist following a recipe. He was interested in fashion, often buying designer clothing during overseas trips and seeking the opinion of his daughter Bec, a fashion designer, on his sartorial choices. He had wide interests—from classical music and opera to theatre and books—and would spend hours reading. ‘He was a walking encyclopaedia, absent minded, terrible in a medical emergency, and he kept his family laughing often at his dorkishness,’ says Dorrie.41 His mind was always on greater challenges.
By the time David died of hemophagocytic lymphohistiocytosis on 18 March 2018, same-sex couples had been given the right to marry in Australia, the cross-sector approach to health was well-established in Australia, people diagnosed with HIV were receiving optimal treatments in the form of a single pill and living long, healthy lives, and regular testing and PrEP had completely transformed the AIDS landscape in Australia and the world. ‘The story of HIV is a modern medical miracle. From despair and tragedy, we have moved into an era of chronic treatable illness, in just 30 years,’ David told The Lancet.42
Speaking at David’s memorial service, his friend the Hon. Michael Kirby said that David was one of Australia’s finest, bravest and best of scientists and citizens.
Doing the right thing at the right time was one of David Cooper’s specialties. He was in Boston when HIV first struck. He returned at once to Australia. What could have been an unmitigated catastrophe, became a brave example to the world. Our nation responded to this cruel epidemic with rare political harmony, based on scientific evidence. David Cooper gave us the evidence to support the rare national energy and determination. He seized the moment … [He] was tireless in the preparation. He was superbly professional. He gathered together a magnificent team. He reached out, beyond our country. We should be proud of such a scientist and of our country, its universities and the institutions, that produced him. His family that nurtured him. His religion that taught him. The patients that loved him. But he was not ours alone. He belonged to the world of science. Today we honour him as a global hero. – The Hon. Michael Kirby, friend.43
David is survived by Dorrie, Ilana (now working for NSW Health), Bec (co-owner of fashion label Bec & Bridge) and her two children, David’s grandsons Max and Teddy.
Conflicts of interest
The authors were friends and professional colleagues of David Cooper, and each co-published with him. Additionally, David Cooper was the PhD supervisor of ADK. The authors declare no other conflicts of interest.
References
Brumby, E. (2014) Communication and health policy creation during the Australian HIV/AIDS crisis, Sydney Morning Herald, 15 July. https://www.smh.com.au/education/communication-and-health-policy-creation-during-the-australian-hivaids-crisis-20140715-3byt8.html, viewed October 2023
Carr, A., and Cooper, D. A. (1998) Lipodystrophy associated with an HIV-protease inhibitor, The New England Journal of Medicine, 339(18), 1296.
| Crossref | Google Scholar | PubMed |
Carr, A., Workman, C., Smith, D. E., Hoy, J., Hudson, J., Doong, N., Martin, A., Amin, J., Freund, J., Law, M., Cooper, D. A., and for the Mitochondrial Toxicity (MITOX) Study Group (2002) Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy: a randomized trial, JAMA, 288(2), 207-215.
| Crossref | Google Scholar | PubMed |
Cooper, D. A., and Dodds, A. J. (1986) Letter to the Editor: Aids and Prostitutes, Medical Journal of Australia, 145(1), 55.
| Crossref | Google Scholar |
Cooper, D. A., and Pantaleo, G. (2015) One year on: vale Joep Lange, Current Opinion in HIV and AIDS, 10(4), 215.
| Crossref | Google Scholar | PubMed |
Cooper, D. A., Duckett, M., Hansen, P., Petts, V., and Penny, R. (1981a) Glucocorticosteroid enhancement of immunoglobulin synthesis by pokeweed mitogen-stimulated human lymphocytes, Clinical & Experimental Immunology, 44(1), 129-136.
| Google Scholar | PubMed |
Cooper, D. A., Gold, J., Maclean, P., Donovan, B., Finlayson, R., Barnes, T. G., Michelmore, H. M., Brooke, P., Penny, R., and for the Sydney AIDS Study Group (1985) Acute AIDS retrovirus infection. Definition of a clinical illness associated with seroconversion, The Lancet, 1(8428), 537-540.
| Crossref | Google Scholar | PubMed |
Delta Coordinating Committee (1996) Delta: a randomised double-blind controlled trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals, The Lancet, 348(9023), 283-291.
| Crossref | Google Scholar |
Delta Coordinating Committee (2001) Evidence for prolonged clinical benefit from initial combination antiretroviral therapy: Delta extended follow-up, HIV Medicine, 2(3), 181-188.
| Crossref | Google Scholar | PubMed |
ENCORE1 Study Group (2014) Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial, The Lancet, 383(9927), 1474-1482.
| Crossref | Google Scholar | PubMed |
Grulich, A. E., Jin, F., Bavinton, B. R., Yeung, B., Hammoud, M. A., Amin, J., Cabrera, G., Clackett, S., Ogilvie, E., Vaccher, S., Vickers, T., McNulty, A., Smith, D. J., Dharan, N. J., Selvey, C., Power, C., Price, K., Zablotska, I., Baker, D. A., Bloch, M., Brown, K., Carmody, C. J., Carr, A., Chanisheff, D., Doong, N., Finlayson, R., Lewis, D. A., Lusk, J., Martin, S., Ooi, C., Read, P., Ryder, N., Smith, D., Tuck Meng Soo, C., Templeton, D. J., Vlahakis, E., Guy, R., and for the Expanded PrEP Implementation in Communities New South Wales (EPIC-NSW) research group (2021) Long-term protection from HIV infection with oral HIV pre-exposure prophylaxis in gay and bisexual men: findings from the expanded and extended EPIC-NSW prospective implementation study, The Lancet HIV, 8(8), 486-494.
| Crossref | Google Scholar | PubMed |
INSIGHT-ESPRIT Study Group and SILCAAT Scientific Committee, Abrams, D., Lévy, Y., Losso, M. H., Babiker, A., Collins, G., Cooper, D. A., Darbyshire, J., Emery, S., Fox, L., Gordin, F., Lane, H. C., Lundgren, J. D., Mitsuyasu, R., Neaton, J. D., Phillips, A., Routy, J. P., Tambussi, G., and Wentworth, D. (2009) Interleukin-2 therapy in patients with HIV infection, The New England Journal of Medicine, 361(16), 1548-1559.
| Crossref | Google Scholar | PubMed |
International AIDS Society (2017), ‘David Cooper’, https://www.youtube.com/watch?v=DDRC6iqfx7M, viewed October 2023.
Kirby, M. (1996) Human rights and the HIV paradox, The Lancet, 348(9036), 1217-1218.
| Crossref | Google Scholar | PubMed |
Kirby, T. (2016) David Cooper: Australia’s fighter against HIV and discrimination, The Lancet, 388(10059), 2469.
| Crossref | Google Scholar | PubMed |
Kirby, M. (2018) ‘Professor David Cooper AC Memorial Service’ (speech), https://livestream.com/accounts/5690925/events/8246707, viewed October 2023.
Mardi Gras and Ward 17 South: 35 years on (n.d.) St Vincent’s Curran Foundation, https://www.supportstvincents.com.au/about-us/our-stories/35-years-since-hiv-aids-ward-opening-17-south/, viewed October 2023.
McLaws, M. L., Brown, A. R., Cunningham, P. H., Imrie, A. A., Wilcken, B., and Cooper, D. A. (1990) Prevalence of maternal HIV infection based on anonymous testing of neonates, Sydney 1989, Medical Journal of Australia, 153(7), 383-386.
| Crossref | Google Scholar | PubMed |
Meuer, S. C., Cooper, D. A., Hodgdon, J. C., Hussey, R. E., Morimoto, C., Schlossman, S. F., and Reinherz, E. L. (1983a) Immunoregulatory human T lymphocytes triggered as a consequence of viral infection: clonal analysis of helper, suppressor inducer and suppressor effector cell populations, Journal of Immunology, 131(3), 1167-1172.
| Google Scholar | PubMed |
Meuer, S. C., Cooper, D. A., Hodgdon, J. C., Hussey, R. E., Fitzgerald, K. A., Schlossman, S. F., and Reinherz, E. L. (1983b) Identification of the receptor for antigen and major histocompatibility complex on human inducer T lymphocytes, Science, 222(4629), 1239-1242.
| Crossref | Google Scholar | PubMed |
Montaner, J. S. G., Reiss, P., Cooper, D., Vella, S., Harris, M., Conway, B., Wainberg, M. A., Smith, D., Robinson, P., Hall, D., Myers, M., Lange, J. M. A., and for the INCAS Study Group (1998) A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: The INCAS Trial, JAMA, 279(12), 930-937.
| Crossref | Google Scholar | PubMed |
SECOND-LINE Study Group, Boyd, M. A., Kumarasamy, N., Moore, C. L., Nwizu, C., Losso, M. H., Mohapi, L., Martin, A., Kerr, S., Sohn, A. H., Teppler, H., Van de Steen, O., Molina, J. M., Emery, S., and Cooper, D. A. (2013) Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study, The Lancet, 381(9883), 2091-2099.
| Crossref | Google Scholar | PubMed |
Solomon, P. J., Wilson, S. R., Swanson, C. E., and Cooper, D. A. (1990) Effect of zidovudine on survival of patients with AIDS in Australia, Medical Journal of Australia, 153(5), 254-257.
| Crossref | Google Scholar | PubMed |
Sonnabend, D., Cooper, D., Fiddes, P. J., and Penny, R. (1972) Fibrin degradation products in thrombo-embolic disease, Pathology, 4(1), 47-51.
| Crossref | Google Scholar | PubMed |
Sydney AIDS Study Group (1984) The Sydney AIDS Project, Medical Journal of Australia, 141(9), 569-573.
| Crossref | Google Scholar |
The INSIGHT START Study Group (2015) Initiation of antiretroviral therapy in early asymptomatic HIV infection, The New England Journal of Medicine, 373(9), 795-807.
| Crossref | Google Scholar |
The Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr, W. M., Lundgren, J. D., Neaton, J. D., Gordin, F., Abrams, D., Arduino, R. C., Babiker, A., Burman, W., Clumeck, N., Cohen, C. J., Cohn, D., Cooper, D., Darbyshire, J., Emery, S., Fätkenheuer, G., Gazzard, B., Grund, B., Hoy, J., Klingman, K., Losso, M., Markowitz, N., Neuhaus, J., Phillips, A., and Rappoport, C. (2006) CD4+ count-guided interruption of antiretroviral treatment, The New England Journal of Medicine, 355(22), 2283-2296.
| Crossref | Google Scholar | PubMed |
Van de Ven, P., Noble, J., Kippax, S., Prestage, G., Crawford, J., Baxter, D., and Cooper, D. (1997) Gay youth and their precautionary sexual behaviors: the Sydney men and sexual health study, AIDS Education and Prevention, 9(5), 395-410.
| Google Scholar | PubMed |
Van de Ven, P., Campbell, D., Kippax, S., Knox, S., Prestage, G., Crawford, J., Kinder, P., and Cooper, D. (1998) Gay men who engage repeatedly in unprotected anal intercourse with casual partners: the Sydney Men and Sexual Health Study, International Journal of STD & AIDS, 9(6), 336-340.
| Crossref | Google Scholar | PubMed |
Ward, J., Guy, R. J., Rumbold, A. R., McGregor, S., Wand, H., McManus, H., Dyda, A., Garton, L., Hengel, B., Silver, B. J., Taylor-Thomson, D., Knox, J., Donovan, B., Law, M., Maher, L., Fairley, C. K., Skov, S., Ryder, N., Moore, E., Mein, J., Reeve, C., Ah Chee, D., Boffa, J., Kaldor, J. M, and on behalf of the STRIVE investigators (2019) Strategies to improve control of sexually transmissible infections in remote Australian Aboriginal communities: a stepped-wedge, cluster-randomised trial, The Lancet Global Health, 7(11), 1553-1563.
| Crossref | Google Scholar | PubMed |
Ziegler, J. B., Cooper, D. A., Johnson, R. O., and Gold, J. (1985) Postnatal transmission of AIDS-associated retrovirus from mother to infant, The Lancet, 325(8434), 896-898.
| Crossref | Google Scholar | PubMed |
Footnotes
3 Letter, D. Cooper, R. Penny, P. Fiddes to editor, ‘Autologous-plasma sensitisation in Behcet’s disease’, in The Lancet, 1 May 1971; 910.
4 Sonnabend and others (1972) pp. 47–51.
6 Cooper and others (1981a, pp. 129–136, 1981b).
10 Brumby (2014).
14 Sydney AIDS Study Group (1984) pp. 569–573.
15 Sydney AIDS Study Group (1984) pp. 569–573.
16 Cooper and others (1985) pp. 537–540.
17 Ziegler and others (1985) pp. 896–898.
19 Kirby (1996) pp. 1217–1218.
20 The other two being the National Centre for HIV Virology Research, Melbourne (now the Australian Centre for HIV and Hepatitis Virology Research [ACH4]), and the National Centre for HIV and Social Research (now the Centre for Social Research in Health, UNSW Sydney).
21 Solomon and others (1990) pp. 254–257.
23 McLaws and others (1990) pp. 383–386.
24 Van de Ven and others (1997, pp. 395–410, 1998, pp. 336–340).
25 Carr and Cooper (1998) p. 1296.
26 Carr and others (2002) pp. 207–215.
27 Delta Coordinating Committee (1996, pp. 283–291, 2001, pp. 181–188). Note: David Cooper was a member of the committee.
28 Montaner and others (1998) pp. 930–937.
30 The INSIGHT START Study Group (2015) pp. 795–807.
31 The Strategies for Management of Antiretroviral Therapy (SMART) Study Group and others (2006) pp. 2283–2296.
32 INSIGHT-ESPRIT Study Group and others (2009) pp. 1548–1559.
34 Ward and others (2019) pp. 1553–1563.
35 ENCORE1 Study Group (2014) pp. 1474–1482.
36 SECOND-LINE Study Group and others (2013) pp. 2091–2099.
37 Cooper and Pantaleo (2015) p. 215.
39 Kirby (2016) p. 2469.
40 Grulich and others (2021) pp. 486–494.
42 Kirby (2016) p. 2469.
43 Kirby (2018).




